Moss Tom. DNA-protein interactions: principles and protocols
Подождите немного. Документ загружается.

358 Naryshkin et al.
11. Acryloyl chloride reacts violently with water. Add acryloyl chloride in 0.5-mL
portions, waiting 30 s between successive additions.
12. BAC is substituted for bis-acrylamide on a mole-equivalent, not mass-equiva-
lent, basis (43–45). The solubility of BAC in water is increased by adding
acrylamide before adding BAC and by performing additions at 60°C.
13. TEMED and ammonium persulfate concentrations are critical variables in the
preparation of polyacrylamide:BAC gels (44,45). (Use of nonoptimal TEMED
and ammonium persulfate concentrations in preparing of polyacrylamide:BAC
results in difficulties in subsequently solubilizing gels.)
14. Siliconize notched glass plate by applying 30 µL SurfaSil siliconizing agent and
spreading evenly with a Kimwipe.
15. Heating during polymerization yields polyacrylamide:BAC gels that are
maximally solubilizable upon the addition of reducing agents (44,45). Heat
the glass plates of the gel assembly evenly. (If necessary, use two task lamps.)
Avoid heating above 70°C, as this can result in the formation of bubbles and/
or detachment of gels from the glass plates.
16. Do not add loading buffer to the reaction mixture. The reaction mixture is suffi-
ciently dense for loading (because of the presence of glycerol).
17. Ultraviolet irradiation is performed with both glass plates in place. The glass
plates exclude wavelengths <300 nm, minimizing photodamage to the protein
and DNA. It is important to verify that the plates exhibit absorbances of ≤1.5 AU
at 320 nm (e.g., by sacrificing a glass plate and placing a piece in the cuvet holder
of a UV/Vis spectrophotometer). Glass plates purchased from Aladin (San Fran-
cisco, CA, USA) have performed satisfactorily.
18. For in-gel UV irradiation of the RNAP–promoter intermediate complex, prechill
photochemical reactor for 15 min in a 15°C cabinet.
19. Do not use tight-fitting X-ray autoradiography cassettes, which can squeeze and
distort the gel on the glass plate during exposure. The Kodak X-ray exposure
holder with intensifying screen has performed satisfactorily.
20. 2–4 M β-mercaptoethanol can be substituted for 1 M DTT.
Acknowledgments
The basic protocol for preparation of derivatized DNA fragments was
developed by T. Lagrange (1), the basic protocol for preparation of RNAP was
developed by H. Tang and K. Severinov (34,46), and the basic protocol for
in-gel UV irradiation was developed by T.-K. Kim (2).
We thank K. Severinov for plasmids and T.-K. Kim, T. Lagrange, D.
Reinberg, and K. Severinov for discussions; and we are grateful for the Howard
Hughes Medical Institute Investigatorship and National Institutes of Health
grant GM41376 to R.H.E. for financial support.
References
1. Lagrange, T., Kim, T. K., Orphanides, G., Ebright, Y., Ebright, R., and Reinberg,
D. (1996) High-resolution mapping of nucleoprotein complexes by site-specific
Protein–DNA Photocrosslinking 359
protein–DNA photocrosslinking: organization of the human TBP–TFIIA–TFIIB–
DNA quaternary complex. Proc. Natl. Acad. Sci. USA 93, 10,620–10,625.
2. Kim, T.-K., Lagrange, T., Wang, Y.-H., Griffith, J., Reinberg, D., and Ebright, R.
(1997) Trajectory of DNA in the RNA polymerase II transcription preinitiation
complex. Proc. Natl. Acad. Sci. USA 94, 12,268–12,273.
2a. Kim, T.-K., Ebright, R., and Reinberg, D. (2000) Mechanisms of ATP-dependent
promoter melting by transcription factor IIH. Science 288, 1418–1421.
3. Lagrange, T., Kapanidis, A., Tang, H., Reinberg, D., and Ebright, R. (1998) New
core promoter element in RNA polymerase II-dependent transcription: sequence-
specific DNA binding by transcription factor IIB. Genes Dev. 12, 34–44.
4. Fidanza, J., Ozaki, H., and McLaughlin, L. (1992) Site-specific labeling of
DNA sequences containing phosphorothioate diesters. J. Am. Chem. Soc. 114,
5509–5517.
5. Yang, S.-W. and Nash, H. (1994) Specific photocrosslinking of DNA–protein
complexes: identification of contacts between integration host factor and its target
DNA. Proc. Natl. Acad. Sci. USA 91, 12,183–12,187.
6. Mayer, A. and Barany, F. (1995) Photoaffinity cross-linking of TaqI restriction
endonuclease using an aryl azide linked to the phosphate backbone. Gene 153, 1–8.
7. Sambrook, J., Fritsch, E., and Maniatis, T. (1989) Molecular Cloning: A Labora-
tory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
8. Wang, Y. and Stumph, W. (1998) Identification and topological arrangement of
Drosophila proximal sequence element (PSE)-binding protein subunits that
contact the PSEs of U1 and U6 small nuclear RNA genes. Mol. Cell. Biol. 18,
1570–1579.
9. Kim, Y., Geiger, J., Hahn, S., and Sigler, P. (1993) Crystal structure of a yeast
TBP/TATA-box complex. Nature 365, 512–520.
10. Kim, J., Nikolov, D., and Burley, S. (1993) Co-crystal structure of TBP recogniz-
ing the minor groove of a TATA element. Nature 365, 520–527.
11. Geiger, J., Hahn, S., Lee, S., and Sigler, P. (1996) Crystal structure of the yeast
TFIIA/TBP/DNA complex. Science 272, 830–836.
12. Tan, S., Hunziker, Y., Sargent, D., and Richmond, T. (1996) Crystal structure of a
yeast TFIIA/TBP/DNA complex. Nature 381, 127–134.
13. Nikolov, D., Chen, H., Halay, E., Usheva, A., Hisatake, K., Lee, D. K., et al.
(1995) Crystal structure of a TFIIB–TBP–TATA-element ternary complex. Nature
377, 119–128.
14. Bartholomew, B., Kassavetis, G., Braun, B., and Geiduschek, E. P. (1990) The
subunit structure of Saccharomyces cerevisiae transcription factor IIIC probed
with a novel photocrosslinking reagent. EMBO J. 9, 2197–2205.
15. Bartholomew, B., Kassavetis, G., and Geiduschek, E. P. (1991) Two components
of Saccharomyces cerevisiae transcription factor IIIB (TFIIIB) are stereospecifi-
cally located upstream of a tRNA gene and interact with the second-largest sub-
unit of TFIIIC. Mol. Cell. Biol. 11, 5181–5189.
16. Braun, B., Bartholomew, B., Kassavetis, G., and Geiduschek, E. P. (1992)
Topography of transcription factor complexes on the Saccharomyces cerevisiae 5
S RNA gene. J. Mol. Biol. 228, 1063–1077.
360 Naryshkin et al.
17. Kassavetis, G., Kumar, A., Ramirez, E., and Geiduschek, E. P. (1998) Functional
and structural organization of Brf, the TFIIB-related component of the RNA poly-
merase III transcription initiation complex. Mol. Cell. Biol. 18, 5587–5599.
18. Bell, S., and Stillman, B. (1992) ATP-dependent recognition of eukaryotic ori-
gins of DNA replication by a multiprotein complex. Nature 357, 128–134.
19. Coulombe, B., Li, J., and Greenblatt, J. (1994) Topological localization of the human
transcription factors IIA, IIB, TATA Box-binding protein, and RNA polymerase II-
associated protein 30 on a class II promoter. J. Biol. Chem. 269, 19,962–19,967.
20. Gong, X., Radebaugh, C., Geiss, G., Simon, S., and Paule, M. (1995) Site-directed
photo-crosslinking of rRNA transcription initiation complexes. Mol. Cell. Biol.
15, 4956–4963.
21. Pruss, D., Bartholomew, B., Persinger, J., Hayes, J., Arents, G., Moudrianakis, E.,
and Wolffe, A. (1996) An asymmetric model for the nucleosome: a binding site
for linker histones inside the DNA gyres. Science 274, 614–617.
22. Robert, F., Forget, D., Li, J., Greenblatt, J., and Coulombe, B. (1996) Localiza-
tion of subunits of transcription factors IIE and IIF immediately upstream of the
transcriptional initiation site of the adenovirus major late promoter. J. Biol. Chem.
271, 8517–8520.
23. Forget, D., Robert, F., Grondin, G., Burton, Z., Greenblatt, J., and Coulombe, B.
(1997) RAP74 induces promoter contacts by RNA polymerase II upstream and
downstream of a DNA bend centered on the TATA box. Proc. Natl. Acad. Sci.
USA 94, 7150–7155.
24. Record, M. T., Reznikoff, W., Craig, M., McQuade, K., and Schlax, P. (1996)
Escherichia coli RNA polymerase (Eσ70), promoters, and the kinetics of the steps
of transcription initiation, in Escherichia coli and Salmonella, vol. 1 (Neidhart,
F., ed.), ASM, Washington, DC, pp. 792-820.
25. deHaseth, P., Zupancic, M., and Record, M. (1998) RNA polymerase–promoter
interactions. J. Bact. 180, 3019–3025.
26. Ebright, R. (1998) RNA polymerase–DNA interaction: structures of intermediate,
open, and elongation complexes. Cold Spring Harbor Symp. Quant. Biol. 63, 11–20.
27. Buc, H. and McClure, W. (1985) Kinetics of open complex formation between
Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a
sequential mechanism involving three steps. Biochemistry 24, 2712–2723.
28. Spassky, A., Kirkegaard, K., and Buc, H. (1985) Changes in the DNA structure of
the lac UV5 promoter during formation of an open complex with Escherichia coli
RNA polymerase. Biochemistry 24, 2723–2731.
29. Kirkegaard, K., Buc, H., Spassky, A., and Wang, J. (1983) Mapping of single-
stranded regions in duplex DNA at the sequence level: single-strand-specific
cytosine methylation in RNA polymerase–promoter complexes. Proc. Natl. Acad.
Sci. USA 80, 2544–2548.
30. Becker, M. and Wang, J. (1984) Use of light for footprinting DNA in vivo. Nature
309, 682–687.
31. Straney, D. and Crothers, D. (1985) Intermediates in transcription initiation from
the E. coli lac UV5 promoter. Cell 43, 449–459.
Protein–DNA Photocrosslinking 361
32. Severinov, K., Mustaev, A., Severinova, E., Bass, I., Kashlev, M., Landick, R., et
al. (1995) Assembly of functional Escherichia coli RNA polymerase containing
β subunit fragments. Proc. Natl. Acad. Sci. USA 92, 4591–4595.
33. Severinov, K., Mustaev, A., Kukarin, A., Muzzin, O., Bass, I., Darst, S., et al.
(1996) Structural modules of the large subunits of RNA polymerase. Introducing
archaebacterial and chloroplast split sites in the β and β' subunits of Escherichia
coli RNA polymerase. J. Biol. Chem. 271, 27,969–27,974.
34. Tang, H., Severinov, K., Goldfarb, A., and Elbright, E. (1995) Rapid RNA poly-
merase genetics: one-day, no-column preparation of reconstituted recombinant
Escherichia coli RNA polymerase. Proc. Natl. Acad. Sci. USA 92, 4902–4906.
35. Martin, E., Sagitov, V., Burova, E., Nikiforov, V., and Goldfarb, A. (1992)
Genetic dissection of the transcription cycle. A mutant RNA polymerase that can-
not hold onto a promoter. J. Biol. Chem. 267, 20,175–20,180.
36. Zalenskaya, K., Lee, J., Chandrasekhar, N. G., Shin, Y. K., Slutsky, M., and
Goldfarb, A. (1990) Recombinant RNA polymerase: inducible overexpression,
purification and assembly of Escherichia coli rpo gene products. Gene 89, 7–12.
37. Ebright, R., Ebright, Y., and Gunasekera, A. (1989) Consensus DNA site for the
Escherichia coli catabolite gene activator protein (CAP): CAP exhibits a 450-fold
higher affinity for the consensus DNA site than for the E. coli lac DNA site.
Nucleic Acids Res. 17, 10,295–10,305.
38. Gilbert, W. (1976) Starting and stopping sequences for the RNA polymerase, in
RNA Polymerase (Losick, R., and Chamberlin, M., eds.), Cold Spring Harbor
Laboratory, Cold Spring Harbor, NY, pp. 193–206.
39. Zhang, X., Zhou, Y., Ebright, Y., and Ebright, R. (1992) Catabolite gene activator
protein (CAP) is not an acidic-activating-region transcription activator protein:
negatively charged amino acids of CAP that are solvent-accessible in the CAP–
DNA complex play no role in transcription activation at the lac promoter. J. Biol.
Chem. 267, 8136–8139.
40. Kunkel, T. (1985) Rapid and efficient site-specific mutagenesis without pheno-
typic selection. Proc. Natl. Acad. Sci. USA 82, 488–492.
41. Staros, J., Bayley, H., Standring, D., and Knowles, J. (1978) Reduction of aryl
azides by thiols: implications for the use of photoaffinity reagents. Biochem.
Biophys. Res. Commun. 80, 568–572.
42. Melancon, P., Burgess, R., and Record, M. (1983) Direct evidence for the prefer-
ential binding of Escherichia coli RNA polymerase holoenzyme to the ends of
deoxyribonucleic acid restriction fragments. Biochemistry 22, 5169–5176.
43. Hansen, J. N. (1976) Electrophoresis of ribonucleic acid on a polyacrylamide gel
which contains disulfide cross-linkages. Anal. Biochem. 76, 37–44.
44. Hansen, J. N. (1980) Chemical and electrophoretic properties of solubilizable dis-
ulfide gels. Anal. Biochem. 105, 192–201.
45. Hansen, J. N. (1981) Use of solubilizable acrylamide disulfide gels for isolation
of DNA fragments suitable for sequence analysis. Anal. Biochem. 116, 146–151.
46. Tang, H., Kim, Y., Severinov, K., Goldfarb, A., and Ebright, R. (1996) Escheri-
chia coli RNA polymerase holoenzyme: rapid reconstitution from recombinant α,
β, β', and σ subunits. Methods Enzymol. 273, 130–134.

Site-Directed DNA Photoaffinity Labeling 363
25
Site-Directed DNA Photoaffinity Labeling
of RNA Polymerase III Transcription Complexes
Jim Persinger and Blaine Bartholomew
1. Introduction
Site-specific DNA photoaffinity labeling is a useful technique for mapping
interactions of proteins with DNA in complex systems such as the yeast RNA
polymerase III (Pol III) transcription complex, which consists of at least 25
different proteins (1,2). This technique allows probing of protein–DNA
interactions across large stretches of DNA and can be done in relatively crude
extracts. The regions or domains of the protein contacting DNA can be
identified by peptide mapping of the photoaffinity labeled protein. Our
discussion of DNA photoaffinity labeling will focus on (1) the synthesis of
photoreactive nucleotide analogs, (2) the manner in which the photoreactive
nucleotide is incorporated into DNA, and (3) experimental details of DNA
photoaffinity labeling.
Our group has used this technique to map the locations of many of the pro-
teins of the Pol III transcription complex to sites within the SUP4 tRNA
Tyr
gene (3–5). Some of the advantages of this approach are (1) detailed mapping
of protein interactions with DNA in large multisubunit protein–DNA com-
plexes and (2) the ability to use crude protein extracts potentially containing
important auxiliary factors that may be lost upon purification. Solid-phase
DNA probe synthesis allows for the synthesis of multiple probes in a single
day, whereas in the past, this process would have taken several days. The syn-
thesis of modified analogs for dATP, dCTP, and dTTP allow for the incorpora-
tion at nearly all positions in DNA. The photoreactive moiety can be changed
on these nucleotides to place the more photoreactive phenyl diazirine into DNA
to better target all potential protein surfaces. These photoreactive groups have
short half-lives of less than 1 ns to approx 5 µs and can be used for kinetic
363
From:
Methods in Molecular Biology, vol. 148: DNA–Protein Interactions: Principles and Protocols, 2nd ed.
Edited by: T. Moss © Humana Press Inc., Totowa, NJ
364 Persinger and Bartholomew
analysis of changes in specific protein–DNA contacts. The 4-thiothymidine
nucleotide has also been used for zero-distance crosslinking of protein to DNA
and may be use useful for probing very close protein–DNA contacts (6).
2. Materials
2.1. Synthesis of Modified Nucleotides
1. Para-azidobenzoic acid (4-ABA) (Molecular Probes).
2. Succinimidyl esters of 4-azidobenzoic acid, 4-azido-2,3,5,6-tetrafluorobenzoic
acid, and 4-benzoylbenzoic acid (Molecular Probes).
3. 5-[N-(3-Aminoallyl)]-deoxyuridine triphosphate (5-aa-dUTP) (Sigma).
4. dCTP (Sigma).
5. Ethylene diamine, dicyclohexylcarbodiimide, anhydrous dioxane (99+%), ethyl
ether, and sodium metabisulfite (Aldrich).
6. DEAE–Sephadex A-25 resin (Pharmacia).
7. Glycine, glycyl glycine, and glycylglycyl glycine (Sigma).
8. pH indicator strip (Panpeha, Schleicher & Schull).
9. Polyethyleneimine (PEI)–cellulose thin-layer chromatography (TLC) plates
(J. T. Baker, with fluorescence indicator).
10. TE: 10 mM Tris-HCl, pH 8.0, 1 mM EDTA.
2.2. Immobilized DNA Templates
1. Buffer A: 10 mM Tris-HCl (pH 8.0), 10 mM MgCl
2
, 50 mM NaCl, and 1 mM
dithiothreitol (DTT).
2. Buffer B: 1 M LiCl, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 0.1% sodium
dodecyl sulfate (SDS).
3. Plasmid DNA pTZ1 containing the SUP4 tRNA
Tyr
gene with promoter-up muta-
tion inserted into pGEM1 (7).
4. Magnetic separation stand for DNA bead isolation (Promega).
5. M-280 Streptavidin Dynabeads (Dynal).
6. Buffer C: 2 M NaCl, 10 mM Tris-HCl (pH 7.5), and 1 mM EDTA (pH 8.0).
7. Buffer D: 30 mM Tris-HCl (pH. 8.0), 50 mM KCl, 7 mM MgCl
2
, 1 mM of 2-mer-
captoethanol, and 0.05% Tween-20.
8. Polystrene chromatography columns (5 in. [12.5 cm]) with a 45- to 90-µm
filter (Evergreen Scientific). These disposable columns are ideal for the 2.5-mL
spin columns.
9. Bio-11–dUTP and Bio-14–dATP (Sigma).
2.3. DNA Probe Synthesis
1. Buffer E: 150 mM Tris-HCl (pH 8.0), 250 mM KCl, 35 mM MgCl
2
, 5 mM of
2-mercaptoethanol, and 0.25% Tween-20.
2. Storage buffer F: 50 mM potassium phosphate (pH 7.0), 5 mM of 2-mercap-
toethanol, and 50% glycerol.
Site-Directed DNA Photoaffinity Labeling 365
3. Storage buffer G: 50 mM KCl, 10 mM Tris (pH 7.5), 0.1 mM EDTA, 5 mM of
2-mercaptoethanol, 200 mg/mL bovine serum albumin (BSA), and 50% glycerol.
4. Site-specific oligonucleotides and upstream oligonucleotide (50-nmol-
scale synthesis).
5. 4-Thiothymidine triphosphate (Amersham/Pharmacia).
6. Exonuclease-free version of the Klenow fragment of DNA Polymerase I
(Amersham/Pharmacia, 5 U/µL) diluted to 0.25 U/µL with storage buffer F.
7. T
4
DNA ligase (New England Biolabs, high concentration form, 2000 U/µL)
diluted to approx 300 U/µL with storage buffer G containing 5 mM of
2-mercaptoethanol.
8. TE: 10 mM Tris-HCl (pH 8.0) and 1 mM EDTA.
9. PBS: phosphate-buffered saline solution (pH 7.4).
10. T
4
DNA polymerase from New England Biolabs, comes stored in 100 mM potas-
sium phosphate (pH 6.5), 10 mM of 2-mercaptoethanol, and 50% glycerol.
2.4. Photoaffinity Labeling
1. Buffer H: 100 mM Tris-HCl (pH 8.0), 25 mM MgCl
2
and 250 mM NaCl.
2. Buffer I: 100 mM NaCl, 40 mM Tris-HCl (pH 8.0), 5 mM MgCl
2
, 1 mM EDTA,
20% glycerol, 10 mM of 2-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluo-
ride (PMSF), 1 µg/mL pepstatin, and 1 µg/mL leupeptin.
3. Zinc acetate solution: 0.5 M glacial acetic acid and 12.5 mM zinc acetate.
4. 5X DB: 10% SDS, 25% 2-mercaptoethanol, 0.3 M Tris-HCl (pH 6.8), 0.4%
bromophenol blue.
5. 3 NTP mix: 2 µL of 100 mM ATP, UTP, and CTP (Boehringer Mannheim), 20 µL
buffer H, 35 µL Buffer I, and 43 µL sterile deionized water.
2.5. Peptide Mapping
1. Formic acid (99%, Sigma).
2. Diphenylamine (ACS 99+%, Aldrich).
2. Cyanogen bromide (97%, Aldrich.
3. Centricon 30 (Millipore).
3. Methods
Synthesis of modified nucleotides and DNA photoaffinity probes is done
with indirect lighting conditions using 40-W incandescent lamps.
3.1. Synthesis of Modified Nucleotides
We have used a variety of modified nucleotides to probe the RNA poly-
merase III transcription complex. This section contains procedures for the syn-
thesis of some of the commonly used nucleotides.
3.1.1. AB–dUTP
AB–dUTP (Fig. 1) is synthesized as follows:
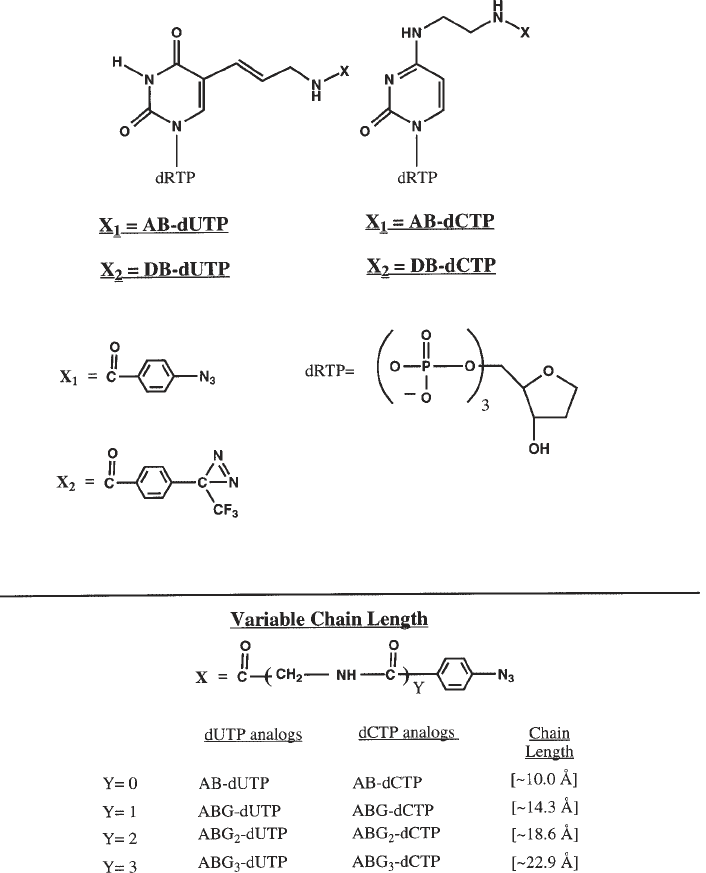
366 Persinger and Bartholomew
1. Add 100 µL of 100 mM 4-azidobenzoic acid N-hydroxysuccinimide solution
(ABA-NHS) in dimethylformamide (DMF) to 100 µL of 20 mM 5-aa-dUTP in
100 mM sodium borate (pH 8.5) (8). The reaction is incubated at 25°C for 4 h,
Fig. 1. Structures of each of the photoreactive nucleotide analogs are shown. The
complete IUPAC names for each nucleotide is given in the text.
Site-Directed DNA Photoaffinity Labeling 367
and the pH of the reaction is checked with pH indicator strips (pH 8.5). Any
excess precipitation that forms can be eliminated by addition of DMF.
2. The coupling reaction is stopped by the addition of 200 µL sterile deionized water
and the product applied to a 0.7 × 8-cm (1.6-mL) DEAE–Sephadex A-25 column
equilibrated in 100 mM TEAB (pH 8.0). The column is washed with 5 mL of the
same buffer at a flow rate of 5 mL/h, and eluted with a 30-mL linear gradient of
0.1 to 1.5 M TEAB (pH 8.0), and 500 µL fractions are collected.
3. Every third fraction from the column is evaporated to dryness by vacuum
centrifugation and resuspended in 250 µL of sterile deionized water. The samples
are dried down and this process repeated a further time.
4. The fractions are resuspended in 50 µL of sterile deionized water and 2 µL of
each analyzed on PEI-cellulose TLC plates (see Note 1). The plates were
developed with 1 M LiCl and visualized with ultraviolet (UV) light source
(254 nM). The reported R
f
values for 5-aa-dUTP and AB–dUTP are 0.54 and
0.098, respectively (8).
5. All of the fractions containing AB–dUTP are combined and TEAB is removed
by repeated drying and resuspension in sterile deionized water. The final product
is resuspended in 200 µL of TE. An estimated extinction coefficient of AB–dUTP
at 270 nm is 10.3 × 10
3
/M/cm at pH 8.0 (based on the sum of the extinction
coefficients of ABA and 5-aa-dUTP at the indicated pH and wavelength). A con-
centrated stock of AB–dUTP is stable at –80°C for several years, and a 0.2-mM
working stock can be stored at –20°C wrapped in foil (see Notes 2–4).
3.1.2. Varied Tether-Length Nucleotides
The tether of AB–dUTP is 9–10 Å in length and places the photoreactive
group near the edge of the major groove of DNA. We have synthesized differ-
ent dUTP and dCTP analogs with varying tether lengths by the addition of
glycine residues into the tether (4). Synthesis of these nucleotide analogs is
similar to that of AB–dUTP and is as follows (Fig. 1).
Para-azidobenzoic acid (4-ABA) was esterified with N-hydroxysuccinimide
(NHS) using the coupling reagent dicyclohexylcarbodiimide (DCI) and the
product was recrystallized from anhydrous dioxane and ethyl ether (1:1) (9).
1. A typical reaction contained 28 mmol ABA and 28 mmol NHS in 50 mL of anhy-
drous dioxane (99+%).
2. The solution is cooled in ice, and DCI in 15 mL dioxane is added and stirred for
approx 24 h at room temperature.
3. Dicyclohexyl urea is removed by centrifugation and the supernatant was evapo-
rated to dryness by vacuum centrifugation.
The ABA-NHS is coupled to glycine, (Gly-Gly), or (GlyGlyGly) to make ABA
derivatives with glycine, Gly-Gly, or Gly-Gly-Gly, respectively, attached to the
carboxylic group of 4-ABA.
4. The reaction is started on ice and contains 2 mmol of glycine, the Gly-Gly, or
Gly-Gly-Gly and 4 mmol of sodium bicarbonate in 4 mL of deionized
368 Persinger and Bartholomew
water to which is added 2 mmol of ABA-NHS in 8 mL of dioxane with con-
stant stirring.
5. The reaction is allowed to proceed for 10–15 min on ice and is then transferred to
room temperature and left with stirring for an additional 24 h.
6. Any insoluble material is removed from the reaction by centrifugation.
7. The pH of the reaction is lowered to 2 with concentrated HCl to precipitate
the product.
8. Products are washed with deionized water.
9. The products are esterified with N-hydroxysuccinimide as described for ABA
(Subheading 3.1.1.) except that dimethyl sulfoxide is used instead of dioxane
for the ABG
3
-NHS because of the limited solubility of this compound.
10. The dimethyl sulfoxide (DMSO) or dioxane is removed by vacuum centrifugation
and the product is recrystallized from dioxane/isopropyl alcohol (1:1). Any residual
solvent is removed by vacuum centrifugation. These products are coupled to 5-aa-
dUTP in the same fashion as described for AB–dUTP (Subheading 3.1.1.).
3.1.3. Varied Photochemistry Nucleotides
We have also varied the photoreactive group attached to 5-aa-dUTP to con-
tain either a phenyldiazirine, tetraflouro aryl azide, or a benzophenone group
to optimize for nonselective crosslinking (5). The coupling reactions of 5-aa-dUTP
to the NHS esters of 4-azido-2,3,5,6-tetrafluorobenzoic acid, 4-benzoylbenzoic
acid (commercially available from Molecular Probes), and 4-[3-9trifluoro-
methyl)diazirin-3-yl]benzoic acid (synthesized as described in ref. 10) are
similar to that for the synthesis of AB–dUTP (Fig. 1).
3.1.4. Synthesis of dCTP Analogs
The synthesis of dCTP nucleotides begins with the synthesis of N
4
-amino-
ethyl deoxycytidine triphosphate (daeCTP) by a bisulfite-catalyzed transami-
nation reaction.
1. A bisulfite-amine solution is made by adding dropwise 2 mL of freshly distilled
ethylene diamine to 4.0 mL of concentrated HCl and 3.5 mL of deionized water
on ice.
2. Next, sodium meta-bisulfite (1.895 g) is added and the pH is adjusted to 5.0 with
concentrated HCl.
3. Then, 100 µL of 1 mg/mL hydroquinone in ethanol is added to the reaction to
scavenge free radicals. Bisulfite-amine solutions are always made up fresh.
4. The transamination reaction is initiated by adding 9 vol of the bisulfite-amine
solution to 1 vol of 100 mM dCTP in 50 mM TEAB (pH 8.0).
5. The sample is incubated with constant vortexing at 42°C for 4 h.
6. The reaction is stopped by adjusting the pH to 8.2 with 5 M KOH.
7. The product is purified by DEAE–Sephadex A-25 chromatography as described
for the purification of AB–dUTP (Subheading 3.1.1.) and dae-dCTP eluted from
0.84 M to 1.0 M TEAB.
