Magill J., Galy J. Radioactivity Radionuclides Radiation
Подождите немного. Документ загружается.


6. Archaeology and Dating
Radioactive Dating
Radioactive dating methods are based on the temporal decrease of the number of
radioactive atoms and/or on the related ingrowth of radioactive or stable daughter
nuclides. These changes are described by the radioactive decay laws and in particular
by the decay constant or half-life. Because the half-life of a given radioisotope is
not affected by temperature, physical or chemical state, or any other influence of
the environment outside the nucleus (apart, of course, from nuclear reactions), then
radioactive samples continue to decay at a predictable rate. This makes several types
of radioactive dating feasible [1, 2].
Fig. 6.1. The Dead Sea scrolls were dated to 1900 y BP (before present) using
14
C dating [3].
© Rights reserved
Comparison of Present and Initial Radioactivity
The simplest form of isotopic age computation is where there are no daughter atoms
present initially and where no mass has been lost from the sample. The equation for
the time-dependence of the parent is given by:
P(t) = P(0)e
−kt
,
where P(t)is the quantity of parent isotope present at time t,P(0) the initial amount
and k the decay constant k = ln 2/τ . It follows that the age can be obtained from
Age = t =
1
k
· ln
P(0)
P(t)
.

106 6. Archaeology and Dating
14
C Dating
Carbon-14 is produced at almost a constant rate in the atmosphere by interactions of
cosmic rays with nitrogen i.e.
14
N(n,p)
14
C. Following production the carbon atoms are
oxidised to form
14
CO
2
which then follows the CO
2
cycle. Following the death of an
organism, the CO
2
exchange is terminated and the
14
C starts to decay. Since the initial
activity is known (activity of
14
C in the atmosphere), and the present value of
14
Cinthe
organism can be measured, the age can be determined.
Example: Take the present
14
C activity to be 900 Bq per gram. In the sample the activity
is 6 Bq per gram. The age of the sample is then
Age =
5730 y
0.693
· ln
900
6
= 41400 y .
Archaeological dating by radiocarbon is limited to about ten half-lives i.e. to about
50,000 BP (before present). Longer-lived nuclides such as
10
Be (1.6 million years),
26
Al
(760,000 years) and
41
Ca (100,000) can be used to date older samples.
So from a knowledge of the parent concentrations at different times, the age can
be evaluated. This age determination can be applied to many of the radionuclides
produced in the atmosphere by the cosmic radiation (cosmogenic radionuclides). The
best known example is
14
C (see inset).
Since the parent atoms decay to daughter atoms, the number of daughter atoms
D(t) = P(0) − P(t)or P(0) = P(t)+ D(t), the “age” t can be obtained from
Age = t =
1
k
· ln
1 +
D(t)
P(t)
.
From a measurement of the parent and daughter concentrations, and the time t , the
age can be evaluated (assuming the decay constant is known).
Radioactive Parent and Stable Decay Product
In this case a parent nuclide decays to a stable daughter. In general one has to account
for the fact that the (stable) daughter nuclide is already present such that the following
relation holds:
D(t) + P(t)= D(0) + P(0).
Since this now adds an additional unknown, D(0), additional information is required
before the age can be determined. There are two main assumptions in the dating
process:
• The amount of daughter isotope at the time of formation of the sample is zero (or
known independently and can be compensated for).
• No parent isotope or daughter isotope has entered or left the sample since its time
of formation.
If one of these assumptions has been violated, the simple computation above yields
an incorrect age.
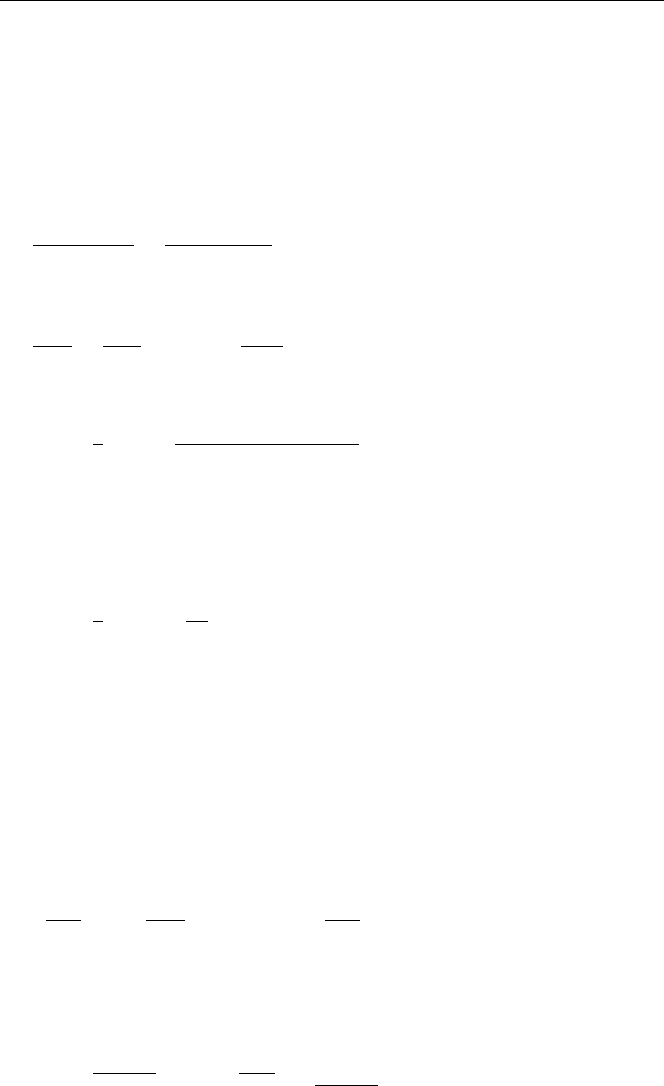
Radioactive Dating 107
Isochron Methodology
Isochron methods avoid the problems which can potentially result from both of the
above assumptions. Such information can be obtained in cases where there are other
stable isotopes of the elements involved in the sample. This stable, non-radiogenic
nuclide can then be used as a reference nuclide. Denoting the concentration of the
stable, non-radiogenic daughter nuclide by D
s
, then the above equation can be written
in the form of ratios [4, 5] e.g.
D(t) + P(t)
D
s
=
D(0) + P(0)
D
s
.
Substituting P(0) = P(t)e
+kt
, the relation can be expressed as
D(t)
D
s
=
P(t)
D
s
[e
kt
− 1]+
D(0)
D
s
.
From which the age is given by
Age =
1
k
ln
1 +
(D(t)/D
s
− D(0)/D
s
)
P (t)/D
s
.
The equation for D(t)/D
s
above has the form
y = x ·[e
kt
− 1]+y
0
.
On a plot of y vs. x, the slope is dy/dx = e
kt
− 1. The age can then be determined
by
Age =
1
k
· ln
1 +
dy
dx
.
If the samples have the same age, a plot of D/D
s
against P/D
s
will give a straight
line with slope (e
kt
− 1) intersecting the ordinate at D(0)/D
s
. Such a plot is called
an isochron and is particularly useful for age determinations on samples of the same
age.
Rubidium-Strontium Dating
In the Rb/Sr method, determination of the parent (
87
Rb) and the daughter (
87
Sr)
are compared to to the stable isotope
86
Sr which is used as the reference nuclide.
Application of the isochron method to the Rb/Sr system gives
87
Sr
86
Sr
t
=
87
Rb
86
Sr
0
(e
kt
− 1) +
87
Sr
86
Sr
0
.
A plot of
87
Sr/
86
Sr against
87
Rb/
86
Sr for various samples of the same age is
shown in Fig. 6.1. From the relation above, using 1/k = τ/0.693, the age can then
be determined i.e.
Age =
47.5Gy
(0.693)
· ln
1 +
0.3
4.51
= 4.41 Gy
.
108 6. Archaeology and Dating
Fig. 6.2. Rb/Sr dating method [2],
HyperPhysics, © C. R. Nave, 2003
Radioactive Disequilibrium
Radioactive disequilbria may arise in a variety of situations in which the parent
and daughter nuclides do not remain together. Following chemical processing, it is
possible to separate the parent and daughter nuclides. Following this separation, the
daughter nuclide will start to grow in again. Information on the time of this processing
or separation may be obtained by comparing the ratio of the parent to daughter
nuclides. An example of this is given later in this chapter for the determination of
the age of plutonium particles. Fresh plutonium particles will, in general, contain the
isotopes
238
Pu,
239
Pu,
240
Pu,
241
Pu, and
242
Pu depending on the production route.
With time, these nuclides decay to
234
U,
235
U,
236
U,
241
Am, and
238
U, respectively.
From the ratios of the parent to daughter nuclides, one can deduce the elapsed time
and hence the age of the particle.
Fission Tracks
Fission tracks, which result from spontaneous fission or by neutron induced fission,
can be used for dating purposes. The method is really only applicable to
238
U where
there is a measurable density of tracks. In a first step, the tracks naturally produced in
minerals by
238
U spontaneous fission are counted. In the second step, the uranium in
the sample is determined by
235
U fission tracks in a reactor with a well characterised
thermal flux. The age of the sample is obtained from the ratios of the tracks, the
reactor neutron flux, etc.
In the following sections, various examples of dating techniques based on ra-
dioactive decay are described in more detail.
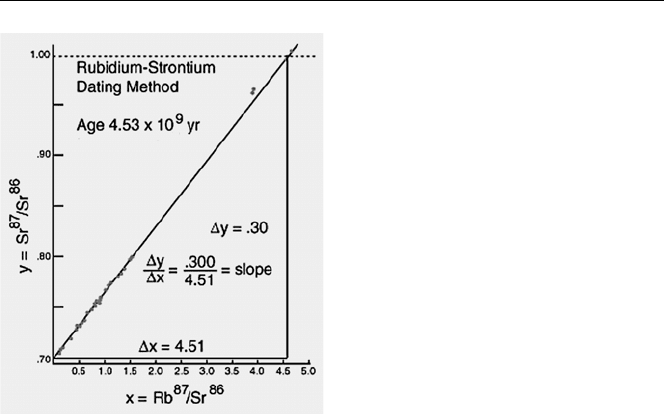
An Astrophysical Clock
Heavy elements such as uranium formed in a supernova more than 6000 million
years ago. From the supernova remnants, the solar system was born around 4600
Age of the Earth 109
Fig. 6.3. The Astrophysical Clock
million years ago. Assuming uranium isotopes were produced in equal amounts in
the supernova the ratios of uranium isotopes can be used as an astrophysical clock.
Today, the natural abundance of
235
U ratio is 0.71%. The natural reactors at Oklo in
Africa occurred around 2000 million years ago at which time the natural abundance of
235
U was around 3%. Evidence indicates that the natural uranium deposits achieved
nuclear criticality and operated for tens of thousands of years or longer. The absence
of
236
U, which has a half-life of 2.342 × 10
7
y indicates that induced fission stopped
at least 10
8
years ago. The recently discovered “Toumai” has been dated to around
7 million years. In the above diagram this time is almost indistinguishable from the
present time.
Age of the Earth
Towards the end of the 19th century, the age of the Earth was of great scientific
controversy [6]. Lord Kelvin had estimated the age to be around 20 million years
based on the rate of cooling assuming that the Earth was originally in a molten
state. Already by 1840, from the work of Hutton and Lyell, it was known that rocks
were laid down on top of one another in an orderly manner. This gave rise to age
estimates based on sedimentation geology. Darwin had estimated the age of the Earth
at 300 million years from cliff erosion assuming an erosion rate of 1 inch per century.
110 6. Archaeology and Dating
Fig. 6.4. Portrait of Lord Kelvin (1824–1907) by
W. Rothenstein. © Hunterian Art Gallery, University
of Glasgow
Shortly after the discovery of radioactivity by Becquerel in 1896, Rutherford and
Soddy showed that through radioactive decay, one element could be transformed into
another (see page 43), e.g. radium (a metal) became radon (a gas).They suggested also
that the observed presence of helium in rocks might be due to radioactive “decay”.
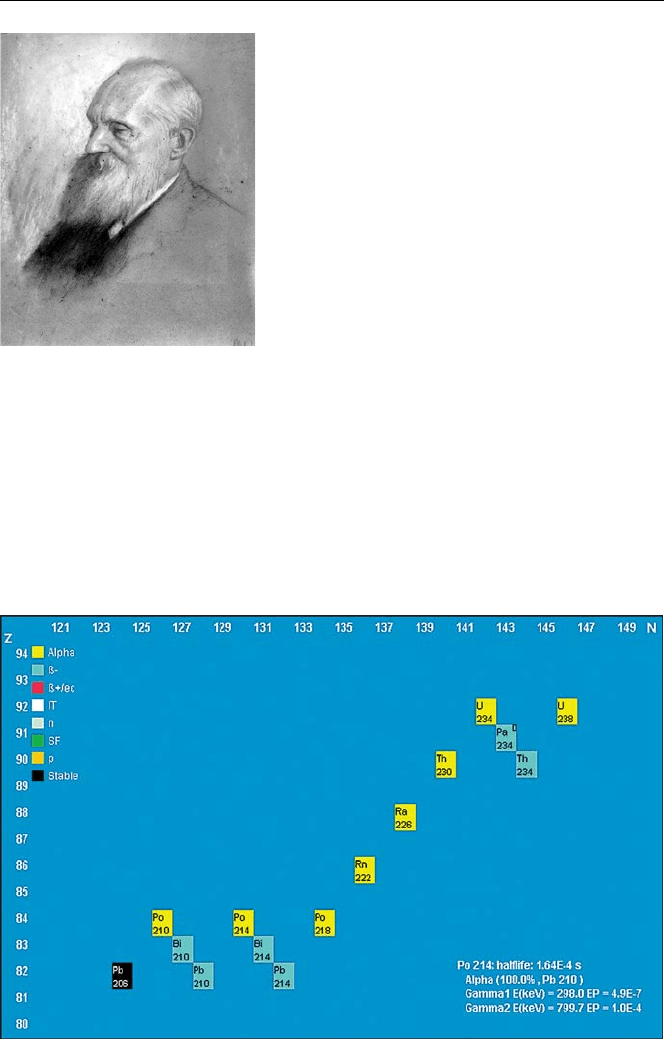
The same authors first established the “decay chain” of the unstable “parent” uranium.
They identified the 14 stages in the decay of uranium, shown in Fig. 6.5, producing
8 atoms of helium.
Based on these observations, Rutherford and Soddy concluded that if the amount
of helium in a rock could be measured, one could estimate how long this process
Fig. 6.5. Decay chain of uranium with the 14 stages established by Rutherford (from the
“Universal Nuclide Chart”, Appendix E)

Prehistoric Cave Art at Altamira, Northern Spain 111
took i.e. the age of the rock. In particular, by measuring the amount of radium and
helium, Rutherford dated the age of a rock at 500 million years. The problem with
this method was, however, that helium gas could escape from the rock and that
an age determination would result in a minimum age. From these results, Kelvin’s
age determination was clearly too low. In 1907, Boltwood had observed that along
with helium, large amounts of lead were found in rocks containing radioactivity. He
postulated that lead was the stable product in the decay of uranium. Based on this,
Arthur Holmes proposed that the age could be determined by measuring the amount
of lead, rather than helium i.e. on a uranium/lead technique. The basic assumption
here was that the “ordinary” lead in a rock was present in much smaller quantities
than the amount produced by the decay of uranium.
Around this time, Soddy discovered “isotopes” – nuclides which had different
atomic masses. The existence of these isotopes would complicate matters consider-
ably in the development of age techniques – they would, however, ultimately result
in an accurate dating technique. Not only did lead have four such isotopes
208
Pb
(parent
232
Th),
207
Pb (parent
235
U),
206
Pb (parent
238
U),
204
Pb (“ordinary” lead) but
natural uranium had also three isotopes (
238
U,
235
U,
234
U with relative abundancies
of 99.28%, 0.72% and 0.0055%, respectively). Following the discovery of the new
isotope of uranium
235
U, which decayed much faster than
238
U, Rutherford in 1929
determined the age of the Earth by assuming that, at formation, equal amounts of
238
U and
235
U were present. The result obtained, 3400 million years, was the first
age determination based on isotope ratios.
It also followed from this new isotope of uranium, that the uranium-lead pathways
contained two geological “clocks” that could be used to check one another i.e. the
decay rate of
238
Uto
206
Pb and the decay rate of
235
Uto
207
Pb. In addition, a third
“clock” based on the ratios of the isotopes
206
Pb,
207
Pb relative to the constant value
of
204
Pb was proposed. This new lead-lead method is still used today in age dating
techniques. The first estimates of the age of the Earth based on these lead ratios (the
isochron method) are due to Paterson in the 1950s. The current value of the age of
the Earth is 4550 ± 70 million years.
Prehistoric Cave Art at Altamira, Northern Spain
The most famous of the Altamira paintings are on the plafond–alowceiling in
one of the caves to the left from the entrance [7]. The total area of the ceiling is
about 100 m
2
. Here the artist skilfully combined pigment painting with the ceiling
relief. The majority of more than 20 animal figures are bison (though there is also
a horse, a boar and a deer). The most common pigments used in these paintings
were red Fe
2
O
3
, black MnO
2
and charcoal. Rather than dating the sample by tradi-
tional
14
C techniques of β activity measurements (where sample requirements would
damage the artwork), accelerator mass spectrometry was used to count individual
carbon isotopes thereby reducing the amount of sample required to a minimum. To
obtain the carbon needed for dating, a scalpel was used to scratch off approximately
20–40 mg from a dark section of the painting. Radiocarbon dating of the charcoal
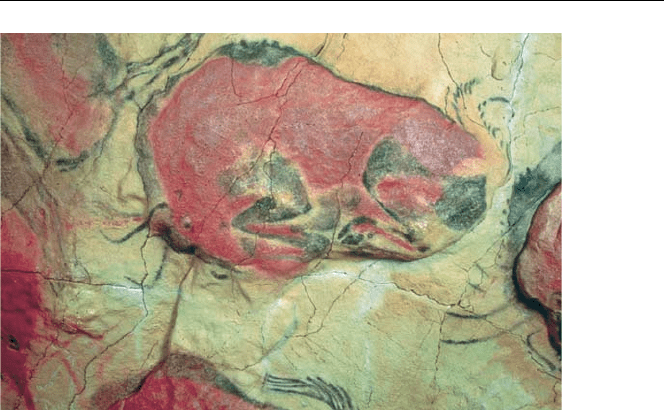
112 6. Archaeology and Dating
Fig. 6.6. Prehistoric cave art at Altamira, Northern Spain. © Museo de Altamira
used to draw the bison shown above found the drawing to be 14,000 ± 400 years
old [8].
The Age of Groundwater – The Oasis Ballad Seet
Groundwater provides one of the most important sources of drinking water world-
wide. In dry climates, in deserts, etc., groundwater is often the only permanent
source of water. Important information for the sustainable cultivation of groundwa-
ter resources is the residence time of the water underground i.e. the “age” of the
groundwater. Groundwater is an archive of historical environmental conditions. Fol-
lowing infiltration, the water can spend thousands of years in the ground, isolated
from the atmosphere. Valuable information can be obtained from the dissolved noble
gases in the water. Heavy noble gases can give information on the temperature which
existed when the water first entered into the ground [9–11].
One of the most reliable methods for dating “young” groundwater (up to ages of
50 y) is based on the rare “stable” isotope
3
He. This isotope is created in water through
the radioactive decay of the hydrogen isotope tritium
3
H (half-life 2.34 y) contained
in the water molecules. This tritium is produced in nature in very small amounts by
cosmic radiation through the reaction
14
N + n →
12
C +
3
H. Tritium was produced
in very much larger quantities in the 1950s and 1960s through atmospheric nuclear
explosions. The resulting “tritium peak” in rainfall was used in the following years
as a useful “marker” in hydrology. Today, the tritium levels have almost returned
to their natural constant values. For this reason, little information can be gained on
residence times of water by measuring the tritium content alone.
Of considerably more interest, however, is the combined measurement of
3
H
and
3
He. Following infiltration of rainwater, groundwater becomes isolated from the
The Age of Groundwater – The Oasis Ballad Seet 113
Age = t = 1/k · ln
1 +
3
He
3
H
3
H →
3
He + β
−
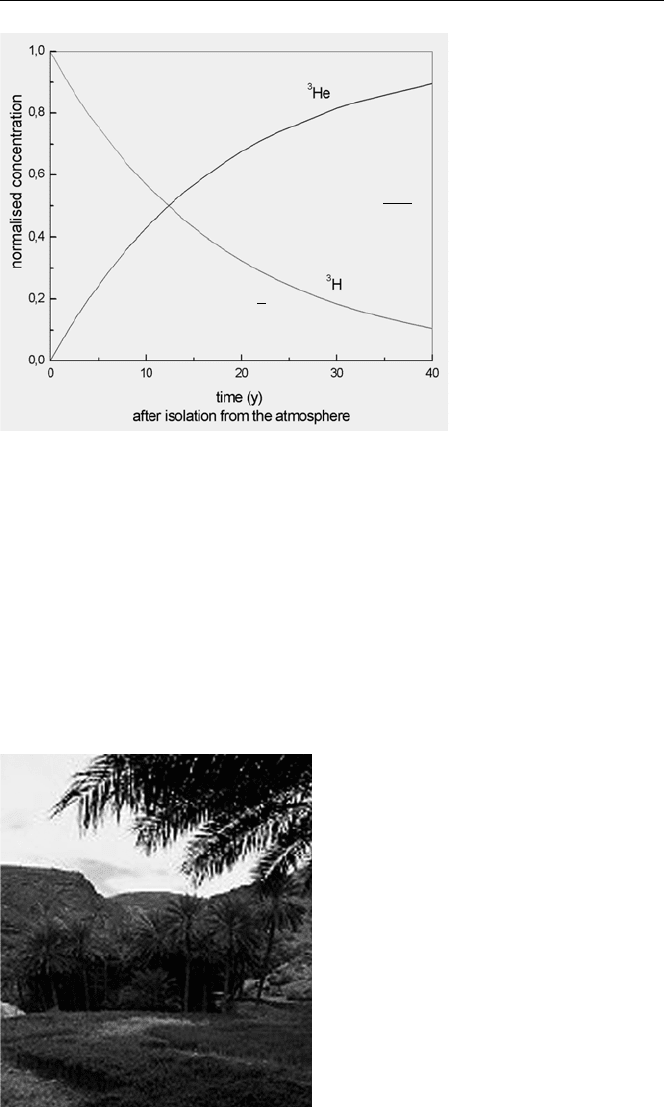
+ ν
Fig. 6.7. Time evolution of
the concentrations of
3
H
and
3
He in water isolated
from the atmosphere
atmosphere and the concentration of
3
He increases through the radioactive decay of
3
H. From the ratio of the
3
H and
3
He concentrations, the “age” of the water can be
calculated.
The “age” of the groundwater refers to the time elapsed since the isolation of
the groundwater from the atmosphere. Surface waters, whose dissolved gases are in
solution equilibrium with the atmosphere, have age zero. After isolation from the
atmosphere, for example water at the bottom of lakes or in groundwater, the gas
exchange is no longer possible and helium gas accumulates in the water.
Even the presence of tritium in such water can be useful information since it
establishes that the formation of groundwater inside the previous 50 y. From the
concentrations of the
3
H and
3
He, the history of the tritium in groundwater can
Fig. 6.8. The Oasis Ballad Seet in Oman [9] –
how did the irrigation work?
© Resa Asarschahab – Source: ZDF
