Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.


Drilling Muds and Completion Fluids
655
where
vw
=
volume percent water in
96
v,
=
volume percent oil in
%
vs
=
volume percent solids in
%
Sm
=
specific gravity of the mud
(Oil is assumed to have
a
specific gravity of
0.8.)
For freshwater muds, a rough measure
of
the relative amounts of barite and
clay in the solids can be made by using Table
4-45.
As
both suspended and
dissolved solids are retained in the retort for muds containing substantial
quantities of salt, corrections are made for the salt
[23].
PH.
Two methods for measuring the pH of drilling mud have been used:
(1)
a
modified colorimetric method using paper test strips, and
(2)
the electrometric
method using
a
glass electrode. The paper strip test may not be reliable if the
salt concentration of the sample is
too
high. The electrometric method is subject
to error in solutions containing high concentrations of sodium ions, unless a
special glass electrode is used, or unless suitable correction factors are applied
if an ordinary electrode is used. In addition, a temperature correction is required
for the electrometric method of measuring pH.
The paper strips used in the colorimetric method are impregnated with such
dyes that the color
of
the test paper is dependent upon the
pH
of the medium
in which the paper is placed.
A
standard color chart is supplied for comparison
with the test strip. Test papers are available in
a
wide range type, which permits
estimating pH to
0.5
units, and in narrow range papers, with which the pH can
be estimated to
0.2
units.
The glass electrode pH meter consists of a glass electrode system, an electronic
amplifier, and a meter calibrated in pH units. The electrode system is composed
of
(1)
the glass electrode, a thin walled bulb made of special glass within which
is sealed
a
suitable electrolyte and an electrode; and
(2)
the
reference electrode,
which is a saturated calomel cell. Electrical connection with the mud is estab-
lished through
a
saturated solution of potassium chloride contained in a tube
surrounding the calomel cell. The electrical potential generated in the glass
electrode system by the hydrogen ions in the drilling mud is amplified and
operates the calibrated pH meter.
Table
4-45
Relative Amounts
of
Barite and Clay in Solids
Specific Gravity Barite,
Percent
Clay,
Percent
of
Solids
by
Weight
by
Weight
2.6
0
100
2.8 18 82
3.0 34 66
3.2 48 52
3.4 60
40
3.6
71
29
3.8 81 19
4.0 89
11
4.3
100 0
656
Drilling and Well Completions
Resistivity.
Control of the resistivity of the mud and mud filtrate while drilling
may be desirable to permit better evaluation of formation characteristics from
electric logs. The determination of resistivity is essentially the measurement of
the resistance to electrical current flow through a known sample configuration.
Measured resistance is converted to resistivity by use of a cell constant. The cell
constant is fixed by the configuration of the sample in the cell and is determined
by calibration with standard solutions of known resistivity. The resistivity is
expressed in ohm-meters.
Chemical Analysis.
Standard chemical analyses have been developed for
determining the concentration of various ions present in the mud
[23].
Test for
concentration of chloride, hydroxide and calcium ions are required to fill out
the API drilling mud report. The tests are based on filtration, i.e., reaction of
a known volume of mud filtrate sample with a standard solution of known
volume and concentration. The end of chemical reaction is usually indicated
by the change of color. The concentration of the ion being tested then can be
determined from a knowledge of the chemical reaction taking place
[7].
Chloride.
The chloride concentration is determined by titration with silver
nitrate solution. This causes the chloride to be removed from the solution as
AgCl, a white precipitate. The endpoint of the titration is detected using a
potassium chromate indicator. The excess Ag' present after all C1- has been
removed from solution reacts with the chromate to form Ag,CrO,, an orange-
red precipitate.
The mud contamination with chlorides results from salt intrusion. Salt can
enter and contaminate the mud system when salt formations are drilled and
when saline formation water enters the wellbore.
Alkalinity and Lime Content.
Alkalinity
is the ability of a solution
or
mixture
to react with an acid. The
phenolphthalein alkalinity
refers to the amount of acid
required to reduce the pH to
8.3,
the phenolphthalein endpoint. The phenol-
phthalein alkalinity of the mud and mud filtrate is called the Pm and P, respectively.
The P, test includes the effect of only dissolved bases and salts while the Pm
test includes the effect of both dissolved and suspended bases and salts. The
methyl orange alkalinity
refers to the amount of acid required to reduce the pH
to
4.3,
the methyl orange endpoint. The methyl orange alkalinity of the mud
and mud filtrate is called the
Mm
and
M,,
respectively. The API diagnostic tests
include the determination of Pm, P,, and
M,.
All values are reported in cubic centi-
meters of
0.02
N
(normality
=
0.02)
sulfuric acid per cubic centimeter of sample.
The P, and
M,
tests are designed to establish the concentration of hydroxyl,
bicarbonate, and carbonate ions in the aqueous phase of the mud. At a pH of
8.3,
the conversion of hydroxides to water and carbonates to bicarbonates is essentially
complete. The bicarbonates originally present in solution do not enter the reactions.
As the pH is further reduced to
4.3,
the acid then reacts with the bicarbonate
ions to form carbon dioxide and water.
The P,
and
Pm test results indicate the reserve alkalinity of the suspended solids.
As the [OH-] in solution is reduced, the lime and limestone suspended in the
mud will go into solution and tend to stabilize the pH. This reserve alkalinity
generally is expressed as an equivalent lime concentration, in lb/bbl of mud.
Total Hardness.
A combined concentration of calcium and magnesium in the
mud water phase is defined as total hardness. These contaminants are often
present in the water available for use in the drilling fluid. In addition, calcium

Drilling Muds and Completion Fluids
657
can enter the mud when anhydrite (CaSO,) or gypsum (CaS0,.2H20) formations
are drilled. Cement also contains calcium and can contaminate the mud. The
total hardness is determined by titration with a standard (0.02
N)
Versenate
(EDTA) solution. The standard Versenate solution contains Sodium Versenate,
an organic compound capable of forming a chelate with Ca2+ and Mg2+.
The hardness test sometimes is performed on the mud as well as the mud
filtrate. The mud hardness indicates the amount of calcium suspended in the
mud as well as the calcium in solution. This test usually is made on gypsum-
treated muds to indicate the amount of excess CaSO, present in suspension.
To
perform the hardness test on mud, a small sample of mud is first diluted to 50
times its original volume with distilled water
so
that any undissolved calcium
or magnesium compounds can go into solution. The mixture then is filtered through
hardened filter paper to obtain a clear filtrate. The total hardness of this filtrate
then is obtained using the same procedure used for the filtrate from the low-
temperature low-pressure API filter press apparatus.
Methylene Blue.
Frequently, it is desirable to know the cation exchange capacity
of the drilling fluid. To some extent, this value can be correlated to the
bentonite content of the mud.
The test is only qualitative because organic material and some other clays
present in the mud also will absorb methylene blue. The mud sample usually is
treated with hydrogen peroxide to oxidize most of the organic material. The
cation exchange capacity is reported in milliequivalent weights (meq)
of
methylene
blue per 100 ml of mud. The methylene blue solution used for titration is
usually
0.01
N,
so
that the cation exchange capacity is numerically equal to the
cubic centimeters
of
methylene blue solution per cubic centimeter of sample
required to reach an endpoint. If other adsorptive materials are not present in
significant quantities, the montmorillonite content of the mud in pounds per
barrel
is
five times the cation exchange capacity.
The methylene blue test can also be used to determine cation exchange
capacity of clays and shales. In the test a weighed amount of clay is dispersed
into water by a high-speed stirrer. Titration is carried out as for drilling muds,
except that hydrogen peroxide is not added. The cation exchange capacity of
clays is expressed as milliequivalents of methylene blue per 100 g of clay.
Oil-Base Muds
[23-251
Specific Weight.
Mud weight of oil muds is measured with a mud balance. The
result obtained has the same significance as in water-base mud.
Viscosity.
The measurement procedure for API funnel viscosity is the same as for
water-base muds. Since temperature affects the viscosity, API procedure recommends
that the mud temperature should always be recorded along with the viscosity.
Plastic Viscosity and Yield Point.
Plastic viscosity and yield point measure-
ments are obtained from a direct indicating viscometer. Due to the temperature
effect on the flow properties of oil-base mud, the testing procedure is modified.
The mud sample in the container is placed into a cup heater
[23].
The heated
viscometer cup provides flow property data under atmospheric pressure and
bottomhole temperature.
Gel Strength.
The gel strength of oil-base muds is measured with a direct
indicating viscometer exactly like that of water-base muds.

658
Drilling and Well Completions
Filtration.
The API filtration test for oil-base muds usually gives an all-oil filtrate.
The test may not indicate downhole filtration, especially in viscous oils. The
alternative high-temperature-high-pressure (HT-HP) filtration test will generally
indicate a pending mud problem by amount of fluid loss or water in the filtrate.
The instruments for the HT-HP filtration test consist essentially of a controlled
pressure source, a cell designed to withstand a working pressure of at least
1000 psi, a system for heating the cell, and a suitable frame to hold the cell
and the heating system. For filtration tests at temperatures above 200"F, a
pressurized collection cell is attached to the delivery tube. The filter cell is
equipped with a thermometer well, oil-resistant gaskets, and a support for the
filter paper (Whatman
No.
50 or the equivalent).
A
valve on the filtrate delivery
tube controls flow from the cell. A nonhazardous gas such as nitrogen
or
carbon
dioxide should be used for the pressure source.
The test is usually performed at a temperature of 300'F and a pressure of
600
psi over a 30-min period. When other temperatures, pressures, or times are
used, their values should be reported together with test results. If the cake
compressibility is desired, the test should be repeated with pressures of 200 psi
on the filter cell and 100 psi back pressure on the collection cell.
Electrical Stability
of
Emulsions.
The electrical stability test indicates the stability
of emulsions of water in oil. The emulsion tester consists of a reliable circuit using
a source of variable AC current (or DC current in portable units) connected to strip
electrodes. The voltage imposed across the electrodes can be increased until a
predetermined amount of current flows through the mud emulsion-breakdown point.
Relative stability is indicated as the voltage at the breakdown point.
Sand Content.
Sand content measurement
is
the same as for water-base muds
except that diesel oil instead of water should be used for dilution.
Liquids and Solids Content.
Oil, water, and solids volume percent is deter-
mined by retort analysis as in a water-base mud. More time is required to get a
complete distillation of an oil mud than of a water mud. Then the corrected water
phase volume, the volume percent of low gravity solids, and the oil-water ratio can
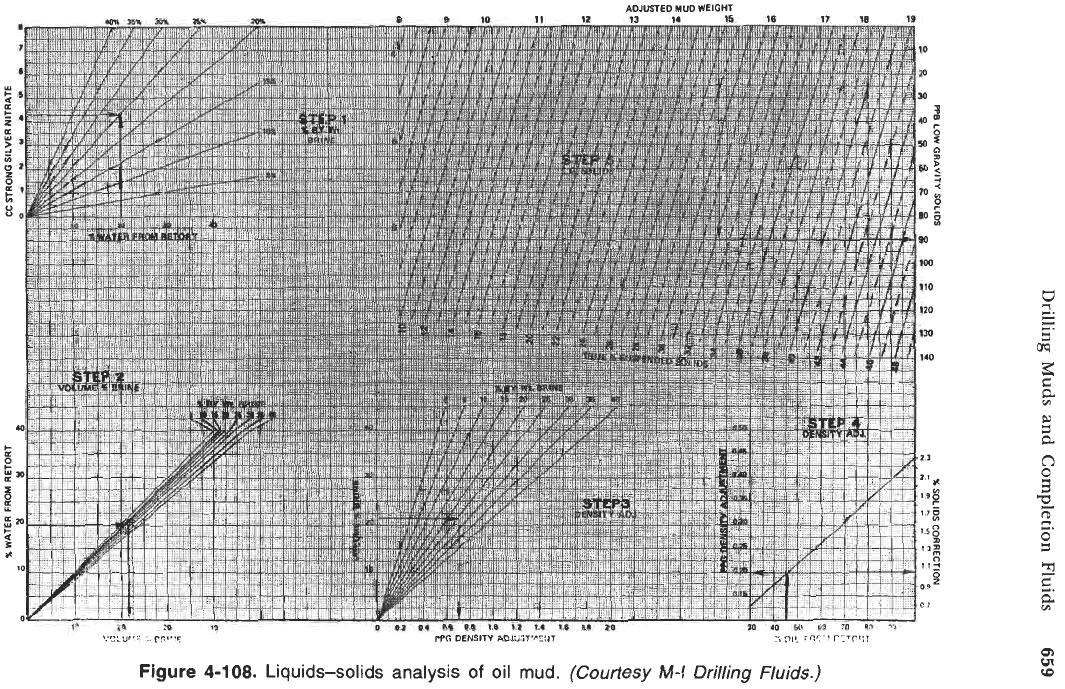
be calculated; the chart in Figure 4-108 can be used for the calculations [24].
Example.
Find the volume fraction
of
brine, the low gravity solids content, the
adjusted mud weight, and the oil-to-water ratio from the test data below (use
Figure 4-107).
Mud weight (specific weight)
=
15.7 lb/gal
Volume
%
water (retort)
=
20%
Volume
%
oil (retort)
=
45%
Strong silver nitrate
=
4.3 ml
(1 ml equivalent to 0.01 g C1)
Step
1.
To determine the percent by weight of calcium
or
of sodium chloride
in the internal phase, locate the intersection of the line drawn horizontally from
the cm' of strong silver nitrate required to titrate 1 cms of whole mud with the
line projected vertically from the volume percent of fresh water by retort.
Percent by weight brine in internal phase:
Strong silver nitrate
=
4.3 ml
Volume
%
water (retort)
=
20%
Read 25% by weight brine in internal phase
Drilling
Muds
and Completion Fluids
659

660
Drilling and Well Completions
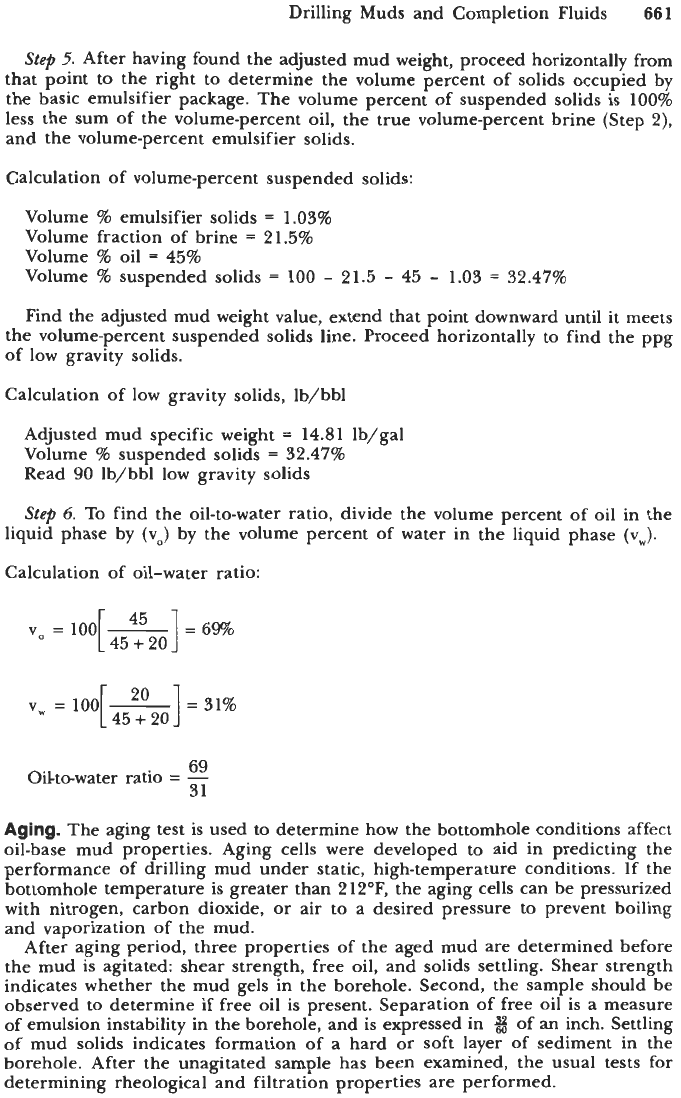
Step
2.
Knowing the weight percent of brine and using the volume percent of
freshwater by retort, the corrected volume fraction, which represents the true
volume percent of brine in the solution, can be determined by running a line
from the volume percent of water horizontally across until it meets the brine
concentration, then dropping vertically to find the true volume percent of brine in
the original mud. This number will always be greater than the volume percent
of
freshwater by retort.
Volume fraction brine in internal phase:
Fvw
=
20%
Weight
%
brine
=
25%
Read
21.5%
volume fraction brine in internal phase
Step
3.
To determine the gravity solids in the drilling mud, it is necessary to
subtract from the mud weight all of the mud components except diesel oil and
low gravity solids.
To do
so,
subtract from the measured mud weight the fraction contributed
by brine and basic emulsifier (this step and Step
4).
Knowing the volume fraction
of brine in the internal phase (from Step
2)
and the weight percent of brine
(from Step
l),
follow the appropriate value for the volume of brine horizontally
to intersect the weight percent of brine. Extend that point vertically down to
determine the weight in lb/gal and subtract that weight from the mud weight
to correct for the weight of the internal phase.
Weight adjustment due to the internal phase:
Volume fraction of brine in internal phase
=
21.5%
Weight percent of brine in internal phase
=
25%
Read 0.69 lb/gal density adjustment
Step
4.
This step corrects the mud weight and the volume percent of sus-
pended solids as a function of the hydrocarbons distilled off by the mud still.
Knowing the volume percent of oil from the mud still, follow this value vertically
until it meets the line representing the system being run. Then extend this
point horizontally to the left to determine the weight to subtract from the initial
mud weight.
Weight adjustment due to distilled hydrocarbons:
Volume
%
oil
=
45%
Read
0.20
lb/gal specific weight adjustment
The initial mud weight less the sum of the weight adjustments from Steps
3
and
4
is the corrected mud weight representing the weight of the diesel oil,
low gravity solids, and barite only.
Calculation of adjusted mud weight:
Weight adjustment-internal phase
=
0.69 lb/gal
Weight adjustment-emulsifier solids
=
0.20
lb/gal
Mud weight
=
15.7
lb/gal
Adjusted mud weight
=
15.7
-
0.69
-
0.20
=
14.81
lb/gal
Drilling Muds and Completion Fluids
661
Step
5.
After having found
the
adjusted mud weight, proceed horizontally from
that point to the right to determine the volume percent of solids occupied by
the basic emulsifier package. The volume percent of suspended solids is
100%
less the sum of the volume-percent oil, the true volume-percent brine (Step
2),
and the volume-percent emulsifier solids.
Calculation of volume-percent suspended solids:
Volume
%
emulsifier solids
=
1.03%
Volume fraction of brine
=
21.5%
Volume
%
oil
=
45%
Volume
%
suspended solids
=
100
-
21.5
-
45
-
1.03
=
32.47%
Find the adjusted mud weight value, extend that point downward until it meets
the volume-percent suspended solids line. Proceed horizontally to find the ppg
of low gravity solids.
Calculation of low gravity solids, lb/bbl
Adjusted mud specific weight
=
14.81
lb/gal
Volume
%
suspended solids
=
32.47%
Read
90
lb/bbl low gravity solids
Step
6.
To find the oil-to-water ratio, divide the volume percent
of
oil in the
liquid phase by
(vo)
by the volume percent of water in the liquid phase
(vw).
Calculation of oil-water ratio:
v,
=
lOO[
-1
20
=
31%
45
+
20
69
Oiko-water ratio
=
-
31
Aging.
The aging test is used
to
determine how the bottomhole conditions affect
oil-base mud properties. Aging cells were developed to aid in predicting the
performance
of
drilling mud under static, high-temperature conditions. If the
bottomhole temperature is greater than
212"F,
the aging cells can be pressurized
with nitrogen, carbon dioxide, or air to
a
desired pressure to prevent boiling
and vaporization
of
the mud.
After aging period, three properties of the aged mud are determined before
the mud is agitated: shear strength, free oil, and solids settling. Shear strength
indicates whether the mud gels in the borehole. Second, the sample should be
observed to determine if free oil is present. Separation of free oil is a measure
of emulsion instability in the borehole,
and
is expressed in of
an
inch. Settling
of
mud solids indicates formation of
a
hard or soft layer of sediment in the
borehole. After the unagitated sample has been examined, the usual tests for
determining rheological and filtration properties are performed.

662
Drilling and Well Completions
Alkalinity and Lime Content.
The whole mud alkalinity test procedure is a
titration method which measures the volume of standard acid required to react
with the alkaline (basic) materials in an oil mud sample. The alkalinity value is
used to calculate the pounds per barrel unreacted “excess” lime in an oil mud.
Excess alkaline materials, such as lime, help to stabilize the emulsion and also
neutralize carbon dioxide or hydrogen sulfide acidic gases.
To approximately 20 ml of a 1:l mixture of toluene (xy1ene):isopropyl alcohol,
add 1 ml
of
oil-base mud and
75
to 100 ml of distilled water. Add
8
to 10 drops
of phenolphthalein indicator solution and stir vigorously with a stirring rod (the
use of a Hamilton Beach mixer is suggested). Titrate slowly with H,SO,
(N/lO)
until red (or pink) color disappears permanently from the mixture. Report the
alkalinity as the number of ml of
H,SO,
(N/10) per ml
of
mud. Lime content
may be calculated as
Lime, ppb
=
(
1.5)(H,S04, ml)
Calcium Chloride
[25].
Calcium chloride estimation is based on calcium
titration. To 20 ml of 1:l mixture of toluene (xy1ene):isopropyl alcohol, add a
1-ml
(or
O.l-ml, if calcium is high) sample of oil-base mud, while stirring.
Dilute the mixture with 75 to 100 ml of distilled water. Add 2 ml of hardness
buffer solution and 10 to 15 drops of hardness indicator solution. Titrate
mixture with standard versenate solution until the color changes from wine-
red to blue. If common standard versenate solution (1 ml
=
20 g calcium
ions) is used, then
CaCl,, ppb
=
(0.4)(standard versenate, ml)
If strong standard versenate solution (1 ml
=
2 g calcium ions) is used, then
CaCl,, ppb
=
(4.0)(strong standard versenate, ml)
Sodium Chloride
[25].
Sodium chloride estimation is based on sodium titration.
To 20 ml of a 1:l mixture of toluene (xy1ene):isopropyl alcohol, add a 1-ml sample
of oil-base mud, stirring constantly and 75 to
100
ml of distilled water. Add 8-10
drops of phenolphthalein indicator solution and titrate the mixture with H,SO,
(N/lO)
until the red (pink) color, if any, disappears. Add
1
ml of potassium
chromate to the mixture and titrate with 0.282N AgNO, (silver nitrate,
1
ml
=
0.001 g chloride ions) until the water portion color changes from yellow to
orange. Then
NaCl, ppb
=
(0.58)(AgNO,, ml)
-
(l.OG)(CaCl,, ppb)
Some other procedures for CaCl, and NaCl content determination are used
by mud service companies. Although probably more accurate, all
of
them are
based on calcium filtration for CaCl, detection and on chlorides filtration for
NaCl detection.
Total Salinity.
The salinity control of oil-base mud is very important for
stabilizing water-sensitive shales and clays. Depending upon the ionic concen-
tration of the shale waters and of the mud water phase, an osmotic flow
of
pure water from the weaker salt concentration (in shale) to the stronger salt
concentration (in mud) will occur. This may cause a dehydration
of
the shale
and, consequently, affect its stabilization.

Drilling Muds and Completion Fluids
663
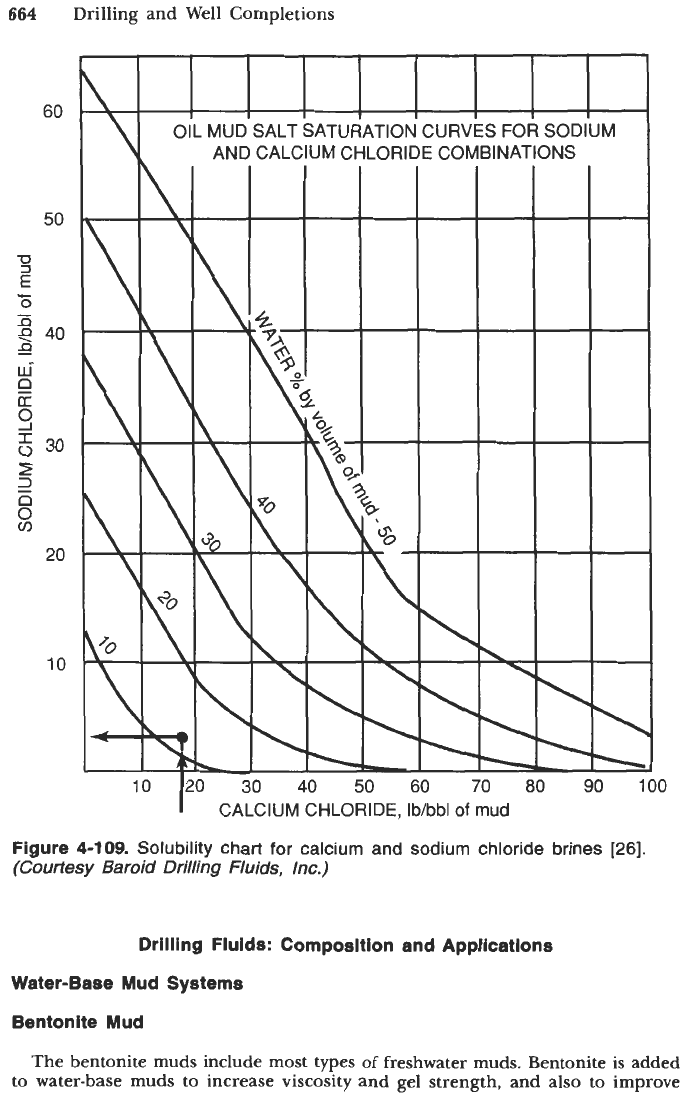
A
standard procedure for estimating the salt content of oil-base muds consists
1.
Determination of calcium chloride concentration, lb/bbl.
2.
Determination of sodium chloride concentration, lb/bbl.
3.
Determination
of
soluble sodium chloride. By entering the graph in
Figure
4-109
with the lb/bbl of calcium chloride at the correct volume
percent of water (by retort) line, the maximum amount
of
soluble sodium
chloride can be found. If the sodium chloride content determined in Step
2
is
greater than the maximum soluble sodium chloride determined from
Figure
4-108,
only the soluble portion should be used for calculating the
total soluble salts.
4.
Determination
of
total mud salinity. The total pounds of soluble salts per
barrel of mud are calculated as
Total soluble salts (lb/bbl)
=
CaCl, (lb/bbl)
-
soluble NaCl (lb/bbl)
5.
Determination
of
water phase salinity. By entering the graph in Figure
4-1
10
with total soluble salts, lb/bbl
of
mud, at the correct volume percent of
water line, the water phase salinity can be read from the left-hand scale.
Example.
Find the total salinity of the oil-base mud using the test data below
and Figures
4-108
and
4-109.
Volume
%
water
=
12%
From Ca titration, the CaCI, concentration is
18
lb/bbl mud
From C1 titration, the NaCl concentration is
9.9
Ib/bbl mud
Step
1.
The maximum soluble NaCl (from Figure
4-109)
=
3
lb/bbl. (There is
Step
2.
The total soluble salts in the mud
=
18
+
3
=
21
lb/bbl.
Step
3.
The water phase salinity (from Figure
4-110)
=
330,000
ppm.
of the following steps
[26]:
excess insoluble NaCl
=
6.9
Ib/bbl.)
Water Wetting
Solids.
The water wetting solids test (oil-base mud coating test)
indicates the severity of water wetting solids in oil-base mud
[24].
The items
needed are
1.
Hamilton Beach mixer
2.
Diesel oil
3.
Xylene-isopropyl alcohol mixture
4.
16-02.
glass jar
Collect a
350-ml
mud sample from the flowline and place the sample in the glass
jar. Allow the sample to cool to room temperature before the test is conducted.
Mix at
70
V
with the mixer for
1
hr. Pour the mud out, add
100
ml diesel oil, and
shake well. (Do not stir with mixer.) Pour the oil out, add
50
ml xylene-isopropyl
alcohol
(1:l)
mixture, and shake well. Empty jar, turn upside down, and allow to
dry. Observe the film on the wall of the jar and report the evaluation as
Opaque film-severe problem, probably settling of barite and plugging of the
Slight film, translucent-moderate problem, mud needs wetting agent immediately.
Very light film, highly translucent-slight wetting problem, mud needs some
No film-no water wetting problem.
drill string.
treatment.
664
Drilling and
Well
Completions
60
50
-0
E'
.c
0
B
&?
40
0
W"
e
6
30
[r
3
5
$
n
20
10
OIL MUD SALT SATURATION CURVES FOR SODIUM
AND CALCIUM CHLORIDE COMBINATIONS
30
40 50
60
70
80 90
100
lo
1"
CALCIUM CHLORIDE,
Ib/bbl
of
mud
Figure
4-109.
Solubility chart for calcium and sodium chloride brines
[26].
(Courtesy
Baroid Drilling Fluids, lnc.)
Drilling Fluids: Composition and Applications
Water-Base Mud Systems
Bentonite Mud
The bentonite muds include
most
types of freshwater muds. Bentonite
is
added
to
water-base muds to increase viscosity and gel strength, and also to improve
