Liu A.L., Tien H.T. Advances in Planar Lipid Bilayer and Liposomes. V.6
Подождите немного. Документ загружается.

(inserted state or I state) [61–65]. The transition from the S state to the I state has a
sigmoidal peptide-concentration dependence indicating cooperativity in the pep-
tide–membrane interactions. Data gained on this transition of alamethicin measured
in three bilayer conditions were explained by a free energy that took into account
the membrane-thinning effect induced by the peptides. This model was extended
to melittin that was shown to form toroidal pores, instead of barrel-stave pores as in
the case of alamethicin, demonstrating that the membrane thinning effect is a
plausible mechanism for the peptide-induced pore formations [66].
The thinning of the bilayer observed is in the range of 2–3 A
˚
and is an average
value over local dimple deformation [60]. However, an atomic force microscopy
study on the effect of MSI-78, a derivative of magainin, on DMPC bilayers revealed
that the bilayer thickness was not reduced uniformly over the entire bilayer area
[67]. Instead, upon binding of the peptide distinct domains were formed, where the
bilayer thickness was reduced by about 11 A
˚
. Increasing the peptide concentration
resulted in a growth of these domains without affecting the reduction of thickness
and the parallel orientation of this a-helical peptide to the membrane.
X-ray studies also yielded structural information on the channel forming pep-
tide Gramicidin D. This peptide is known to form well-defined dimeric channels in
lipid bilayers [68]. Gramicidin D apparently stretches DLPC bilayers and thins
DMPC bilayers toward a common thickness of 32.470.3 A
˚
owing to hydrophobic
mismatch between the peptide and the hydrophobic core of the individual bilayer
[69,70]. However, further studies with the a-helical synthetic WALP peptides,
which consist of a hydrophobic sequence of leucine and alanine of varying lengths
bordered at both ends by tryptophan as membrane anchors, did not reveal a bilayer
thickness adjustment as observed in the presence of Gramicidin D [71]. Since the
energy cost of hydrophobic mismatch is greater than the energy cost of membrane
Figure 6 Illustration showing the e¡ect of an amphipat ic peptide with a spatial location parallel
to the bilayer su rface. Neighboring phospholipids will be character ized by increased trans-gauche
isomer ization of the hydrocarbon chains to ¢ll the void in the hydrophobic core resulti ng in
membrane thinning.
K. Lohner et al.114
deformation [70], this result is intriguing. The authors suggest that this energy
argument is not applicable for single helix since the way the lipids pack around it
differs greatly from larger proteins like Gramicidin.
2.3. Detergent-Like Effect – Micellar Lipid–Peptide Disk Formation
Another mechanism by which antimicrobial peptides can destroy their target
membrane involves micellization via a detergent-like mode of action [37]. The
character of particularly linear amphipathic a-helical peptides and their interactions
with membranes show analogies to detergent molecules. For example, melittin with
its overall amphipathic character composed of a cluster of cationic amino acids at
the C-terminus and a stretch of hydrophobic amino acids, resembles the features of
many detergents being characterized by a polar/charged head group and a hydro-
phobic moiety. Melittin was found to exhibit pronounced effects on the phase
behavior of DPPC already at very low peptide concentrations (lipid-to-peptide
molar ratio of 1000/1) [72,73]. A similar concentration-dependent behavior was
reported for the detergent cetyltrimethylammonium chloride [74]. However, at
high melittin concentrations (lipid-to-peptide molar ratio of 15/1), disk-shaped
particles were found for the DMPC/melittin mixture [75,76], suggesting a deter-
gent-like solubilization of the membrane under these experimental conditions. The
idea of gradual membrane disintegration is also supported by data gained from
staphylococcal d-lysin/DMPC multilamellar vesicles [77,78]. Modeling of X-ray
data further suggested that the disk-like micelles were surrounded by a ring of
peptide of about 1 nm thickness as sketched in Fig. 7 [78]. There has been some
debate about the peptide arrangement that surrounds the hydrophobic core, i.e., if
the helix axes of the peptide is located normal or parallel to the bilayer plane of the
disk-micelle. Evidence for a location parallel to the disk-plane was shown in case of
discoidal micelles formed by apolipoproteins [79]. However, as the peptide
Figure 7 Drawing of a disk-like lipid micelle with the helical peptide surrounding the disk
preventing exposure of the hydrophobic core of the lipid micelle. Note that the peptide could be
also arranged parallel to the disk surface.
Liposome-Based Biomembrane Mimetic Systems 115

orientation cannot be determined by X-ray, small-angle neutron scattering exper-
iments with deuterated peptide would be necessary to clarify this point.
2.4. Promotion or Prevention of Non-Lamellar Phases
As mentioned above, PE is a major component of bacterial cell membranes. Al-
though PE like PC is zwitterionic, they feature very different molecule geometries.
PC is shaped cylindrically, whereas PE is characterized by a truncated cone shape
owing to its small head group as compared to the cross-section of its hydrocarbon
chains [80,81]. Therefore, PE possesses a large spontaneous curvature making the
lipid prone to the formation of non-lamellar structures [82]. The balance between
lamellar and non-lamellar phase promoting lipids is thought to be essential for many
membrane-associated processes such as function of membrane proteins and mem-
brane fusion [83–85]. Incorporation of small molecules like antimicrobial peptides,
which are able to shift this balance to one direction or the other, is therefore bound
to have a large impact on membrane function and integrity [36]. Peptides that
impose a negative curvature stress on the bilayer can potentiate the intrinsic negative
curvature of these bilayers leading to the formation of non-lamellar lipid structures,
however, peptides having the contrary effect, i.e., stabilizing the PE bilayer by
intercalation into the interfacial region, are also described in the literature [86].
As outlined in Section 1.1, microorganisms such as E. coli or A. laidlawii have
been shown to precisely regulate the amounts of non-lamellar phase preferring
lipids such as PE, DPG or monogalactosyl-diglyceride in a narrow window close to
a lamellar to non-lamellar phase boundary [7,87,88]. AMPs interfere with the
packing constraints arising from the coexistence of lamellar and non-lamellar phase
forming lipids and may lower the lamellar to non-lamellar phase boundary leading
to membrane rupture. In this regard, X-ray diffraction is a highly convenient
technique to detect the presence of non-lamellar phases, with the apparent ad-
vantage over e.g.,
31
P-NMR that the supramolecular structure of the lipid aggre-
gates can be analyzed in all structural details [89].
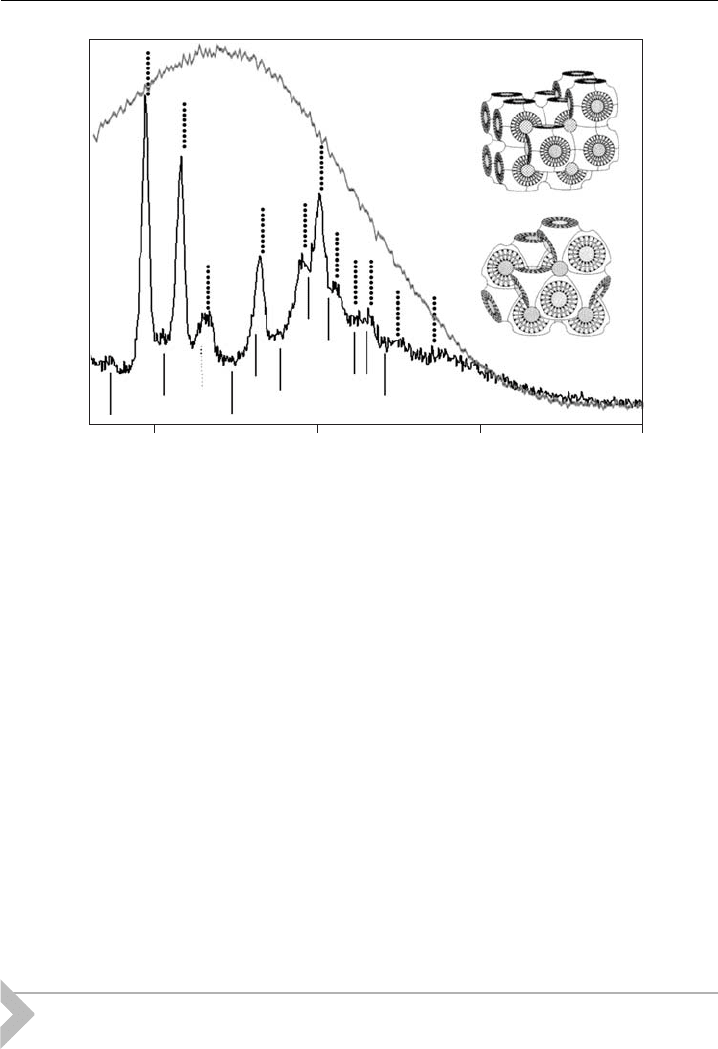
The cyclic peptide Gramicidin S from B. brevis, for example, was shown to
disrupt liposomes composed of lipid extracts from E. coli and A. laidlawii by de-
creasing the energetic barriers against the formation of cubic phases by promoting
negative curvature (Fig. 8) [49].
AMPs were also shown to promote the formation of non-lamellar lipid struc-
tures in pure PE model systems. Alamethicin induces a cubic phase, when incor-
porated in small amounts in dielaidoyl PE [90] and an inverse hexagonal phase in
dioleoyl PE liposomes [91]. It was suggested that alamethicin induces such lipid
structures by changing the thickness and/or flexibility of the lipid bilayer. In dip-
hytanoyl PC model membranes alamethicin causes membrane thinning with a
concomitant increase in chain disorder over a large area [62,92]. Therefore, it was
proposed that the decrease in membrane thickness is compensated by an increase of
the hydrophobic cross-sectional area of the lipid acyl chains, which in case of PE
would further enhance the existing mismatch between the cross-sectional areas of
the head group and hydrocarbon chains. This could be a promotive force for the
lipid monolayer to curl.
K. Lohner et al.116
Promotion of an inverse hexagonal phase was observed for nisin in dioleoyl- and
palmitoyloleoyl-PE, respectively [50]. The formation of this non-bilayer structure
can be explained in analogy to hydrophobic molecules such as squalene that also
strongly decrease the phase boundary between fluid lamellar and inverse hexagonal
phase [93]. Thus, the insertion of the large hydrophobic section of nisin (segments
1–19) will lead to an increase of the hydrophobic volume in the bilayer interior and
in turn promote negative curvature. The role of insertion of a large hydrophobic
volume into the bilayer can also be deduced from the synthetic cecropin B analog
CB3, which in contrast to the natural cecropin B was composed of two hydro-
phobic a-helices [94]. Whereas for cecropin B pore formation was proposed, CB3
induced rapid formation of irregularly shaped, non-lamellar structures.
Examples of peptides inducing positive curvature strain in a membrane thereby
inhibiting the formation of non-lamellar phases include e.g., d-lysin [51], LL-37
[95] and magainin [96].
3. General Aspects of Model Membranes
3.1. Global and Local Membrane Properties
Having outlined the need for studying mimetics of biological membranes in the
introductory section, it appears obvious to look for novel ways to attain a better and
Intensity [a.u.]
0.1 0.2 0.3 0.
4
s [nm
-1
]
Im3m
Pn3m
Figure 8 Small-angle X-ray di¡ractogram of E. coli membrane total lipid extract (gray line)
showing t he typical scattering of uncorrelated bilayers and in the presence of (4 mol%)
Gramicidin S (black line) recorded at 251C. Position of hkl-re£ections corresponding to a cubic
phase of space group P n3m (dotted) and Im3m (solid), respectively, are indicated in the
di¡ractogram.
Liposome-Based Biomembrane Mimetic Systems 117
characterization of the systems throughout. In doing so, it is important to realize the
different time and length scales involved in the problem. With respect to length
scales, membrane properties may be divided into local and global features, which
are, however, intimately coupled to each other [97,98]. Local membrane properties
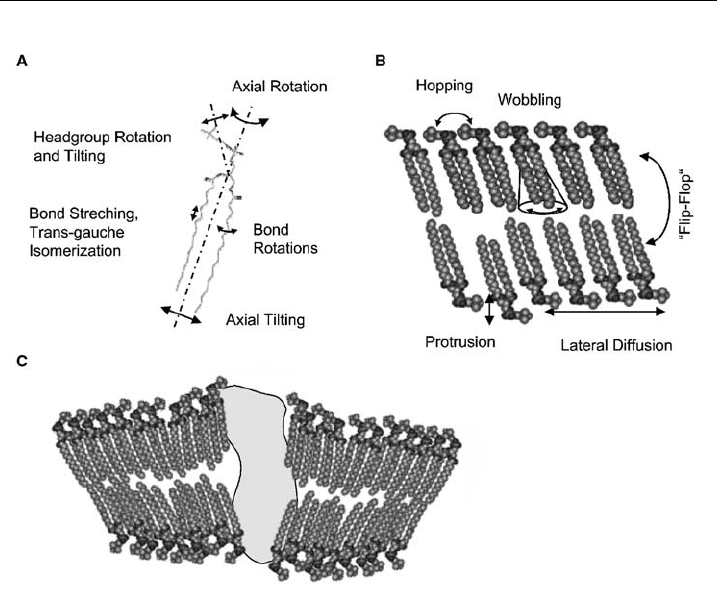
would be, for example, different modes of molecular motions and vibrations,
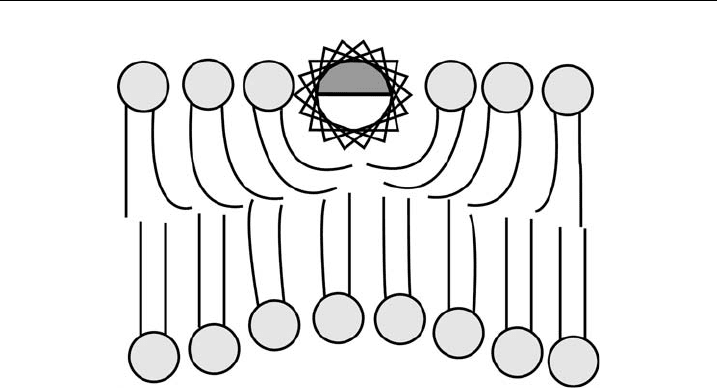
diffusion, chain tilt, but also any disorder in the vicinity of defects (Fig. 9).
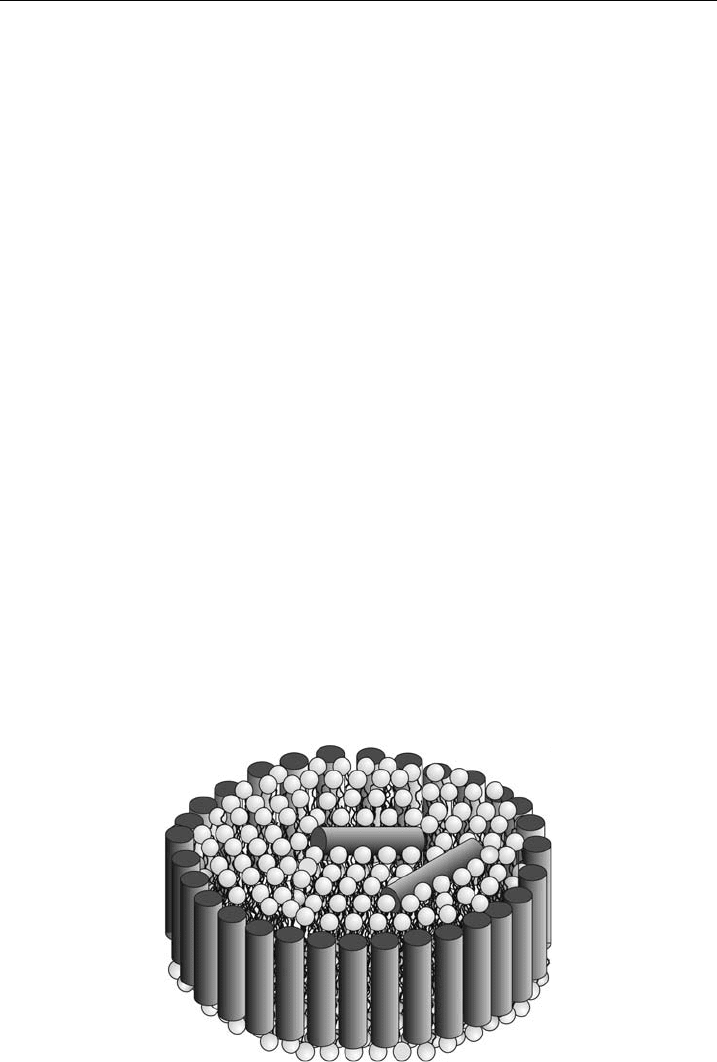
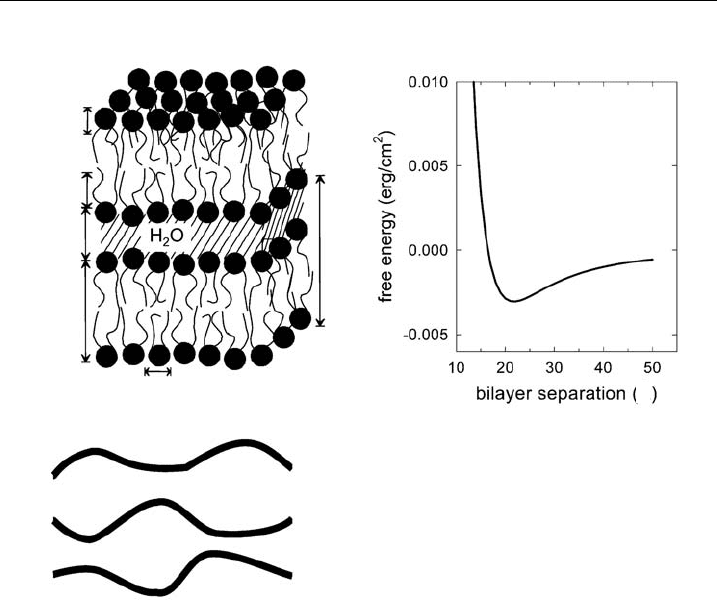
In turn, global membrane properties are the overall bilayer structure, bilayer
interactions, elasticity, global curvature, chain packing, to name but a few (Fig. 10).
This general distinction between global and local membrane properties is clearly
artificial and is due to experimental techniques that address either the one or the
other aspect.
Spectroscopic techniques and all-atom molecular dynamics (MD) simulations
are traditionally applied to study membrane properties on the local scale. Important
distinctions between the different spectroscopic techniques come from the different
timescales involved that range from fast bond vibration modes with correlation
times on the order of 10
14
s to transbilayer diffusion of lipids (‘‘flip-flop’’), which
may take up to several days. Fourier transform infrared (FTIR) and Raman
spectroscopy are the techniques of choice to study the vibrational bands exhibiting
Figure 9 Schematic examples of local bilayer properties including s everal modes of vibrations
and rotations within a single lipid molecule (A), single molecular motions (B) and local
membrane perturbation by the inclusion of a membrane protei n (C).
K. Lohner et al.118
the fastest dynamics, whereas magnetic resonance methods such as electron par-
amagnetic resonance (EPR) and nuclear magnetic resonance (NMR) cover the
‘‘slower’’ motions, such as, molecular wobbling or axial rotations. Here, EPR is
sensitive to faster molecular motions than NMR. On the other hand, EPR requires
the incorporation of nitroxide spin-labels in the membrane, whose concentration
needs to be kept low in order to avoid effects on the bilayer structure. Of recent
interest are also dynamic scattering techniques such as coherent inelastic X-ray
and neutron scattering [99–101], neutron backscattering [102] and X-ray photon
correlation spectroscopy [103].
Global membrane properties, such as the overall structure can be determined
applying elastic X-ray or neutron scattering [104–106], but also H
2
NMR spectra
can yield information on the hydrocarbon chain length of the lipid bilayer [107].
Bilayer interactions can be measured by the surface force apparatus, atomic force
microscopy, pipette aspiration or the osmotic stress method [108,109]. Also bilayer
elasticity can be studied by a range of techniques including micropipette aspiration
[110], mode-analysis of bending fluctuations [111] and electric-field-induced de-
formations on large unilamellar vesicles [112], but also others such as, e.g., optical
C
BA
d
B
d
W
d
C
d
H
A
Å
d
Figure 10 Schematic of some global membrane properties like, t he overall structure with the
lamellar repeat distance d, membrane thickness d
B
, bilayer separation d
W
, hydrocarbon chain
length d
C
, head group thickness d
H
and lateral area per lipid molecule (A). Panel B shows a
bilayer interaction potential and panel C col lective bending £uctuations.
Liposome-Based Biomembrane Mimetic Systems 119

dynamometry [113], optical force measurements [114] or line-shape analysis of
Bragg peaks [115–118] have been applied. Depending on the applied experimental
technique, the reported bending rigidity K
C
values show some scatter, but are
usually in the range of 10
20
J-10
19
J for phospholipid bilayers.
3.2. Domains-Rafts
Biological membranes are compounds of several lipid components (and proteins,
sugars, etc.). Thus, often binary or ternary mixtures of synthetic lipids are studied in
order to address the complex lipid composition in natural membranes. The mixtures
then have to accommodate the properties of the individual components, which may
lead to a demixing or formation of lipid domains. In turn, a demixing of lipid
components may also be induced by interactions with membrane active com-
pounds, such as, peptides [36,119]. Lipid domains have been observed in model
systems already some time ago [120–123]. However, due their possible implication
in several cellular functions in natural membranes, such as endocytosis, protein
trafficking and signal transduction [124,125], domains or better ‘‘rafts’’ as they are
called these days have recently seen a tremendous increase of research interest. Again
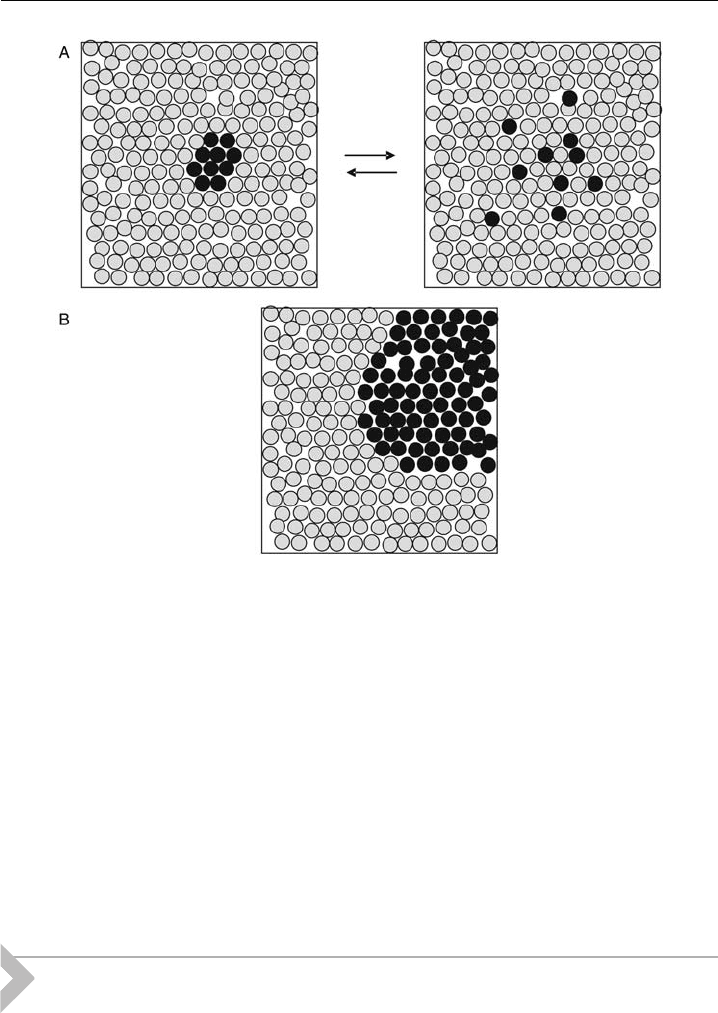
it is important to realize the different time and length scales. Domain formation may
occur as a dynamic process at short-time and small-length scales, respectively. In this
case, the formed domains are small in size and not stable but constantly create and
dissipate and are often referred to as micro-domains (Fig. 11A).
On the other hand, lipid molecules may also segregate and form large areas
composed of single lipids that are stable over longer timescales. This type of do-
mains is often referred to as macroscopic domains. It is, however, important to note
that in the thermodynamic limit only the latter case of domain formation may be
called a phase separation. A lack of this strict distinction has led to severe
controversies in literature with the most prominent example being binary mixtures
of phospholipids with cholesterol. Early phase diagrams [126], based on NMR data
[127], show the existence of a miscibility gap within the fluid phase of
phospholipid/cholesterol mixtures. This fluid–fluid phase separation has been sup-
ported by several further experimental studies such as, [128–131]. Although
McMullen and McElhaney [130] criticized using the terminology of phase diagram
in the context of lipid/cholesterol binary mixtures, their differential scanning
calorimetry (DSC) data clearly showed the co-existence of two phases in a certain
composition range, which can be interpreted according to [126] as the L
d
(liquid-
disordered) and L
o
(liquid-ordered) phases, where the latter phase is induced at high
cholesterol concentrations due to its rigid character. In contrast, groups using other
techniques, such as X-ray diffraction [132] or more recently fluorescent microscopy
[133] found no direct evidences for phase-coexistence in such binary mixtures.
Only stable and large domains within the fluid phase have been reported in ternary
mixtures of lipids with cholesterol, using similar techniques [133–135].
While these more recent results will most likely lead to a revision of the original
phase diagrams of binary cholesterol/lipid mixtures, there is another general lesson
to learn from this example. Global techniques, such as, X-ray (or neutron) diffrac-
tion, or fluorescence microscopy average over a large period in time and hence are
K. Lohner et al.120
unable to detect the signal from unstable microscopic domains. On the other hand,
spectroscopic techniques may also observe density fluctuations from the creation
and dissipation of such ‘‘nuclei’’ and DSC seems to be able to detect both. Rather
than thinking in advantages and disadvantages of the individual techniques it is
more important to realize that only through the application of more than one
technique, questions related to domain formation can be addressed adequately, i.e.,
a technique sensitive to a long timescale should be combined with a technique
sensitive to short timescales. In the case of lipid–peptide interactions, the com-
bination of small- and wide-angle X-ray scattering (SWAXS) and DSC has been
proven to be particularly useful as described in the previous section.
4. X-ray Diffraction in Combination with a Global Data
Analysis
Several recent reviews have dealt with the determination of structural para-
meters from lipid bilayer systems using SAXS [98,136,137]. We will therefore
restrict ourselves here only to the essentials and refer for more details to the above-
listed articles.
Figure 11 Schematic of microscopic (A) and macroscopic domains ( B), where each circle
represents a single lipid molecule. Microscopic domai ns involve only a few lipid molecules and
are highly unstable. Macroscopic domains in cont rast exhibit sizes upto microns and are stable
over an extended time period.
Liposome-Based Biomembrane Mimetic Systems 121
X-rays are known to interact with the electron cloud of atoms and scatter in the
elastic case without loosing or taking up energy from the atoms. The contrast in
X-ray scattering experiments comes from the number of electrons per volume
( ¼ electron density) and its modulation across the sample. Thus, the real space
information that is obtained from scattering experiments is obtained by calculating
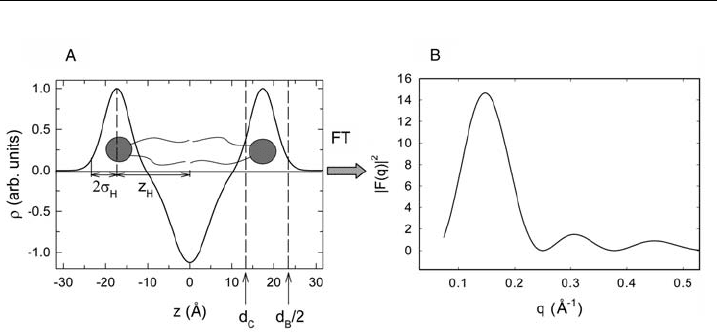
the electron density from the scattering data. In the case of lamellar lipid phases, the
electron density varies only across the lipid bilayer. The corresponding electron
density profile can be modeled to sufficient accuracy by the summation of
three Gaussian distributions [138–140], two centered at the position of the elec-
tron-dense lipid head groups and one of negative amplitude in the middle of the
bilayer, where the hydrocarbon chains meet (Fig. 12A).
In order to compare with experimental data, one needs to Fourier transform the
electron density profile, which can be calculated analytically. The thereby derived
function is called the form factor F (q), which varies as a function of the modulus of
the scattering vector q, which is related to the X-ray photon wavelength l and the
scattering angle y by q ¼ 4p sin(q)/l. Figure 12B shows the absolute square of
the form factor, which corresponds to the intensity recorded in a diffraction
experiment.
Frequently, lipid bilayers self-assemble into a stack of several bilayers. In this case,
positional correlations between the bilayers leading to the observation of Bragg
peaks in scattering patterns need to be considered. The shape of the peaks is affected
due to the significant disorder present in the lipid multibilayers. This is accounted
for by two theoretical descriptions. The paracrystalline theory [141,142] takes into
account a Gaussian distribution of distances between idealized solid layers, which
additionally display some thermal fluctuations (Fig. 13B). This leads in comparison
to thermal fluctuations of the layers around regularly distributed distances
(Fig. 13A) to an increase of the Bragg peak width with diffraction order. The
decrease of peak intensity is also observed in the case of simple thermal fluctuations.
Figure 12 Electron density pro¢le of lipid bilayers across the membrane (panel A) and the
absolute square of its Fourier transform given by the form factor ( panel B). z
H
is the position of
the lipid headgroup and s
H
its width. Panel A further shows de¢nitions for the membrane
thickness d
B
and the hydrocarbon chain thickness d
C
.
K. Lohner et al.122
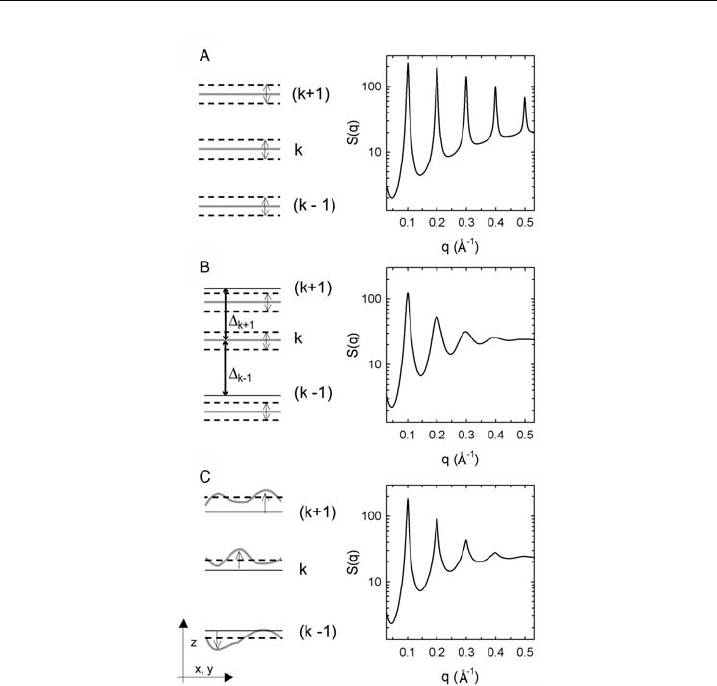
The second theory, named after Caille
´
[143,144], considers the flexibility of the
bilayers yielding bending fluctuations. This translates into cusp-like diffraction peaks
(Fig. 13C), which also broaden with increasing diffraction order. It has been shown
experimentally that the Caille
´
theory accounts for the shape of Bragg peaks of
multilamellar vesicles in the fluid L
a
phase [145]. In turn, bilayer elasticity decreases
by about one order of magnitude upon transforming lipid bilayers into the gel phase.
Thus, in the lamellar gel phase the bilayer can be considered as solid, and the
paracrystalline theory should apply. Nevertheless, there seem to be some inconsist-
encies in fitting higher order Bragg peaks [98], which may be due to the simplifying
assumption of a Gaussian distribution of bilayer separations. Nevertheless, we have
been successful in applying this theory in several cases as will be detailed below.
In X-ray scattering experiments on a liposomal dispersion, one records the
scattered intensity as a function of wave vector q. For lipid–peptide interaction it is
preferable to work under physiological relevant conditions, i.e., at full hydration
and adjusting pH and ionic strength of the buffer solution. In this case, SAXS
Figure 13 Disorder in lamella r stacking and its consequences on the Bragg peaks. (A) Thermal
disorder, (B) paracrystalline theory and (C) Caille
Ł
theory.
Liposome-Based Biomembrane Mimetic Systems 123
