Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


THERMAL BATTERIES 21.11
FIGURE 21.3 Thicknesses of thermal battery cells.
21.4.1 Cup Cells
The typical cup cell features a two layer anode (calcium or magnesium) having active anode
material on both sides of a central current collector. On either side of the anode is an
electrolyte pad made of glass tape impregnated with eutectic electrolyte. Next to each elec-
trolyte are depolarizer pads consisting of cathode material (CaCrO
4
or WO
3
) in an inorganic
fiber matrix (paper). The cell is enclosed in a nickel foil cup and cover that are tightly
crimped (Fig. 21.4a). Some designs also incorporate inorganic fiber mat gaskets and nickel
‘‘eyelets’’ to help prevent the molten electrolyte from leaking out of the activated cell. Zr/
BaCrO
4
heat paper pads located on either side provide heat to the cup cell.
Cup cells have the advantage of large reactive surfaces (they are two-sided or bipolar),
and can contain relatively large amounts of reactive materials. Their disadvantages are that
they are difficult to seal against electrolyte leakage and they have low heat capacity. The
Ca/ CaCrO
4
cell chemistry is also prone to ‘‘alloying’’ (producing excess molten Ca-Li alloy),
which can short-circuit the cells. In order to obtain required short activation times, cup cells
typically have to be pre-melted or ‘‘pre-fused’’ prior to assembly into cell stacks. Inter-cell
electrical connections are accomplished by spot-welding the cell output leads between each
cell, which presents a potential reliability problem.
Currently, cup cells have limited application, and are found primarily in older battery
designs.
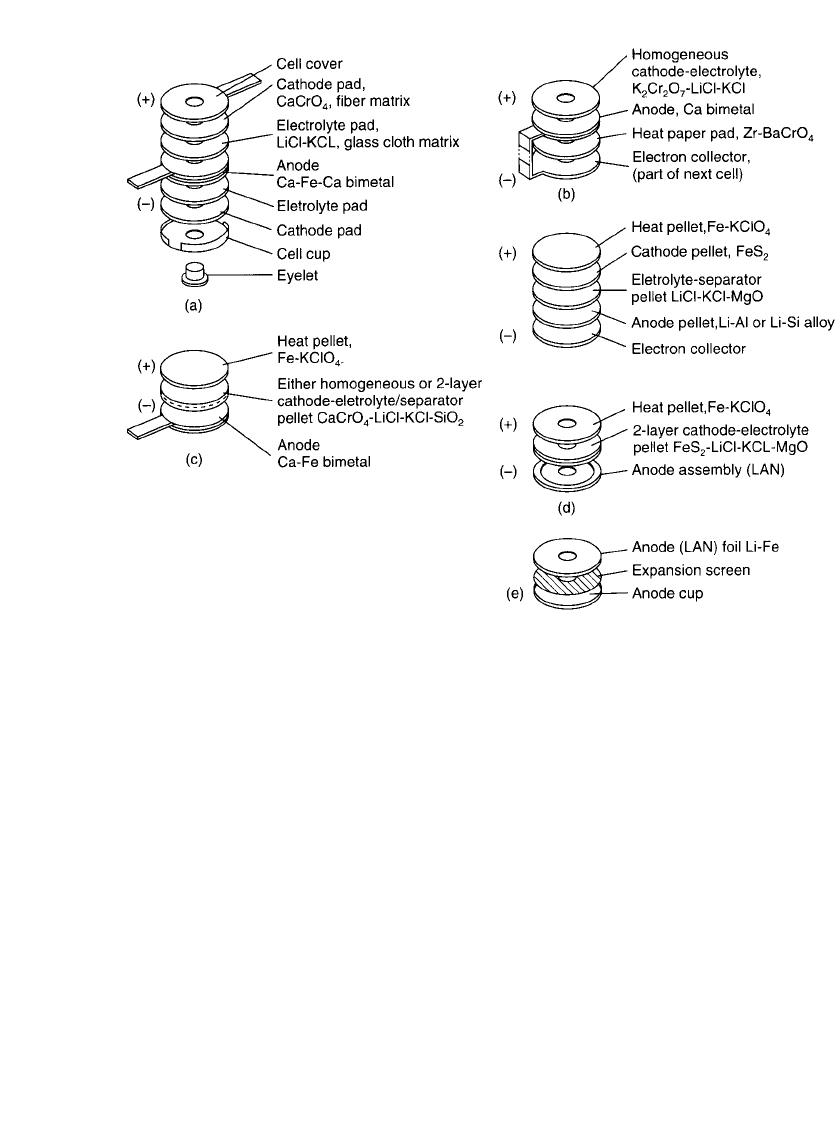
21.12 CHAPTER TWENTY-ONE
FIGURE 21.4 Variations in cell configurations: (a) Cup cell. (b) Open cell. (c) Ca/ CaCrO
4
pellet cell. (d )
Li alloy/ FeS
2
and Li / FeS
2
pellet cell. (e) Li metal /FeS
2
, (LAN) anode assembly.
21.4.2 Open Cells
The open-cell design is similar in construction to the cup cell, except that it is not enclosed
in a cup (Fig. 21.4b). Elimination of the cup is possible because the amount of electrolyte
is reduced to the extent that practically all of it is bound to the glass fiber cloth matrix by
surface tension. Some designs use homogeneous electrolyte-depolarizer pads; others have
discrete parts. Open-cell designs typically incorporate a combination anode-electron collec-
tor, usually in the shape of a ‘‘dumbbell.’’ This combination part has anode material vacuum-
deposited on one end (which serves as anode in one cell), while the other end is an electron
collector in the next, series-connected cell. A narrow bridge connects the two ends of the
dumbbell. The bridge serves as an inter-cell connector, eliminating the need for spot welds.
Zr/ BaCrO
4
heat paper pads heat the open cells, which are assembled between the folded
dumbbells.
The open-cell design is used in relatively short-life applications and in pulse batteries.
Their parts can be made very thin to promote very rapid heat transfer and obtain short
activation times.

THERMAL BATTERIES 21.13
21.4.3 Pellet Cells
In pellet cells, the electrolyte, cathode, and heat source are in pellet (wafer) form. Anodes
can be of different configurations, depending on which electrochemical system is used. For
pellet production, the cell component chemicals are processed into powders, and the powders
are uniaxially pressed into the parts. Electrolytes, which melt at cell operating temperatures,
are combined with inert binders, which hold the molten salts in place by capillary action or
surface tension, or both.
A typical pelletized Ca /CaCrO
4
cell, as shown in Fig. 21.4c, is made up of the following:
1. A calcium anode—either calcium foil (on nickel or iron foil collector) or calcium bimetal
(deposited on either iron or nickel collector)
2. A pelletized electrolyte powder blend—consisting of LiCl-KCl eutectic salts and either
SiO
2
or kaolin as binders
3. A pelletized cathode powder blend—consisting of CaCrO
4
, LiCl-KCl eutectic salts, and
SiO
2
or kaolin binder
4. A pelletized heat source—a blend of iron powder and KClO
4
. (Alternatively, this may be
a non-pelletized heat source assembly made up of Zr-BaCrO
4
heat paper in a nickel or
iron foil dumbbell with the anode of the next cell on the outside—similar to the anode
and heat source in open-cell designs.)
Variations of this cell design include 1) the use of a two-layer pellet with discrete elec-
trolyte and cathode layers formed into one part; and 2) the use of a homogeneous pellet that
has the electrolyte and cathode powders blended together (depolarizer-electrolyte-binder or
DEB pellet).
18
A typical Li/ FeS
2
cell, as illustrated in Fig. 21.4d, is made up of the following:
1. A lithium anode—of either pelletized lithium alloy powder or a lithium metal anode
assembly
2. A pelletized electrolyte powder blend—consisting of a salt mixture and MgO binder. The
salts may include mixtures such as LiCl-KCl eutectic, LiBr-KBr-LiF, or LiCl-LiBr-KBr
3. A pelletized cathode powder blend—of FeS
2
and electrolyte with either MgO or SiO
2
binder
4. A pelletized heat powder blend—of pyrotechnic-grade iron powder and KClO
4
5. An electrical collector—of iron or stainless steel foil, located between the heat pellet and
the lithium alloy anode pellet. This part is not used with a lithium metal anode assembly,
which has an integral metal foil cup. In some cases, especially in longer-life batteries, a
second metal foil ‘‘collector’’ is placed between the FeS
2
cathode and the heat pellet to
buffer or prevent the cathode from exposure to excessive heat.
The pressure used for pelletizing the cell components is critical. In the case of Ca/ CaCrO
4
designs, the forming pressures, and hence the resultant densities of the electrolyte and cath-
ode pellets, have a profound effect on the reactivities of the cells. The components of the
Li/ FeS
2
systems, except for the heat pellets, are less sensitive to variations in density. Heat
pellet ignition sensitivity and burning rate are significantly affected by changes in density,
however, with high density decreasing ignition sensitivity and rate. The design parameters
of a representative Li(Si)/ FeS
2
cell are shown in Table 21.3.
19
The use of pellet-type cell construction has significantly increased the performance ca-
pability of thermal batteries. Pellet designs have particular advantages in longer-activated-
life, high-current-drain applications. They are structurally very rugged, can operate reliably
over wider ambient temperature ranges, and are generally less expensive to manufacture than
older designs. There are applications, however, such as those requiring fast activation times
and high-voltage pulses, where open-cell designs with Ca /LiCl-KCl /K
2
Cr
2
O
7
or Ca/LiCl-
KCl/ PbCrO
4
cell chemistries and heat paper are more suitable.
21.14 CHAPTER TWENTY-ONE
TABLE 21.3 Cell Components of 3400-A /s, Li-Si /FeS
2
, Thermal Battery Cell (From Street
19
)
Pellet
Component Chemical composition
Chemical ratio
w/% 1
Density
(g/ cm
2
)
0.05
Forming
force,
tons
Thickness,
cm Weight, g
0.1
1 Heat pellet Fe /KClO
4
88/ 12 3.40 60 0.14 22
2 Cathode current
collector
SST-304 — 7.75 — 0.013 4.6
3 Cathode pellet FeS
2
/LiCl-KCl /SiO
2
64/ 16/ 20 2.9 200 0.06 8.5
4 Separator pellet LiCl-KCl-Li
2
O/ MgO 65 /35 1.75 90 0.06 4.5
5 Anode pellet Li / Si 44 / 56 1.0 115 0.1 4.5
6 Anode current
collector
SST-304 — 7.75 — 0.013 4.6
21.5 CELL-STACK DESIGNS
All thermal batteries are designed to satisfy a specific set of performance requirements, each
of which includes output voltage, current drain, and activated life. In designing a battery,
the output voltage determines the number of cells that must be connected in series. Since
each cell produces a fixed maximum voltage (from 1.6 to 3.3 V on open circuit, depending
on the cell chemistry used), the battery output will be in multiples of discrete cell voltages.
Batteries containing over 180 series-connected cells with an overall output voltage near 400
V have been successfully manufactured. Typical batteries contain 14 to 80 cells, and have
an output voltage of 28 to 140 V. Figure 21.5 illustrates two different cell-stack configura-
tions, one with cup cells and the other with pellet-type cells.
The current-carrying capacity of each cell is determined by the reactive surface area of
the cell, which is directly related to the cell size (diameter). As with cell voltages, the
maximum useful current densities (Amperes per unit area) differ greatly among cell chem-
istries (see Tables 21.4 and 21.5). The effective cell area, and hence the current-carrying
capacity of a battery, can be adjusted by electrically connecting any number of cells in
parallel.
Thermal batteries can be designed to provide multiple output voltages by electrically
connecting the required number of cells in series. The multiple-voltage outputs can be drawn
either from cells that are common to more than one output or from isolated cells whose
output is not shared. An electrically isolated group of cells must be used for circuits that
cannot tolerate ‘‘crosstalk’’ from other circuits in a system. It is also possible to combine
cell-stack sections with different cell chemistries in the same battery. Such combinations
yield the specific performance characteristics of both chemistries from a common unit. An
example of this is a battery that combines a cell stack with a chemistry that has a very short
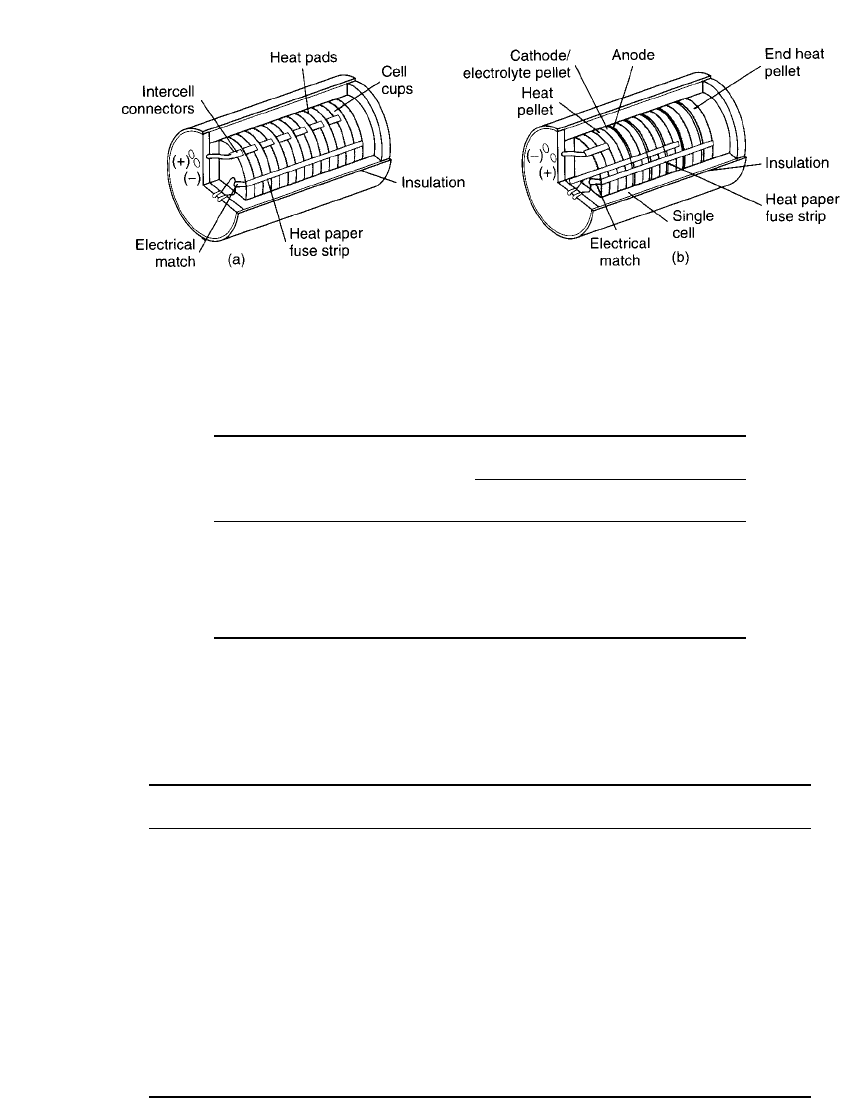
THERMAL BATTERIES 21.15
FIGURE 21.5 Typical thermal battery assemblies. (a) Cup cells. (b) Pellet cells.
TABLE 21.4 Attainable Current Density of Different Cell Designs
Cell design
Current density, mA /cm
2
10-s rate 100-s rate 1000-s rate
Cup cell 620 35
Open cell / dichromate 54
Pellet cell/ two-layer Ca/CaCrO
4
790 46
Pellet cell/ DEB Ca/ CaCrO
4
930 122
Pellet cell/ Li/ FeS
2
⬎2500 610 150
TABLE 21.5 Typical Power and Energy Densities of Li/ FeS
2
Thermal Batteries
Battery volume, cm
3
Power density, W/ cm
2
Energy density, Wh/ L Activated life, s
20 11.25 46.87 15
29 1.44 34.20 85
70 2.59 35.97 50
108 0.65 32.41 180
170 1.98 109.80 200
171 10.64 118.26 40
183 2.29 63.75 100
306 0.51 39.65 280
311 2.25 75.03 700
552 0.15 67.63 1600
1176 0.40 101.19 900
1312 0.17 85.37 1800
3120 1.11 83.30 270
21.16 CHAPTER TWENTY-ONE
start time with a different cell stack that can provide a high current over a long activated
life. Where such combinations are used, the outputs from the different cell-stack sections
are often diode-isolated to prevent one section from charging the other. Some thermal battery
designs combine two or more discrete batteries into an assembly that may have a number
of different, mutually isolated voltage outputs with widely varying current capabilities.
Cells comprising a cell stack are typically held in place by the closing compression
applied when the battery cover is secured by welding it to the battery case. Some battery
designs incorporate an inner case to maintain compression on the cell stack while the outer
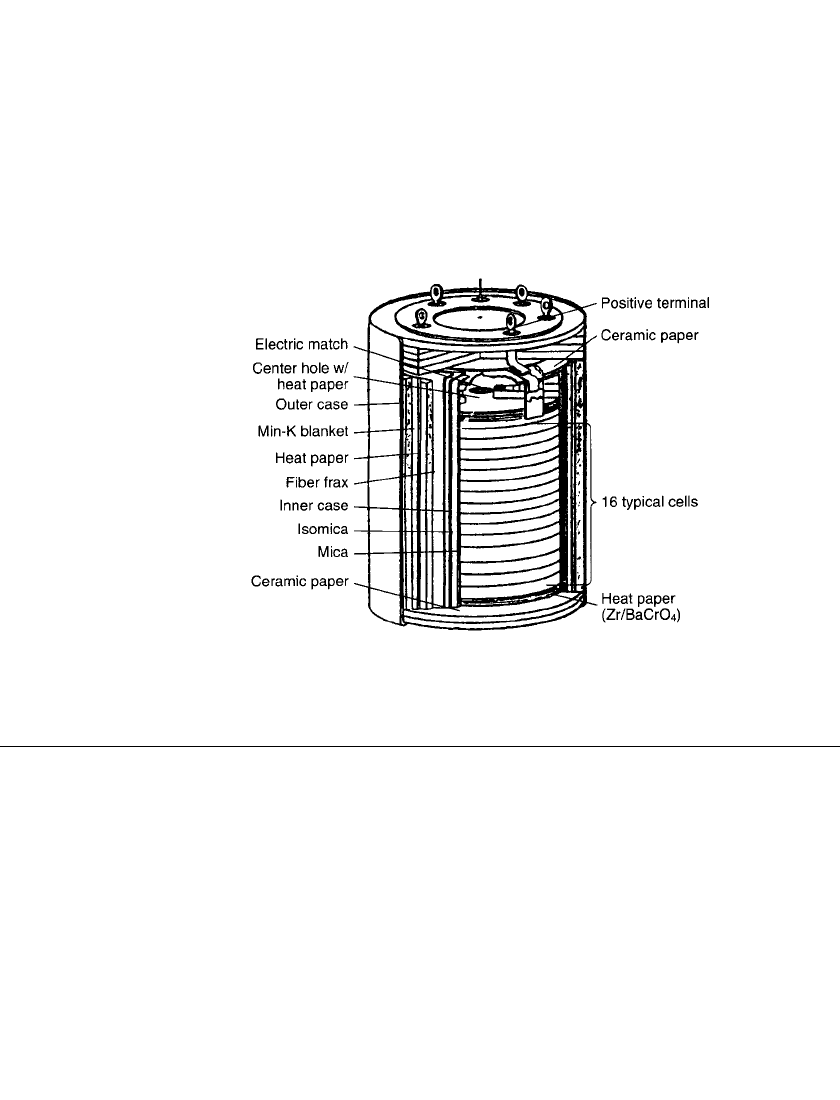
case and cover combination provides hermetic enclosure for the unit. Figure 21.6 pictures a
battery design that employs an inner cell stack case.
FIGURE 21.6 Typical thermal battery assembly with inner case.
(Courtesy of SAFT America, Inc.)
21.6 PERFORMANCE CHARACTERISTICS
Thermal batteries are custom-designed to satisfy a specific set of performance requirements.
These include not only output voltage, current, activated life, and voltage rise time (start),
but also storage and activated-life environments, mounting, surface temperature, activation
method and energy, and others. For this reason it is very important that the user or systems
designer have a close technical interface with the battery designer during the design and
development phases of the battery.
21.6.1 Voltage Regulation
Thermal battery output voltages are not linear. After reaching a peak level, typically within
1 second after activation, the voltage starts to decay until it eventually drops below the
minimum useful level. Voltage regulation is the range between the specified minimum and
maximum limits. Typically, the minimum voltage limit is 75% of the peak voltage. The
battery output profile (consisting of the rise time, peak voltage, and rate of decay) depends
on the cell chemistry, and is strongly affected by the operating temperature and applied load.
Figure 21.7 illustrates the effects of discharge load on a typical battery output profile.
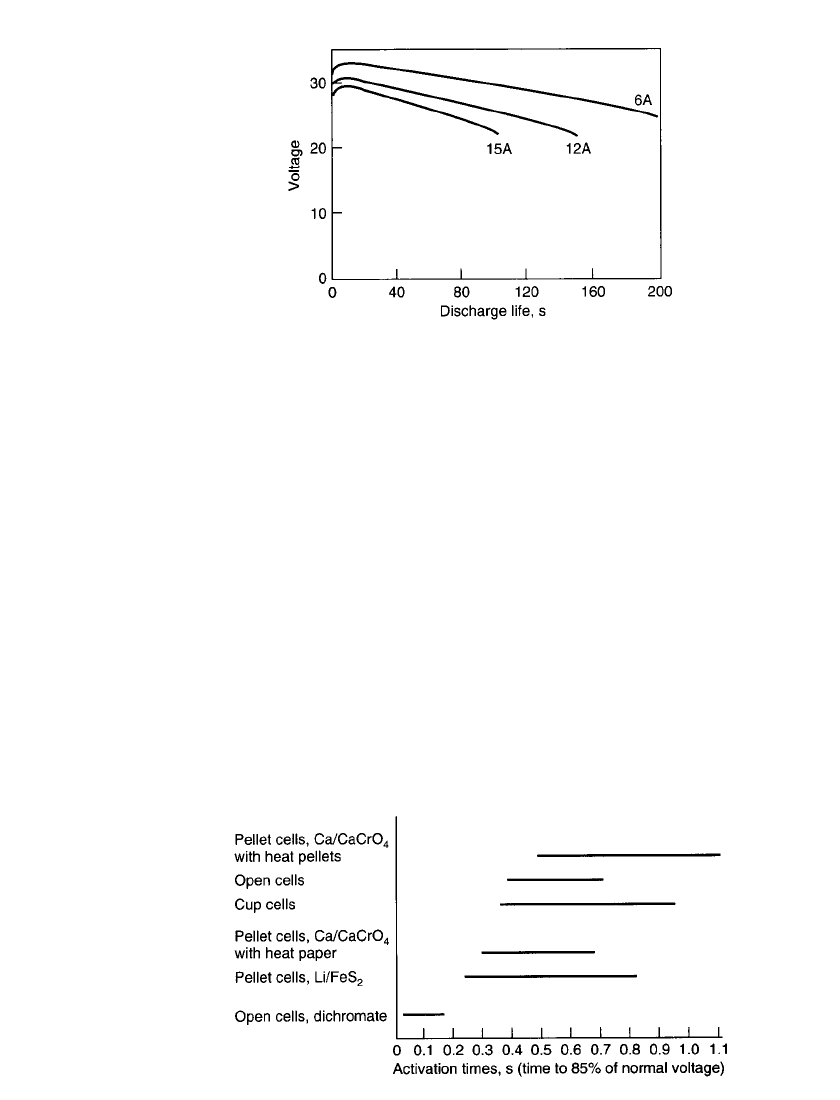
THERMAL BATTERIES 21.17
FIGURE 21.7 Discharge voltage curves of typical Li /
FeS
2
thermal battery at three different current drain rates.
21.6.2 Activation Time
The activation time (rise time) is the time interval from the application of energy to the
initiation device until the battery output voltage reaches the minimum specified limit. The
activation time is affected by the operating temperature, applied load, and cell chemistry
used. Lowering the operating temperature or increasing the load typically increases the ac-
tivation time. Typical Li/FeS
2
batteries have activation times from 0.35 to 1.00 s. Large,
high-capacity batteries can have activation times as long as 3 s. (Large diameter heat pellets
take longer times to burn.) On the other hand, fast-activating chemistries such as Ca /K
2
Cr
2
O
7
can yield activation times as short as 12 ms. Figure 21.8 shows activation time ranges for
various cell chemistries and Fig. 21.9 illustrates the effects of ambient temperature.
FIGURE 21.8 Activation times of different thermal battery cell designs.
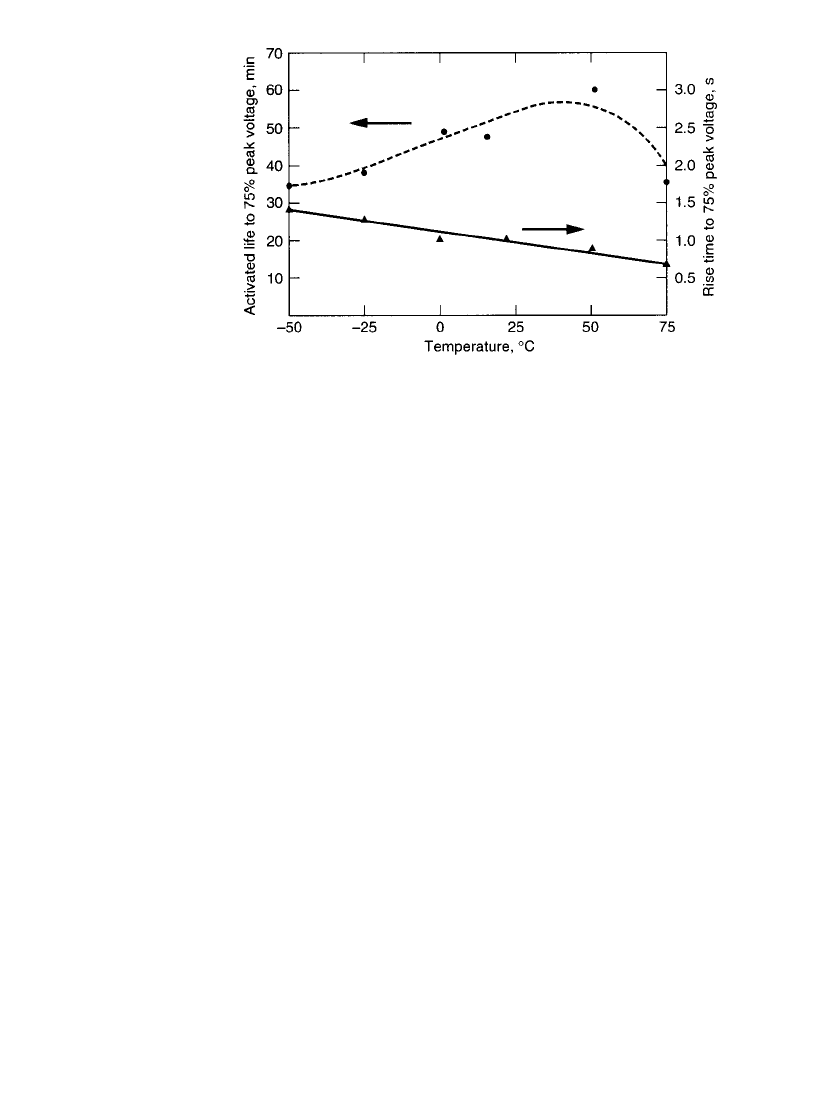
21.18 CHAPTER TWENTY-ONE
FIGURE 21.9 Activated life and rise time of Li / FeS
2
battery. (From
Quinn and Baldwin.
20
)
21.6.3 Activated Life
The activated (operating) life is typically specified as the time from the initial application of
the activation energy until the battery voltage drops below the minimum specified limit.
Activated life is affected by the cell chemistry used, the operating temperature environment,
and the current drain. Typically, thermal batteries are thermally balanced (total cell mass vs.
caloric input) to have the longest activated lives between the high and low operating tem-
perature limits, or near room ambient. Lives will get shorter near each temperature limit. At
the low limit, the electrolyte will start freezing sooner, whereas at the high limit the thermal
degradation of FeS
2
occurs at a faster rate, depleting active materials.
21.6.4 Interface Considerations
The following performance and design characteristics must be noted when designing a sys-
tem that interfaces with a thermal battery:
1. An unactivated battery has a very high internal resistance (megOhms). Once activated,
an individual cell’s resistance is between 0.003 and 0.02 Ohm, depending on the cell
design. The internal resistance of the battery is equal to the sum of the resistances of all
series-connected cells.
2. Some cell chemistries, such as Li/ FeS
2
, are tolerant of backcharging from an external
power source. Others, however, such as Ca /CaCrO
4
must not be subjected to backcharg-
ing at all.
3. Electric actuators contain bridge wires that may not burn through during activation and,
if not disconnected, may act as a parasitic load on the external ignition circuit.
4. Leakage paths that can adversely load the battery may develop in an activated battery
between electrically ‘‘live’’ components and the battery case or activator circuits. System
requirements, such as case grounding, cell-stack common output, and activator circuit
grounding must be specified so that special insulation provisions can be incorporated into
the battery design.
THERMAL BATTERIES 21.19
5. The surface temperature of an activated battery may reach 400⬚C. The type of battery
mounting, the heat transfer properties of the mounting, the effects of high temperature
on the surrounding components, and the proximity of combustible materials must be
considered. The battery surface temperature can usually be reduced significantly by in-
corporating added (or more efficient) thermal insulation. This is achieved, however, at
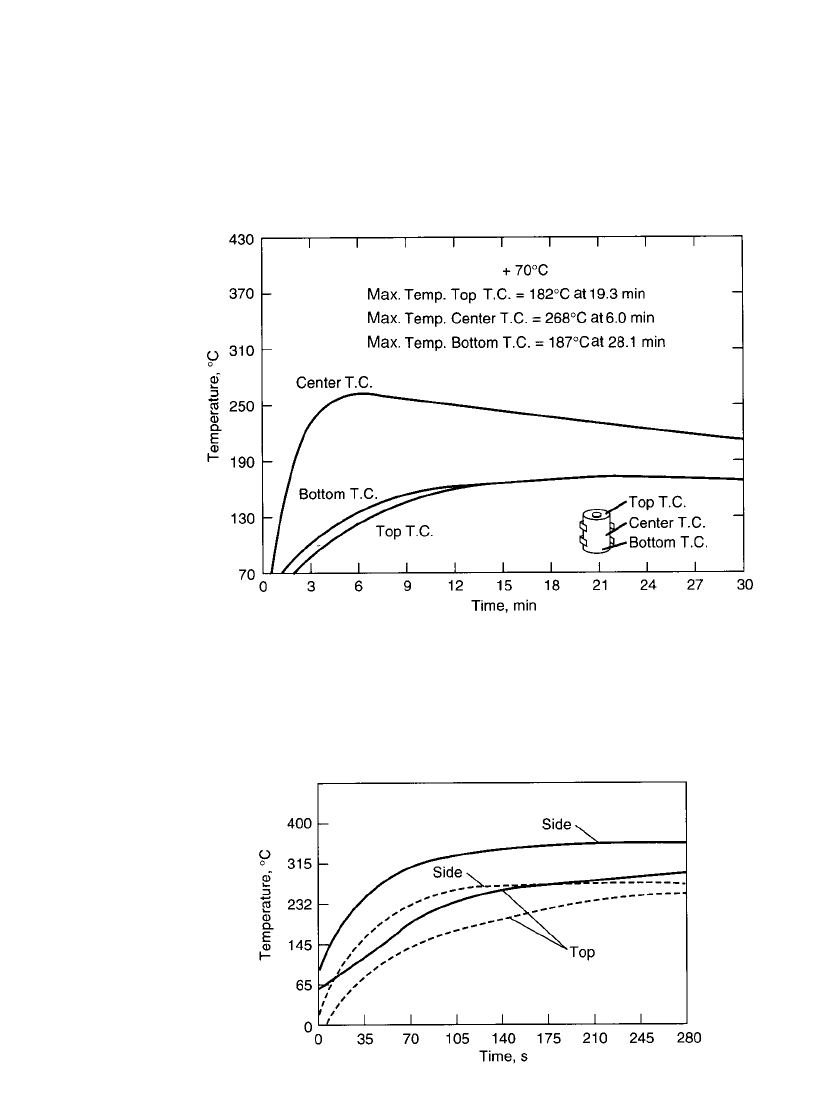
considerable cost and increase in battery volume. Figures 21.10 and 21.11 illustrate typical
surface temperatures of thermal batteries.
FIGURE 21.10 Surface temperature profiles for long-life thermal battery. (From
Street.
19
)
FIGURE 21.11 Surface temperature profiles for a medium-life
thermal battery. Solid line-tested at 71⬚C; broken line-tested at
⫺53⬚C.
21.20 CHAPTER TWENTY-ONE
21.7 TESTING AND SURVEILLANCE
The safety and reliability of thermal batteries has been a matter of continuing study since
they were first developed. To identify defective units, most designs are 100% tested for
hermeticity, polarity, electrical insulation resistance, and activation circuit resistance (if ap-
plicable) on manufacture. Most units are also radiographed. Prior to commencement of pro-
duction, a sample group of 10 to as many as 500 batteries is subjected to qualification tests.
This series of tests includes the most severe environmental and discharge conditions to which
the particular battery design will be exposed in actual field use. Almost all thermal batteries
are fabricated in homogeneous groups or lots, and samples from each lot are discharged to
demonstrate compliance with the performance requirements. Usually the samples are dis-
charged at maximum specified loads, often with concurrently imposed environmental forces.
By using such test programs, reliability values greater than 99% and safety values greater
than 99.9% have been demonstrated innumerable times in the last five decades.
Lithium thermal batteries designed for use in U.S. Navy systems are subject to safety
tests per Navy technical manual S9310-AQ-SAF, ‘‘Battery, Navy Lithium Safety Program
Responsibilities and Procedures.’’ These tests are designed to assure that the battery design
is safe not only in proper storage and use, but also when subjected to inadvertent misuse
and conditions caused by accidents, such as backcharging, short circuits, and fires.
21.8 NEW DEVELOPMENTS
The primary aim of new research and development in the thermal battery area has been to
increase the energy density and specific energy of the practical unit. Two possible approaches
to this goal are to: (1) decrease the total volume and mass of the battery; and (2) increase
the voltage or the current-carrying capacity per cell volume and mass.
In the area of decreasing battery mass, investigations are being conducted in substituting
lighter-weight materials for the currently used stainless steel battery housings. Titanium,
aluminum, composites, and other materials have been suggested and tried with varying de-
grees of success. Titanium cases and headers have been successful but suffer from higher
cost.
Efforts to deposit thin FeS
2
films by plasma-spraying of powders onto stainless steel
substrates have been yielded promising results.
21
This technology could potentially reduce
the mass and volume of a thermal battery by virtue of the higher active material densities
afforded by this technique.
Development efforts to produce cells with higher voltage have demonstrated the potential
of employing molten nitrate electrolytes with lithium anodes.
22
This system has the added
benefit of lowering the battery operating temperature by more than 200
⬚C.
Recent development effort has also been directed toward increasing the activated life of
batteries past 2 hrs up to 4 hrs. This effort has required the development of more efficient
thermal insulation, such as use of double-walled vacuum enclosures (cases) and multi-layered
insulating blankets, as well as lower melting point electrolyte compositions.
REFERENCES
1. G. O. Erb, ‘‘Theory and Practice of Thermal Cells,’’ Publication BIOS / Gp 2 /HEC 182 Part II,
Halstead Exploiting Centre, June 6, 1945.
2. O. G. Bennett et al., U.S. Patent 3,575,714, Apr. 20, 1971.
3. B. H. van Domelen, and R. D. Wehrle, ‘‘A Review of Thermal Battery Technology,’’ Intersoc Energy
Convers. Conf., 1974.
4. F. Tepper, ‘‘A Survey of Thermal Battery Designs and Their Performance Characteristics,’’ Intersoc.
Energy Convers. Conf., 1974.
