Lewin Benjamin (ed.) Genes IX
Подождите немного. Документ загружается.

jfrd
@
Binding
to the
Response
Etement
Is Activated
by Ligand-Binding
r
Binding
of tigand
to the
C-terminal
domain increases
the affinitv
of the DNA-
binding
domain for its
speciiic
target site
in
DNA.
Steroid Receptors
Recognize
Response
Etements
by a CombinatoriaI
Code
o
A steroid response
etement
consists of
two
short half
sites that
may be
pa[indrom'ic
or directly repeated.
r
There
are onty
two types
of half sites.
o
A receptor
recognizes
its
response
etement
by the
orientation
and spacing
of the hatf
sites.
.
The
sequence
of the hatf
sjte is recognized
by the first zinc
finger.
o
The
second zinc
finger is
responsibte for
dimerization, which
determines
the dis-
tance between
the subunits.
o
Subunit
separation in
the receptor
deter-
mines
the recognition
of
spacing
in
the
response
e[ement,
.
Some steroid receptors
function
as homo-
dimers,
whereas
others form heterodimers.
o
Homodimers
recognize
patindromic
response
etements;
heterodimers
recog-
nize response
etements with
directty
repeated
hatf
sites.
Homeodomains
Bind
Retated Tarqets
in DNA
r
The
homeodomain
is a DNA-bindinq
domain of 60
amino acids
that hasihree
cr-
he[ices.
r
The
C-terminaI
a-hetix-3 is 17
amino acids
and
binds
in
the major
groove
of
DNA.
o
The N-terminal
arm
of the homeodomain
projects
into
the minor
groove
of
DNA.
bind
inverted repeats in DNA.
Summary
r
Proteins
containing
homeodomains may
be
either activators or
reDressors
of
tra nscri
ption.
Hetix-Loop-Helix Proteins Interact
by
Com bi natoriaI Association
o
Helix-toop-helix
proteins
have a motif of
40 to 50 amino acids
that comprises
two amphipathic
q-helices
of 15 to
L6 residues
separated
by a Loop.
o
The hetices are resDonsibte
for
dimer
formation.
.
bHLH
proteins
have a basic sequence adja-
cent to the
HLH motif that
is
responsible
for
binding to
DNA.
r
Ctass A bHLH
proteins
are ubiquitously
expressed. Ctass
B
bHLH
proteins
are
tissue-soecific.
.
A class B
protein
usuatty
forms
a
het-
erodimer with a class
A
orotein.
r
HLH
proteins
that lack the basic
region
prevent
a bHLH
partner
in
a
heterodimer
from
binding
to DNA.
o
HLH
proteins
form
combinatoriaI
associa-
tions that may be changed
during devet-
opment by the
addition or
removal
of
specific
proteins.
Leucine Zippers
Are Involved in Dimer
Formation
The
leucine
zipper
is
an amphipathic
helix
that dimerizes.
The zipper
is
adjacent
to a basic
region
that binds
DNA.
Dimerization
forms the bZIP motif
in
which
the two
basic regions symmetricatly
Introduction
r
Eukaryotic
gene
expression is
usualty
controlted at
the [eve[
of
initiation
of
transcriotion.
The
phenotypic
differences
that distinguish
the
various kinds
of cells in
a higher
eukaryote are
Iargely
due to differences in
the expression
of
genes
that code for
proteins,
that is,
those tran-
scribed by RNA
polymerase
II. In
principle,
the
expression
of these
genes
might
be regulated
at
any one of several
stages. We
can distinguish
(at
least) five
potential
control
points,
which
[orm
the following
series:
Activation
of
gene
structure
J
Initiation
of transcrintion
Processing the transcript
Transport
to
cytoplasm
Translation
it
-**o
As we see in
FIGURE 25.1,
gene
expression
in
eukaryotes is largely controlled
at the initiation
of transcription. For most
genes,
this
is
the
major
control
point
in their expression.
It involves
changes in the structure
of chromatin at
the
promoter (see
Section
30.11,
PromoterActiva-
tion Involves
an Ordered
Series
of Events),
accompanied by the binding
of the basal
tran-
scription apparatus
(including
RNA
poly-
merase II) to the
promoter.
(Regulation
at
subsequent stages of transcription
is rare in
eukaryotic
cells.
Premature
termination
occurs
at
some
genes
and is counteracted
by a
kinase,
P-TEFb, but otherwise
antitermination
does
not
seem to be employed.)
25.1
Introduction 641

Control of transcription initiation: used
for
most
genes
Local structure of the
gene
is
changed
General transcription apparatus
binds to
promoter
RNA
is modified and
processed:
can control expression of alternative
products
lrom
gene
Finally, the translation
of an mRNA in the
cytoplasm can be specifically
controlled. While
employment
of this
mechanism is
uncommon
adult somatic cells,
it does occur in some embry-
onic situations.
This can involve localization of
the mRNA to specific sites
where it is expressed
and/or the blocking of
initiation of translation
by specific
protein factors.
Regulation of tissue-specific
gene
transcrip-
tion lies at the
heart of eukaryotic differentia-
tion.
A regulatory transcription factor serves to
provide
common control of
a large number
of
target
genes,
and we seek to answer two
ques-
tions about this
mode of regulation: How does
the transcription
factor identify its
group
of tar-
get genes,
and how is the activity of the tran-
scription factor
itself regulated in response to
intrinsic or extrinsic signals?
There Are
Several
Types
of
Transcri
ption
Factors
The
basa[ apparatus
determines the startpoint for
transcription.
Activators determine
the frequency
of
transcri
ption.
o
Activators work by making
protein-protein
contacts
with
the basal
factors.
e
Activators may work via coactivators.
.
Some components of
the transcriptional
apparatus
work
by changing
chromatin structure.
Initiation of
transcription involves
many
protein-protein
interactions
among transcrip
-
tion factors bound at the
promoter
or at an
enhancer, as well as with RNA
polymerase.
We
can divide the factors required for transcrip-
tion into several classes, which are described
in the following 1is1.
;jdiifil J:,;.:
summarizes
their
properties.
.
Basal factors, together
with RNA
poly-
merase,
bind
at the
startpoint and TAIA
box
(see
Section 24.10, The Basal
Appa-
ratus Assembles at the Promoter).
.
Activators are transcription
factors that
recognize
specific short consensus
elements. They
bind to sites in the
promoter
or
in
enhancers
(see
Sec-
tion 24.11, Short Sequence
Elements
Bind Activators). They
act by increas-
ing the efficiency with
which the
basal
apparatus
binds to the
promoter.
They
therefore increase the frecuencv
of tran-
mRNA is exported from nucleus to cytoplasm:
not regulated
mRNA
is translated:
regulated in
amphibian development
i:i=,::iii ;::.
-i
Gene expression is controtled
principal.l"y
at
the
initiation
of transcriotion. It is rare for the subse-
quent
stages of
gene
expression to be used to determine
whether
a
gene
is
expressed, aLthough controI of
process-
ing
may be used to determine which form
of a
gene
is rep-
resented
in mRNA.
The
primary
transcript is modified
by cap-
ping
at the 5'end,
and
in
general
also is modi-
fied
by
polyadenylation
at the 3' end.
Introns
must be excised from
the transcripts of inter-
rupted
genes.
The mature RNA must be
exported from the nucleus
to the cytoplasm.
Regulation
of
gene
expression
by selection of
sequences
at the level of nuclear RNA might
involve
any or all of these stages,
but the one
for which
we have most
evidence concerns
changes in splicing;
some
genes
are expressed
by means
of alternative splicing
patterns
whose
regulation
controls the type
of
protein product
(see
Section 26.12, Alternative
Splicing Involves
Differential
Use of Splice Junctions).
CHAPTER 25 Activating
Transcription
scription,
and
are required
for
a
pro-
moter to function
at an adequate
level.
Some activators
act
constitutively
(they
are
ubiquitous),
whereas
others have
a
regulatory
role
and
are synthesized
or
activated
at specific
times
or in specific
tissues.
These
factors
are therefore
responsible
for
the control
of transcrip-
tion
patterns
in time
and
space. The
sequences
that
they bind
are called
response
elements.
Members
of another group
of factors
necessary
for
efficient
transcription
do
not
themselves
bind DNA.
Coactiva-
tors
provide
a connection
between
acti-
vators and
the basal
apparatus
(see
Section 25.5,
Aclivators
Interact
with
the Basal Apparatus).
They work
by
protein-protein
interactions,
forming
bridges between
activators
and the
basal
transcription
apparatus.
Some coactivators
and
other regulators
act to make
changes in
chromatin
(see
Section 30.7,
Acetylases
Are Associated
with Activators).
The
diversity
of elements
from which
a
functional
promoter
may
be constructed,
and
the variations
in their
locations
relative to
the
startpoint,
argues
that the
activators have
an
ability to interact
with one
another by
protein-protein
interactions
in
multiple
ways.
There
appear to be no
constraints
on the
poten-
tial
relationships
between
the elements. The
modular nature
of the
promoter
is illustrated
by experiments
in which
equivalent
regions of
different
promoters
have
been exchanged.
Hybrid
promoters,
for
example,
between the
thymidine kinase
and
p-globin
genes,
work
well.
This
suggests that the main
purpose
of the ele-
ments is
to bring the
activators
they bind into
the vicinity of the initiation
complex, where
protein-protein
interactions
determine the
effi-
ciency
of the initiation
reaction.
The
organization of RNA
polymerase
II
pro-
moters
contrasts with
that of
bacterial
promot-
ers,
where all the transcription
factors
must
interact
directly with
RNA
polymerase.
In the
eukaryotic
system, only the
basal factors inter-
act directly with the
enzyme. Activators
may
interact with
the basal factors,
or they may inter-
act
with coactivators that in
tum interact
with the
basal
factors.
The construction
of the
apparatus
through layers
of
interactions
explains the flex-
ibility with which
elements may
be arranged and
the distance over which
thev can
be disnersed.
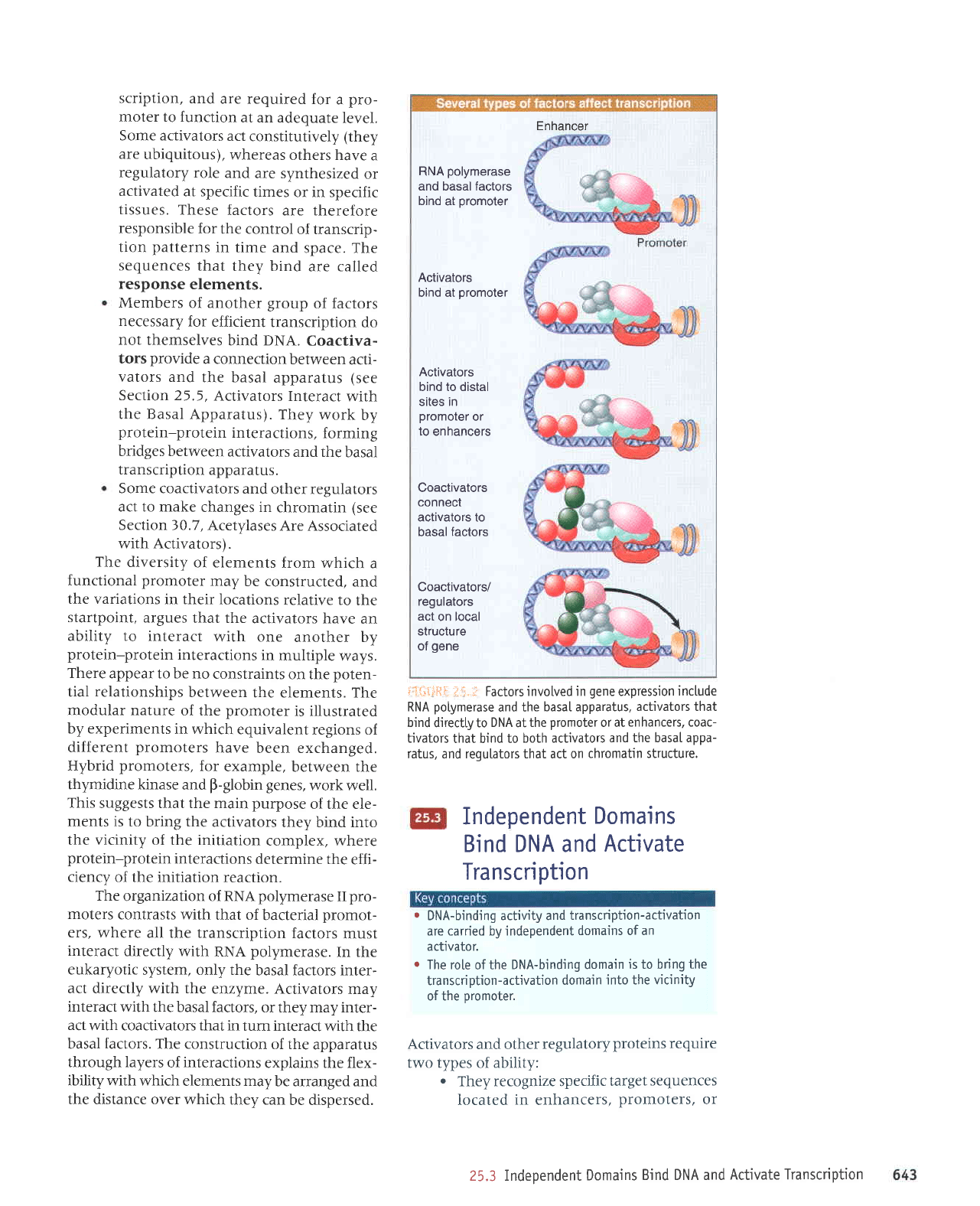
Enhancer
RNA
polymerase
and basal factors
bind
at
promoter
Activators
bind at
promoter
Activators
bind to distal
sites in
promoter
or
to enhancers
Coactivators
connect
activators to
basal
factors
Coactivators/
regulators
act
on local
structure
of
gene
i
!*liit[:
'l:1."]
Factors involved in
gene
expression
include
RNA
polymerase
and the
basaI apparatus,
activators that
bind
directLy to
DNA
at
the
promoter
or at
enhancers, coac-
tjvators
that bind to
both activators
and the basal appa-
ratus, and requlators that act on
chromatin structure.
Independent
Domains
Bind DNA and
Activate
Transcription
r
DNA-binding activity and
transcription-activation
are carried
by
independent
domains of an
activator.
r
The role
of the
DNA-binding domain
is
to bring
the
tra
nscri
ption
-activation
doma'i
n i nto the vici
nity
of the oromoter.
Activators
and other
regulatory
proteins require
two types
of
ability:
o
They recognize specific
target
sequences
located
in
enhancers,
promoters,
or
25.3 Independent
Domains
Bind DNA and
Activate Transcription
other regulatory elements that affect
a
particular
target
gene.
.
Having
bound
to DNA, an activator
exercises
its function by binding to other
components of the transcription
ap-
paratus.
Can we characterize
domains in the acti-
vator
that are
responsible for these activities?
Often an activator
has
separate domains
that
bind DNA and activate transcription. Each
domain behaves as a separate
module
that
func-
tions independently when
it is linked
to
a
domain of the other type. The
geometry
of the
overall transcription
complex
must allow the
activating domain to contact the basal appara-
tus irrespective
of the exact
location
and orien-
tation of the DNA-bindine domain.
Upstream
promoter
elements may be an
appreciable distance
from the
startpoint,
and in
many
cases
may be oriented
in
either direction.
Enhancers may be even
farther
away and
always
show orientation
independence. This organi-
zation has implications
for both the DNA and
proteins.
The DNA
may
be
looped
or condensed
in
some
way
to allow the formation of the tran-
scription complex.
In addition, the domains of
the activator may be connected
in
a
flexi-
ble way, as
illustrated diagrammatically in
ilESi.Jftf 85.3. The main
point
here is that the
DNA-binding and activating domains are
inde-
pendent,
and are connected in a way that allows
the activating domain to
interact
with the basal
apparatus
irrespective of the orientation and
exact location of the
DNA-binding
domain.
Binding to DNA is necessary for activating
transcription, but
does activation depend on
the
p
articul ar
DNA-binding
domain?
FIfri.lRil E$.,{,
illustrates an experiment to
answer this
question.
The activator
Ga14
has
a DNA-binding domain that recognizes a UAS
and an
activating domain that stimulates initi-
ation at the target
promoter.
The
bacterial
repressor LexA
has
an N-terminal
DNA-
binding domain
that recognizes a specific oper-
ator. When LexA binds to this operator, it
represses
the adjacent
promoter.
In
a
"swap"
experiment, the
DNA-binding domain
of
LexA
can be substituted
for the DNA-binding
domain
of Gal4. The hybrid
gene
can then be introduced
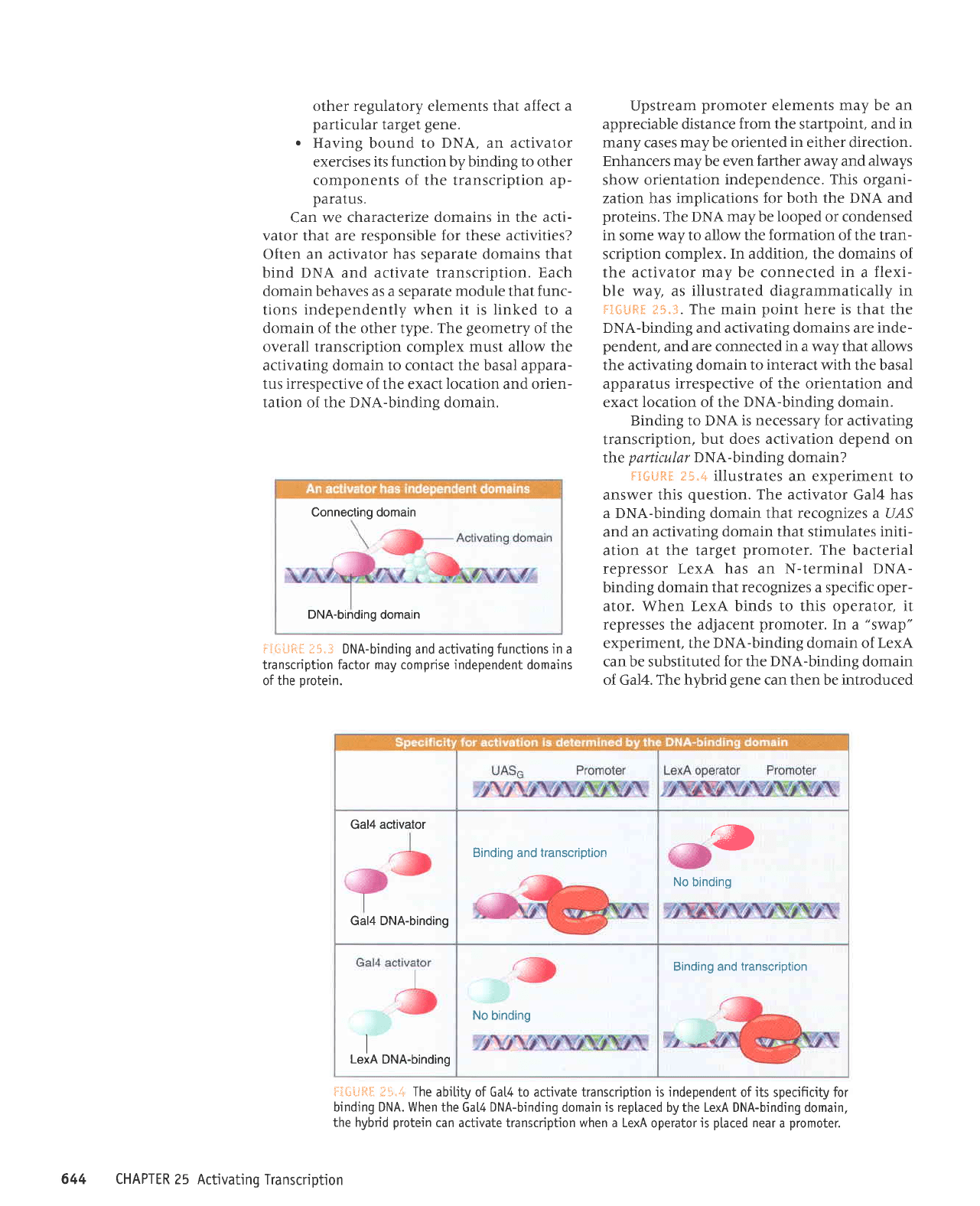
Connecting domain
DNA-binding
domain
if*LJfr{
I5"3 DNA-binding
and activating
functions in a
transcription factor may comprise independent domains
of the
protein.
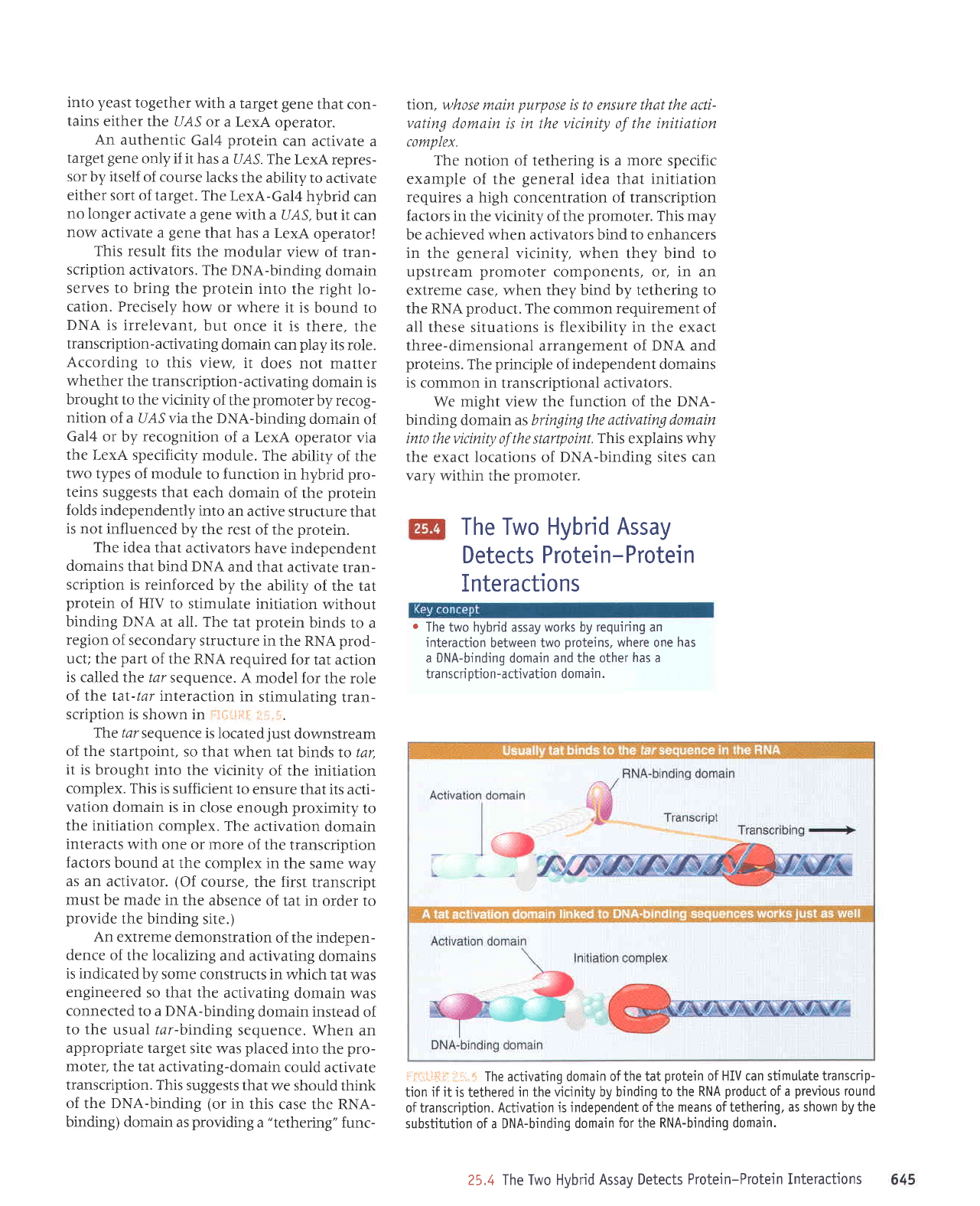
Gal4
activator
Gal4 DNA-binding
Binding
and
transcription
@
No
binding
I
LexA
DNA-binding
No
binding
Binding and
transcription
i:Ifil.Jft{ fh.r"i The
abitity of Gat4 to activate transcription is independent
of
its
specificity for
binding DNA. When
the Gal.4
DNA-binding
domain
is
reptaced bythe LexA DNA-binding domain,
the hybrid
protein
can activate transcription when a LexA
operator
is
ptaced
near
a
promoter.
CHAPTER 25 Activating
Transcription
644
into
yeast
together
with
a target gene
that con-
tains either
the U1S
or a LexA
operator.
An authentic
Gal4
protein
can
activate a
target
gene
only if it has
a
rJ
AS. The LexA
repres-
sor
by
itself
of course
lacks
the ability
to activate
either
sort of target.
The
LexA-Gal4
hybrid
can
no longer
activate
a
gene
with a
UAS, but it
can
now
activate
a
gene
that
has a
LexA
operatorl
This
result fits
the modular
view of tran-
scription activators.
The
DNA-binding
domain
serves to bring
the
protein
into
the right
Io-
cation. Precisely
how
or
where it is
bound to
DNA
is irrelevant,
but
once it
is there,
the
transcription-activating
domain
can
play
its
role.
According
to this view
it
does not matter
whether the transcription-activating
domain is
brought to
the vicinity
of the
promoter
by recog-
nition of a
U,4S via the DNA-binding
domain
of
Gal4
or by recognition
of a LexA
operator via
the LexA specificity
module.
The
ability of
the
two types
of module
to function
in hybrid
pro-
teins
suggests that
each
domain of
the
protein
folds
independently
into an
active
structure that
is
not influenced
by the rest
of the
protein.
The
idea that
activators
have independent
domains
that bind DNA
and
that activate
tran-
scription is reinforced
by the ability
of rhe rar
protein
of HIV to stimulate
initiation
without
binding DNA
at all. The
tat
prorein
binds
to a
region
of secondary
structure
in the RNA
prod-
uct; the
part
of the RNA
required
for tat action
is
called rhe tar
sequence.
A model for
the role
of the taI-tar interaction
in
stimulating
tran-
scription is shown in
i:,r-'iJil!,:
tiij.lr.
The /ar
sequence is located
just
downstream
of the
startpoint, so that
when
tat binds to /ar,
it is
brought into
the vicinity
of the initiation
complex. This is
sufficient
to ensure
that its acti-
vation domain is in
close
enough
proximity
to
the initiation
complex. The
activation
domain
interacts
with one
or more of
the transcription
factors
bound
at the complex
in the
same way
as an
activator.
(Of
course,
the first transcript
must be made
in the
absence
of tat in order to
provide
the binding
site.)
An
extreme demonstration
of the indepen-
dence
of the localizing
and activating
domains
is indicated
by
some constructs
in which
tat was
engineered
so that the
activating
domain was
connected to a DNA-binding
domain instead
of
to the
usual /ar-binding
sequence.
When an
appropriate target
site was
placed
into the
pro-
moter, the tat
activating-domain
could
activate
transcription. This
suggests that
we should think
of
the
DNA-binding (or
in this
case the RNA-
binding) domain
as
providing
a
"tethering"
func-
tion,
whose main
purpose
is to ensure that the acti-
vating
domain
is
in
the
vicinity of the initiation
complex.
The
notion of tethering is a more specific
example of the
general
idea that initiation
requires
a
high
concentration
of transcription
factors
in the
vicinity
of the
promoter.
This
may
be achieved
when
activators bind to enhancers
in
the
general
vicinity, when they bind to
upstream
promoter
components, or, in an
extreme
case, when
they bind by tethering to
the RNA
product.
The
common
requirement of
all
these situations is flexibility
in
the exact
three-dimensional
arrangement
of DNA and
proteins.
The
principle
of independent domains
is common in
transcriptional
activators.
We might view the
function
of the
DNA-
binding domain as bringing the activating
domain
into thevicinity 0f the rtarryoint.This explains why
the
exact locations of
DNA-binding sites can
varv
within the oromoter.
The Two Hybrid
Assay
Detects Protei
n- Protei n
Interactions
r
The
two
hybrid
assay
works by requiring an
interaction
between two
oroteins,
where one
has
a
DNA-binding
domain
and the other has a
transcriotion-activation domai
n.
i:r.tii.ilil
,lli"::i
Theactivatingdomainofthetatproteinof
HlVcanstimutatetranscrip-
tion
if it is
tethered
in the vicinity by binding
to the RNA
product
of a
previous
round
of transcription.
Actjvation is independent
of the means of tethering,
as shown by the
substitution of a DNA-bindinq
domain
for
the
RNA-bindinq domain.
25.4 lhe Two Hybrid
Assay Detects
Protein-Protein Interactions
The model of domain independence
is the basis
for an extremely useful assay
for
detecting
protein
interactions. In effect, we
replace the
connecting domain
in Figure 25.3 wiLh'
a
protein-protein
interaction.
The
principle
is
illustrated in
i:ii.r-:i.t:
'.i1.t-:.
We fuse one of
the
proteins
to
be
tested to a DNA-binding domain.
We fuse
the other
protein
to a transcription-
activating domain.
(This
is
done by
linking the
appropriate coding sequences
in each case and
making synthetic
proteins
by expressing each
hybrid
gene.)
I{
the two
proteins
that
are being
tested can
interact
with one another, the two
hybrid
pro-
teins will interact. This is reflected in the
name
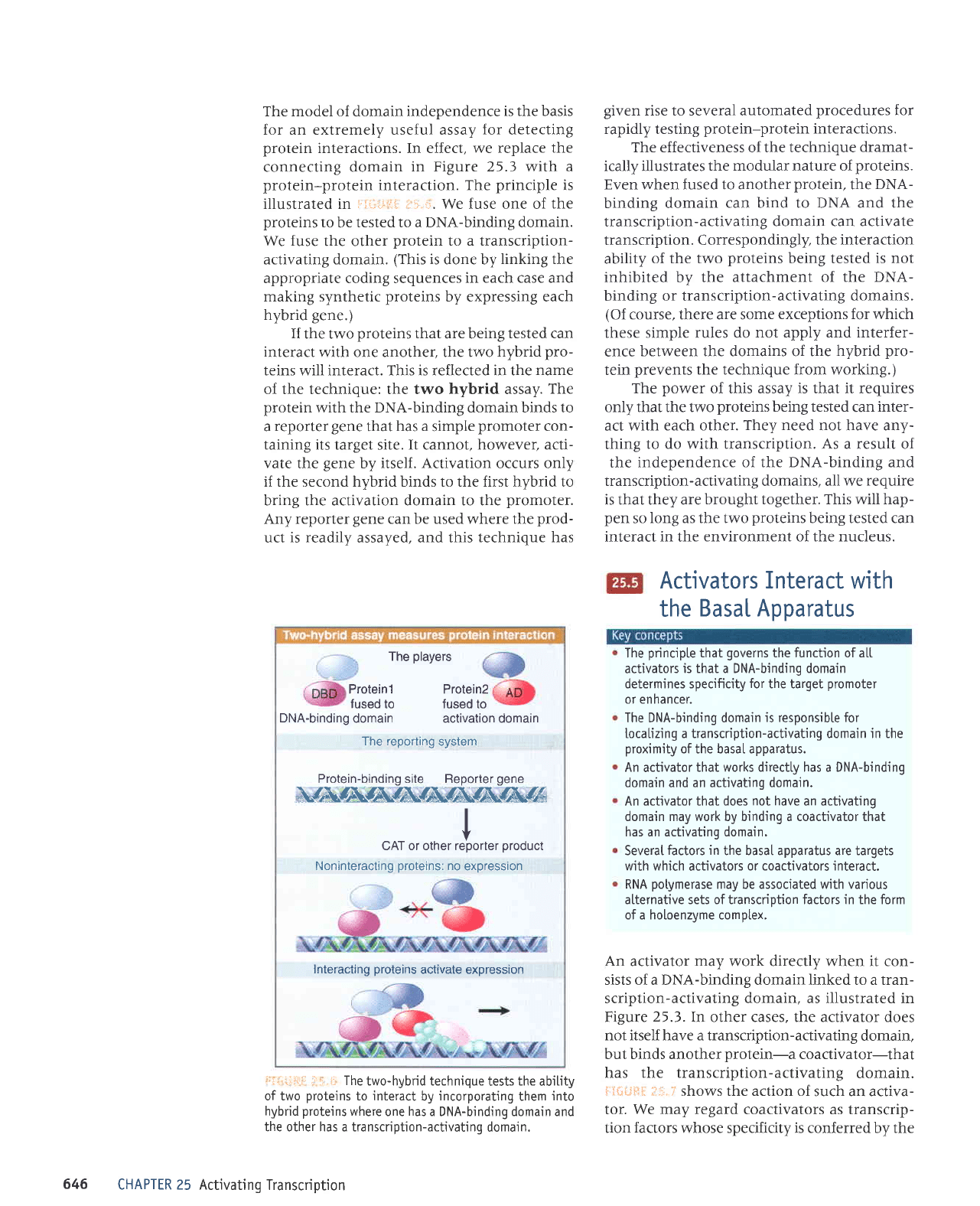
of the technique: the two hybrid assay.
The
protein
wirh the DNA-binding domain binds to
a
reporter
gene
that
has a
simple
promoter
con-
taining its
target site.
It
cannot,
however, acti-
vate the
gene
by itself. Activation occurs only
if the second hybrid binds to the first hybrid to
bring the activation domain to the
promoter.
Any reporter
gene
can be used where the
prod-
uct
is readily
assayed, and this
technique has
i
i,.,,,ii1rr i:;.i. The
two-hybrid technique
tests
the
abil.ity
of two
proteins
to interact
by
incorporating
them
into
hybrid
proteins
where
one
has
a DNA-binding domain and
the other has a transcription-activating
domain.
CHAPTER 25 Activating
Transcription
given
rise to several automated
procedures
for
rapidly testing
protein-protein
interactions.
The effectiveness of the technique
dramat-
ically illustrates
the modular nature of
proteins.
Even when fused to another
protein,
the
DNA-
binding domain
can bind to DNA and the
transcription-activating
domain can activate
transcription. Correspondingly,
the interaction
ability
of the two
proteins
being tested
is not
inhibited by the attachment
of the DNA-
binding or transcription-activating
domains.
(Of
course, there are some
exceptions for which
these
simple rules do
not apply and interfer-
ence
between the domains of the
hybrid
pro-
tein
prevents
the technique from working.)
The
power
of this assay is that it requires
only that the two
proteins
being tested
can
inter-
act
with each other.
They need
not have any-
thing
to do with transcription. As a result of
the
independence of the DNA-binding and
transcription-activating domains, all we
require
is that they are
brought together. This will hap-
pen
so
long as the two
proteins
being tested can
interact
in
the
environment of the nucleus.
Activators Interact
with
the
BasaI Apparatus
The
principte
that
governs
the functjon of a[[
activators
is that a DNA-binding domain
determines specificity
for
the target
promoter
or ennancer.
The DNA-binding domain
is responsible for
localizing a transcription-activating domain
jn
the
proximity
of the basaI apparatus.
An
activator
that works directty has a DNA-binding
domain and an activating domain.
An act'ivator that does
not have
an activating
domain may work by binding a coactivator that
has an activating domain.
Several
factors in
the basal apparatus are targets
with which
activators
or coactivators interact.
RNA
polymerase
may be associated wjth various
atternative sets of transcriotion factors in the form
of a holoenzyme complex.
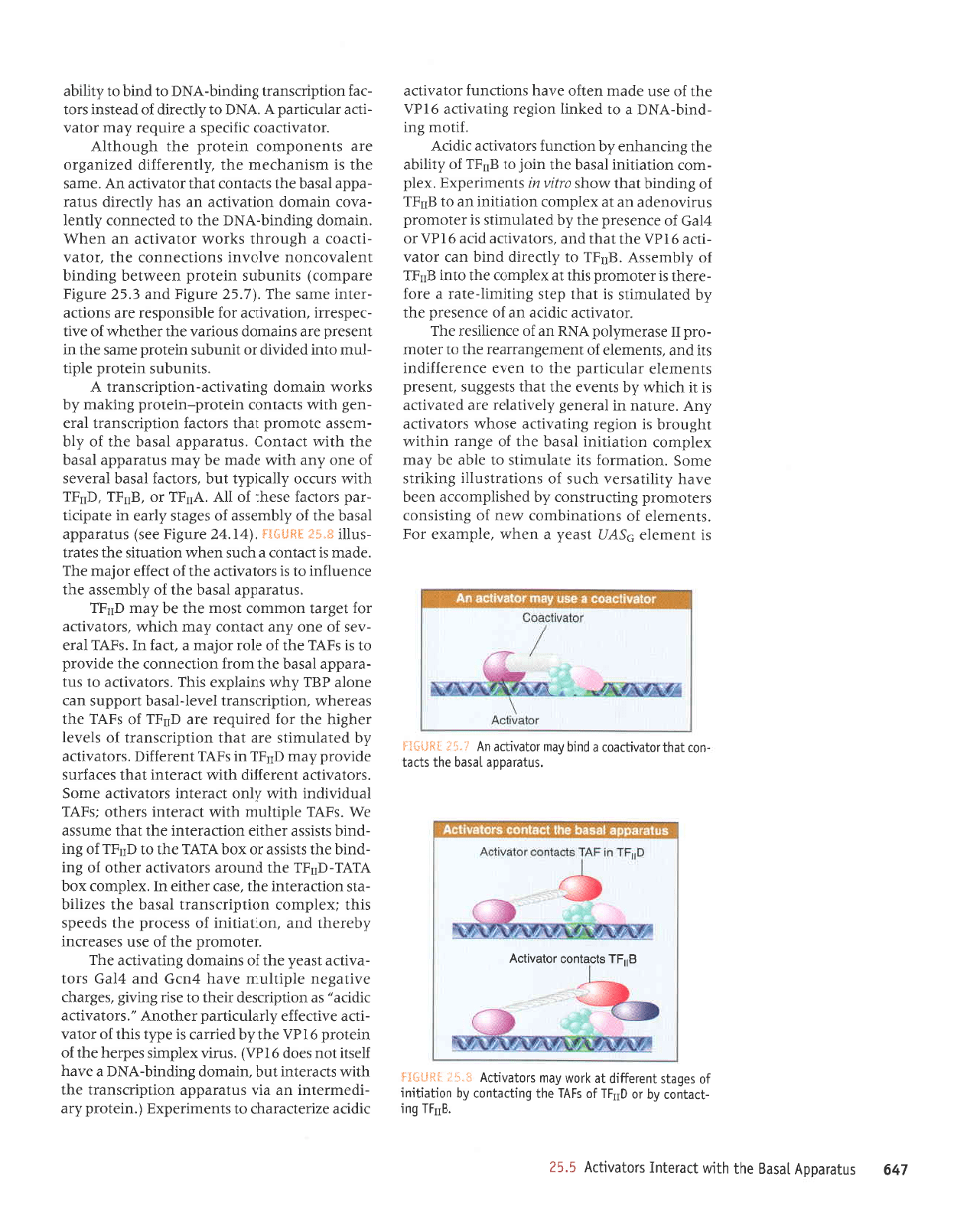
An
activator
may
work directly when it con-
sists of a DNA-binding domain linked to a tran-
scription-activating domain, as illustrated in
Figure
25.3.
ln other cases, the activator does
not itself have a transcription-activating
domain,
but binds another
protein-a
coactivator-that
has the transcription-activating
domain.
liirti!{[
l1ii. ,'" shows the action of
such an activa-
tor. We may regard coactivators as transcrip-
tion
factors
whose specificity is conferred by the
The
players
Proteinl Protein2
fused
to fused to
DNA-binding
domain activation domain
The reporting
system
Protein-bindingsrte Reportergene
=\'ffi*.ffi
I
Y
CAT or other reporter
product
Noninteracting
proteins:
no
expression
I nteracting
proteins
activate expression
646
L'9
snlplpddv
lespg
aql
qlm
lleralul
srolp^ltlv
g.gz
.€rrl1
bu!
-llpluol
Aq
lo
0IIII
Jo
slvj
aql Eurlreluor Aq uorlerlruL
1o
sa6e1s
lua.laJJtp
le
lloM
feu
srolenrlry
t"tji: rtl*ili*g*
gllgl
slceluoc
role^tlcv
'snle.lPddP
lPsPq
aql sllel
-uor
lpqlrolp^qlpol
p
purq
r\eLu rolerrq:e
uV
e1.*rl
i]tr**i:l
sI
luaurelJ
c5yp
lseaz(
p
ueqM
'aldruexa
ro4
'slueruJla
]o
suortpurqruoJ
Mau
Jo
Sullslsuol
sJeloruord
Surlrnrlsuor
dq paqsrldruoJJe
uaeq
aneq z(ltTtlesJa^ q)ns
Jo
suorleJlsn11r
Sur>Ilrts
eluos
'uolleluroJ
s1r Jlelnurls
ol JIqp aq
.{eru
xalduror
uortellrul
Ipspq
erll
;o
a8uer urqlrM
lq8norq
sr uor8ar
Surle,Lrlre
JSoqM sJolelrlJe
,{uy
'arnleu
ur
leraua8
dlanqelar
arp
pJle^rl)e
sl
lI
qJrqM
z(q
sluazra
Jr{l
lpqt
stsaSSns
'tuasard
slueurele
relnrrlred
Jql
ot uene JJueJalJrpur
sll
pue
'slualuale
yo
luaruaSueJJpJr
Jql 0l JJlOru
-ord
1
aseraruLlod
yNU
ue
Jo
JtuJIIrseJ JqJ
'JolelrlJp
JrprJp
up
;o
etuaserd aql
dq parelnulrs
sr
rpql
dals Surtrrurl-aler e JroJ
-eJJql
sr raloruord
slqt
lp
xelduol
eql olq
gIIdJ
yo
,{lqruassy
'gIIdJ
ol
,{lDarrp purq
ue) rolel
-lDe
9 14n
Jql
leql
pue
'srole^rlre
prrp
g
I dA
Jo
71eC
Jo
aruasard
aql
,{q
palelnurts
sr ratoruord
SnJIAouJpe
ue
tP
xelduoJ
uollelllul up ol
gIIdJ
;o
Sutpurq
teql
,lror{s
zJlt^ ut
sluarurradxg
'xa1d
-ruoJ
uopellrut
1eseq
aq1 uroI
ot
gIIdI
yo
.dtlgqe
aqt
Suouequa Lq
uorpunJ
sJolelrDp JIpIJV
';lloru
8ut
-pulq-VNO
p
ot
pe>lurl
uor8ar Surlenrpe
914n
aql
Jo
asn apeu uauo
a^eq suorl)unJ JolenrlJP
JrprJe JZrJepeJpqJ 01 sluerurJJdxg
('uralord
[re
-rperurJtul
ue erl snlereddB uorldrnsue;1
aql
qlIM
speJelul
lnq
'ureruop
Eurpurq-yN(
p
aleq
JIJSIT
tou
sJop
9IdA)
'snrn
xaldrurs
sad-raq aqt;o
uratord
9 I dA
eql ,{.q
parrrer
sr ad[t
slqt
Jo
Jolpn
-rtJp
JAT1JJJJa Lpelnrrged
raqtouv,,'sJolelrtJe
)rpr)p,, se uoudrJJsap reql 01 asu SurArB
'sa8req;
enrlp8au a1dr11nru eleq
tu)t
pup
71eC
srol
-elrtf,p
1sea,{
aql
Jo
sureuop Supezrlne aq1
'ralourord
aql
Jo
Jsn sasPeJJur
.{qa:aqt
pup
'uorlpprur
Jo
ssaJord
aql spaads
srql
lxaldruor
uortdrnsueJl
Ieseq
aql sezrlrq
-e1s
uorpPJJlur 3q1
'esp)
Jeqlra
u1
'xaldruo;
xoq
VJyJ-CIII4J
aql
punoJe
sJotelrl)e raqlo
1o
3ur
-purq
aql slsrssp ro xoq
VJVJ
rqlol
(IIdJ
Jo
3ul
-purq
slsrsse Jaqlra
uortJerJtur aql
lpql
aurnsse
J14
'sdVJ
aldrllnru
qllm
Deretur
sraqlo lsdvJ
Ienprnrput
qrpl
dpo
tJerelur
sJotp^rDe aruos
'sJole^rlJe
lueJaJJrp
qu1!t
1JeJJlul 1eql
sJJeJJns
apnord,{.eru
qIIdJ
uI sdvJ
lueJeJJrq
'sJolplrlJe
.{q
palelnrults
JJp
tpqt
uoltdlJJsuprt
Jo
sIJAJI
raq8q eql roJ
pa:rnbar
are
qIIdJ
Jo
sdVJ
eql
seJJJqM
'uorldrnsueJl
la^al-leseq
uoddns
uel
auole
dgJ
z(qm
surcldxa srqJ
'sJolpnrlJe
01 snl
-eredde
Ieseq
Jr{1 ruoJJ uorlJauuoJ Jqt apmord
01 sr s{vJ eql
Jo
JIor rolpru e
'lJeJ
uI
's{VJ
IpJe
-^as
Jo
auo
^up
lJeluoJ
,{eru
qlqazr
'sJolpnrlJp
ro1
1a8rel
uoruruoJ
lsoru
eql ae,r{eur
qIrgl
'snleredde
Ipseq
aqt;o
dlqruasse
aqt
eJuenlJur 01 sr sJolelrlJe Jql
Jo
lra;;a
roleru
eql
'Jpeu
sr
lJeluo)
e
q)ns
uJqM uorlenlls
Jql selPJl
-snlll
S'$ff
jHfisij
'ftyVZ
arn8rg aas) snleredde
Ieseq
Jql;o,{lqruasse
yo
sa8els d1:ea ur aledolt
-red
srope; esaql
Jo IIV
'YII{J
Jo
'gIIdJ '(ndJ
r{llM srnJJo .{1prrd,fu
tnq
'srolJeJ
Ieseq
lpra^es
Jo
auo z(ue
qlr,u
Jperu Jq l(eru snleredde
pseq
eqt
qrlm
tletuoJ
'snleredde
Ieseq
eqt
Jo
^lq
-Luesse
aloruord
lpql
sJolJpJ uopdrnsuerl
pra
-ua8
qluzr.
stf,etuoJ uralord-uralord Suqeru dq
s>lJom ureruop Surle.ulre-uorldrnsuerl
V
'slrunqns
urJloJo
alorl
-Fru
olur
paprlrp
Jo
llunqns
uralord
arups aql ur
luasard
eJe sureruop snorJpl aqt Jaqlaqu
Jo
eAIl
-radsarrr
'uorlplrlJe
ro; alqrsuodsal eJe suorl)p
-Jelur
eurps aq1-'(L'SZ arn8rg
pue
f'SZ
arn8rg
aredruor)
slrunqns uralo:d ueemlJq Supurq
lualP^oJuou
eAIoAur suorlJJuuoJ Jql
'JoleA
-ltlpoJ
e
q8norql
s>lroM rolelrl)e ue uaq6
'ureruop
Surpurq-y1trq
rr{t ot
parrauuor
d1lua1
-Pno)
ureruop uorle^rl)e uP sPq
,{lDarrp
snlBr
-edde
pseq
Jql speluoJ
lpr{l
Jolp^ltJp uV'erues
aql sl lusruPqrrtu
Jq1 ,(1tuaral;rp
pazrueSro
are sluauoduroJ ureloJd aqr
q8noqrly
'rolelrlJpoJ
rr;neds e arrnbar deru role.r.
-rpe
JelnJrued
v
'VN(
ol
,{pralp;o
peJtsur
sJol
-re;
uorldr.rrsuerl Surpurq-vNq 01
purq
o1 d111qe
uorldulsuerl
6urlp^!]lv
gZ
UlIdVHl
srql JoJ elqrspJJ
tl
sI
'suleloJd
gtr<
Jo
stslsuoJ
leql
snleredde a3re1
Lrarr e
la8
a.,tzr-srolelllJe
-oJ
pue
'sJotplrtJe
'aseraru.dlod
VNU
'sJo1
-JeJ
Ipspq-uortdrnsuerl
lueIJIJJe
ro;
pattnbar
sluauodruoJ eq1
IIe
dn
ppe
eM
urq6
'ralouord
aqt
te
aseraru,{1od
VNU
}o
uolteJluJ)uot
aAIDJJJJ
Jql
aseJnur
ol sr
tJJJJa
elos sll
teql
stsaSSns
srql
'uorldrnsueJt
ul
JSeJrJur
due
arnpord
or
palpy
rolenrlJp aq1 ^ltuJlrlJJns
pJSeeJlul
sem
eseJaur
-,{1od
ypg
Jo
uorleJlueJuoJ
aqt uaq6'uo1tel
-l]Je
JoJ
lunoJJe
ueJ
luJrulInJJJJ lPql
peMoqs
tsea.{
ur JSe) Juo
ut slepou JSJqI
Jo
lset
V
',{ruaog;a
s1r sasPeJ)uI
q)lqm
'arudzua
Jql
Jo
uolteruJoJuoJ
aql ur
'aldruexa
ro;
'xaldtuo)
IPuoIl
-drnsuert
aql ur a8ueqf, aruos
sJJnpq
tl
leql
asoddns ol sl
IJporu
eAlleuJal{p
uf o
'ralouord
eql ot esPrau[1od
y51g
;o
Surpurq
Jql aspaJJur ol sl
1IJJJJ
JIos
str
leql
san8re
lJporu
luerullnJf,al
JVJ .
:lapolu
;o
sad,{1
leraua8
o.ral aut8erul
upJ eM
Zuoll
-drnsuerl
elplnrurls JolplrlJe
up seop
.ryroH
'lseaL
ur slJeluoJ
IpruJou
slr selquJsJr
teqt
snterpdde
uottdtns
-uerl
uerleruueru Jql
Jo
arnleJJ
aruos azruSo
-f,JJ
tsnur
ulatord
TlpD
aql
'salorfuelna;aq8tq
pue
lseaL
ueJMleq
pe^rasuoJ
uaeq eleq ol JroJ
-JJJql
sruJJS rJloruord
aql Jlp^tDe
01 sJsn
TleO
SUPJLU Je^eleqM
'llJr
palnlln)
uPllPrurueru
e
ul
Tlec
.riq
palenrpe
eq ue)
auaS srql
'aua3
trlo
-Lrelna
raq8rq
p
Jo
reloruoJd
aql reau
pauJSuI
819
aqr Aq
parsaSSns
st uorDBrJtul slql
Io
aruetrodtut
aq1
'sn1e;edde
leseq
Jqt
Jo
stueuodruor raqto
qlpu
SurlrerJtur tuorJ
tl luJAJld
01
dgI
ot spurq
teql
reurporJlrq e
'I
dVUC/ I
r(I/Zf
N
rossardar
1eqo13
eql sr ese) euo
'umou4
are saldruexa
JluOS
lnq'arer
r(1anr1e1ar
are uoudlJrsueJl
>lJolq
ol srossardar
IerJaDeq
?u\te-suau a>lrl uorDunJ
leql
sulJloJd roleln8ar lJrnl)nrls ulleluoJql
Suouangur
Jo Ia^JI
eql
te
peqsrldruole
L11era
-ua8
sr salodre>1na ur uorldrrlsuerl;o uorssardag
'srelouord
rgLrads o1 6uLpurq
fiq
pe
1eq1
srosseldar aie araql
lnq
'arnlln.lls
urlpuolql
6uLlrege
fiq
panarqre
r\11ensn sr uorssaldag
o
srossajdau erv
suralojd
6urpurg-ralouord auos
@
-rrporuru,n"-o,u,
1:T?",1*,iiiil#;i:il
-oJqJ
Jo
eJntf,nJls Suqelndrueru Lq >Fo,u sJeqlo
1nq
'snleredde
leseq
aqt ro aserarudlod
y51g
V1IM
Sutperalut .dq ,{lDarrp uortdtrlsuert eJua
-nlJur
sJolJel uorldursuerl
eruos
'uorle8uola
suels JserJruLlod e uaq.tr
pesealeJ,{1qeqo:d
sr
t1
'ase:aur[1od
VNU
eql o1 sluauodtuor ueartsdn
ruorl slJaJJe Surssardar ro
Surlearlre rJqlrJ
lnu
-suert
ue)
11
'aseraru.{.1od
VXU
Jo
(q13)
ureuop
IeuruJet-)
eq1
qtrM
stJerJtur
U
uJqM a8ueqr
puorleuJoJuol
e sao8rapun JolerpapsauaB
rrloL,re4na raq8rq
tsoru
Jo
uortdrnsuert
eql
ro;
parrnbar
are saxaldruor
snoSoloruog
'sauaB
lsBar(
lsoru
Jo
uorldtDsupJl
roJ Lressarau sr Jolp
-rpew'sJole^Ipe
Jo
S]JJJJJ
Jql JlprpJru or,{tr[qe
str Lq
parsaSSns
se,u
erupu ar{J
'(g
aseraru.dlod
VNU
ul suollptnru
;o
srossarddns se
parJrtuJpr
LlleurSrro
ara^,u saua8 rraql
Jo
Lueru asneraq
peueu
os) no1
gyg
aruos Surpnpur
'uorldursuerl
>lJolq suo4elnru
qJrqM
ur seuaS
IpJJ^es Jo
slJn
-pord
sapnpul JotprpeW'Jotplpaur
pa11er
xald
-luo)
llunqns-02
e
qlIM pJlelf,osse
aseraurr(1od
VNU
Jo
stslsuo)
(sluauodruoJ
Ieuorlrppe
tno
-qlptt
uopdtnsueJl Suuertrur
yo
alqeder Suraq se
paulyap)
tsea,{
ur
,,xalduror
aur,{zuao1oq,,
tuau
-rruord
tsoru
JqJ
'sJoDeJ
uortdrnsuerl snorJel
qllM
pelerJosse
sr arudzuJ
Jqt
qJrqM
ur
punoJ
uJJq JAeq aseraurdlod
y51U
Io
stuJoJ
IeraAaS
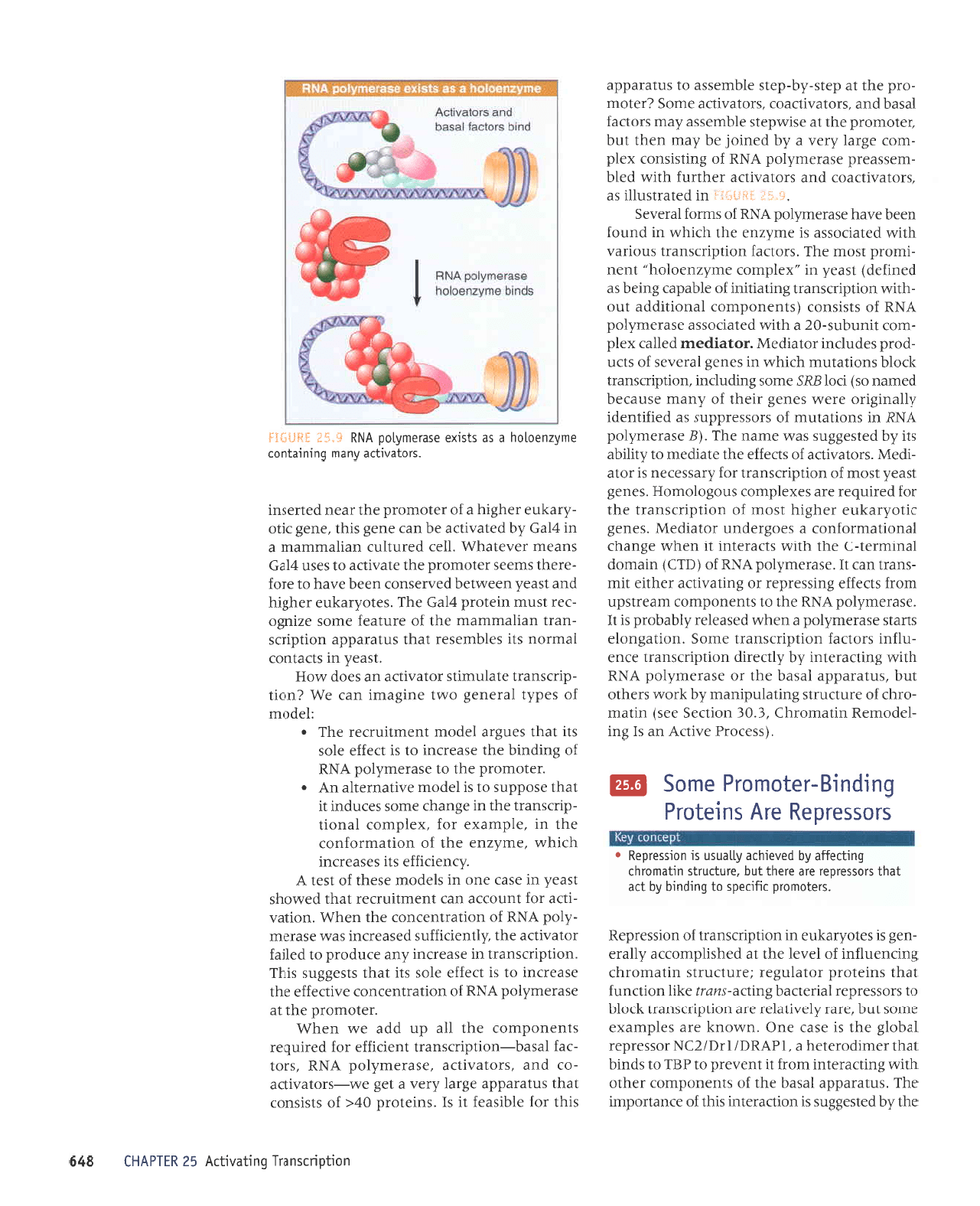
'{'rii:
;iijili,:j:i
uI
pelPI]Snlll
sB
'srole^r]f,eof,
pup
sJolp^rlJp JaqunJ
qll,!1. pJIq
-ruasseard
aseraur,{1od
vNU
Jo
Surlsrsuor xald
-ruor
a8rel
L:a.r
e Lq
paurot
aq ,{.eru uaql
tnq
taloruord aqt
le
astrrzrdals
alquJSSe Aeru srotrey
IPSeq
puP
'srole^rpPof,
'sJolPArDe
eluos
zJalou
-ord
aqt
1e
dals-dq-dals
JIqruJSSp ol snlBredde
's.loJPnrllP
fueu 6uru reluor
auAzueoloq e sp
slsrxo aserauAlod
VNU $.'jf, $+.9{j5:j
6V9
srolp^r.llv
Aq
pazru6orau
alv
sluaual3
asuodsaS
1.g7
sJteJlsnllr
saua8
lroqs
tpaq
eqt
Jo IonuoJ
aqJ
'svNuru
Jo
uorlelsupll
Jr{l ul
sa8ueqr sJsnpf,
pue
'sauaE
>IJoqs
leeq
aql
Jo
uortdrnsuerl
uo
surnl
'saua8
eruos
Jo
uorldrrtsuert
JJo
suJnl
arnleradrual
ur
JsealJur
uy
:uorssJrdxa
aua8
]o
sloJluoJ
a1drl1nru
sJAIo^ur
pue
saloLrelna
pue
sa1o.,{relord
;o
JBUPJ JprM
p
ol uoru
-Luo)
sr srql
'asuodsal
>lJoqs
leeq
aqt .{q
paprrr
-ord
sr roDe;
a13urs
e
, q pa11oDuof,
aJe saueS
z(ueru qrrqaa.
ur uorlpnlrs
e;o
alduexe uv
_
'passJJoxa
Jq
ol sr aua8
aql
uJqM
JunuJJlap suorlrpuoJ
JqI
'suolllpuo)
uIelJJJ
JJpun .{.luo
anrlre sr ro
elqelrele
JerIlrJ
sr uralord
Jql
tpql
sr
slolelr]f,p
enrlle,{1anr1n1r1suo)
uoJJ JJuJreJ}rp
aqJ'uorl
-dtnsuert
alertrur
ot aseraruLlod
VNU
Molle ol
parmbar
sr
luJruJIJ
asuodsar
Jql
ol JolplrlJe ue
;o
Surpurg
'aldnur:d
leraua8
eues eqt.{q uorl
-JunJ
stueuala
asuodsal
IIe
tpqt
Junsse JM
'JJJuequJ
ue
ur
punoJ
sMug e
seeJJqM
'Jelou
-ord
e ur
punoJ
sl
gSH
ue ,{11ensn :JJqlo
eq1
upql
JJrltpJ
euo
ur
punoJ
,{11elrdz(1
JJp slueur
-JIa
Jo
sadu(l aruos
'sre)uequJ
ur ro
slJlour
-ord
ur
pJtpJol
aq.{eu
stuJruele
asuodsag
'sardor
a1drl1nu
are JJeql
seurrleruos
1nq
'asuodsar
LrolelnBar
Jqt JaJuoJ
ol
IUJIJIJJns
sr ,{1ensn
luarualJ
a13urs e
Jo
JJuJ
-sard
aq1
'tl
Jo
ueartsdn
dq
ggg>
,{11ensn
are
lnq'luloduPts
eqt
uoJJ sJJuptsrp
paxrJ
te
luasard
lou
JJp
slueruJlJ
Jql
'srJloruord
u1
'aruanbas
sns
-uJSuoJ
Jql
Jo
Jprs
Jeqlre uo
J)uelsrp
lJorls
p
JoJ
spuJtxJ
rolJey
aqr.dq
punoq
uor8a; JqJ'leJrluepr
dFressarau
lou
lnq'peleleJ
Llasop are saua8
lueJJJJrp
ur
punol
stuJruJIJ
asuodsar
Jql
Jo
sardot lsaruanbas
snsuasuo)
uoqs
ureluo) slueru
-a1a
asuodsag
'(1uarua1a
asuodsar
urnras)
gUS
aqr
pue'(1uatua1a
asuodsar
pIoJIUoJ
-ornp)
trUD
rql
'(1uarua1a
asuodsar >IJoqs
lpeq)
gSH
eql
eJe saldruexa ltuJLuJIJ
asuodsar
p
pJIIpJ
sr JollpJ
e qJns
ot
puodsar
tll JuJS e
sasneJ
leql
luJuele
uv
'.t)lqNila
ua '{q
panuSont
s!
ru41
ruawtala
(ntua4ua
n)
nlotuotd a anuls far1l
leql
sI
loJluof,
uoruuroJ
repun sauaS;o sdnor8
Sutztratlerer{J
tuoJJ sa8rarua
leql
aldourrd
aq1
'suorleurquol
utpilal
ur to Alluapuadapur
uoqdursuerl
ele^rltp l\eu
urnl ur
qlrqM
'sluouralo
asuodset
Aueu
eneq fieu telouord
y
lolPA!1rP
rgoads
e r{q
pezru6oral
sr
}uaulela
asuodsar
qre3
'slaluPuua
ro sralouro.rd
ur
palerol
aq Aeu
sluauale asuodsag
'uorldrnsuerl
aua8 ol
luelalaJJr
uJAJ
.ro iossardar
p
tole^rl)p
ue aq.deru
1I
:pJrunsse
Jq
louueJ
lueruJle
raloruord uMou>l
e ol
8ul
-pulq
ul uralord e
Jo
uorl)unJ aqt
leqr turod
aqt
e>leru osp stlnsJr
asaql'snohqo sr ralourord
aql
le
Suueqrul ruoJJ eseJeru,{1od
y111g
Suuuanard
ur rossardar
lprraDeq
p
Jo
paJJe
Jqt
qrlM
^3o
-leue
aqJ
'pelquasse
Suraq uror; xaldruor
uorl
-drnsuerl
e sluanard ralourord Jql ruoJJ
JoDeJ
Supurq-1yy) eqt
Jo
uorsnlJxJ eql
'Jnssrl
Jruo
-.drqrua
uI'sa)uJnbJs Jarrrepo
pue
'sexoqJW)
'xoq
VJVJ
eql
le
sJope; uorldrnsuerl ,{q
punoq
sr raloruord
Jql
'susel
u1
'uorssardxa
aua8
ro;
saruanbasuoJ Jql sJleJtsnfir
ij
i'
ii
i1 :ii,.i ir* {:!
'wa41
6utztu6nat wott
to1
-o^tpo
atltr 6u4ua^ard f.qata4l'sJxoq
JyV)
eql 01
spulq
'(dql)
ularord
luaurareldstp-JyV)
rql
pJIIp)
'urJlord:aqloue
sanssrl lruoz(Jqrua
Jr{l uI
'xoq
Jvy)
Jqt ol
pulq
ueJ rrurroJ
aql
,{1uo
rnq
'sJnssrl
rruo,{rqua uorJ
oslp
pup
Jnssrl srlsal
ruorJ
patJeJtxa
eq upf, sJolf,pJ Surpurq-1yy3
'urqf,Jn
pJS
e ur srsauaSolerurads Surrnp zlpo
passa:dxa
\lr-q1(Ze'VZ
arn8r4 aas)
g3g
auolsrq
ro;
aua8 e
Jo
Jaloruord aqt ur
punoJ
eJp
tuaur
-elJ
srql;o sardor olAl'uoueln8ar ro;
ta8Jel
p
sr
aruanb a s
rYY )
aqr'ffi
ijlllli:i::H;"1,
"o
Surlrqrqur ur JIoJ Jlrl)e ue eleq z(e,lr
srql ur
>lJoM
leql
srossardag
'lsead
ur rossarda:
eq1 JoJ
apoJ
teql
saua8 aql ur suouelnru
1nu
yo,{r1pqta1
'ul.lol
louuPl
xalouoJ
e^qlP
uP
os
'6urpurq
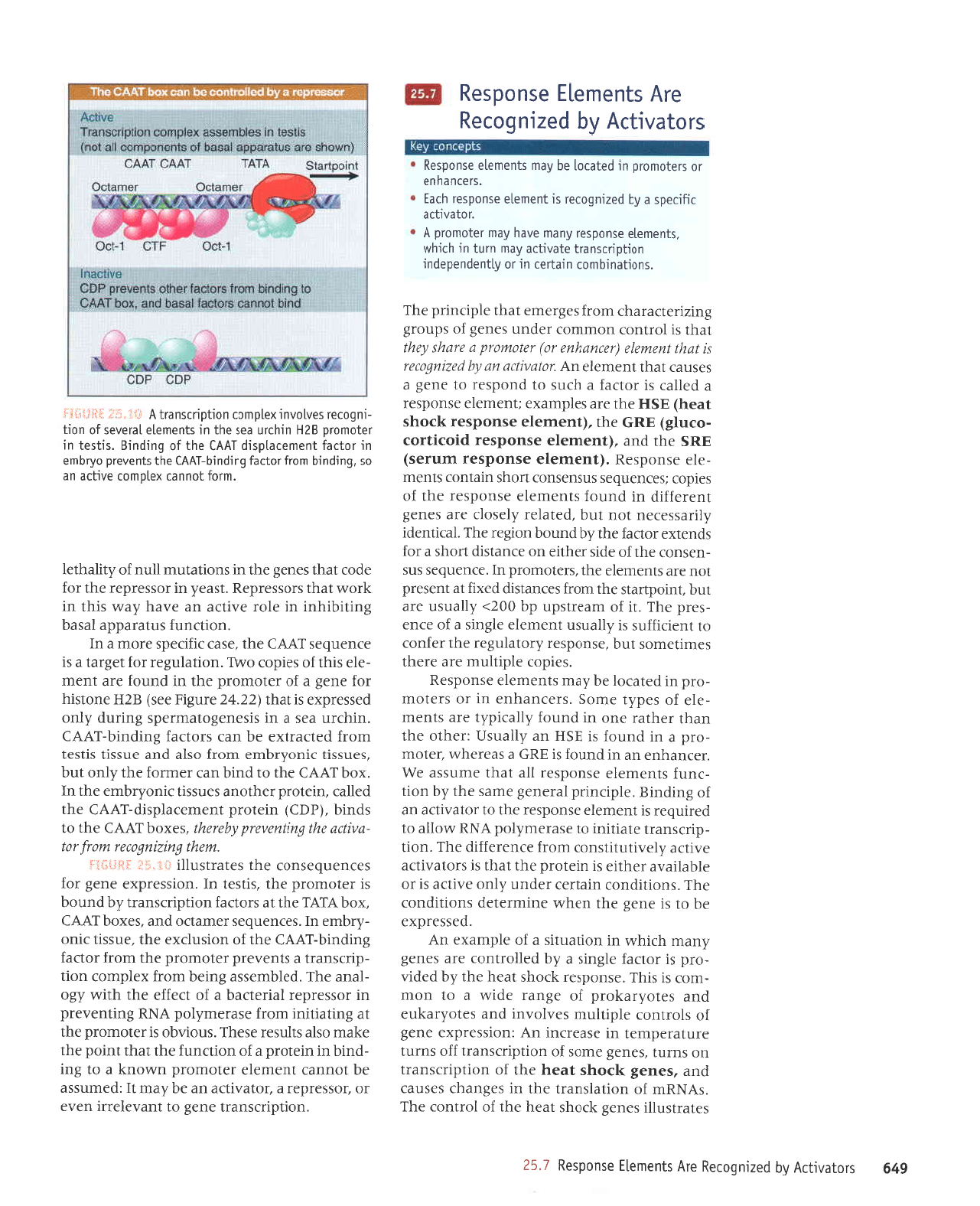
uorJ.lolleJ 6uLpurq-1yy3 eq1 sluanard oArqtua
ur. rollpJ
lueuereldsrp
lVVl
aqt
yo
6urpurg
'srlsal
ur
.lalourojo
8zH
urql.ln eas aql ur sluau0l0
lera^es Jo
u0rl
-ru6orar
sanlo,ruL
xaldtuoc
uorldulsue:l
V
il;
i.'i,i
ri,tJillti::
sroJP^tllv
Aq
pazru6olau
eJV
slueulall
esuodsau
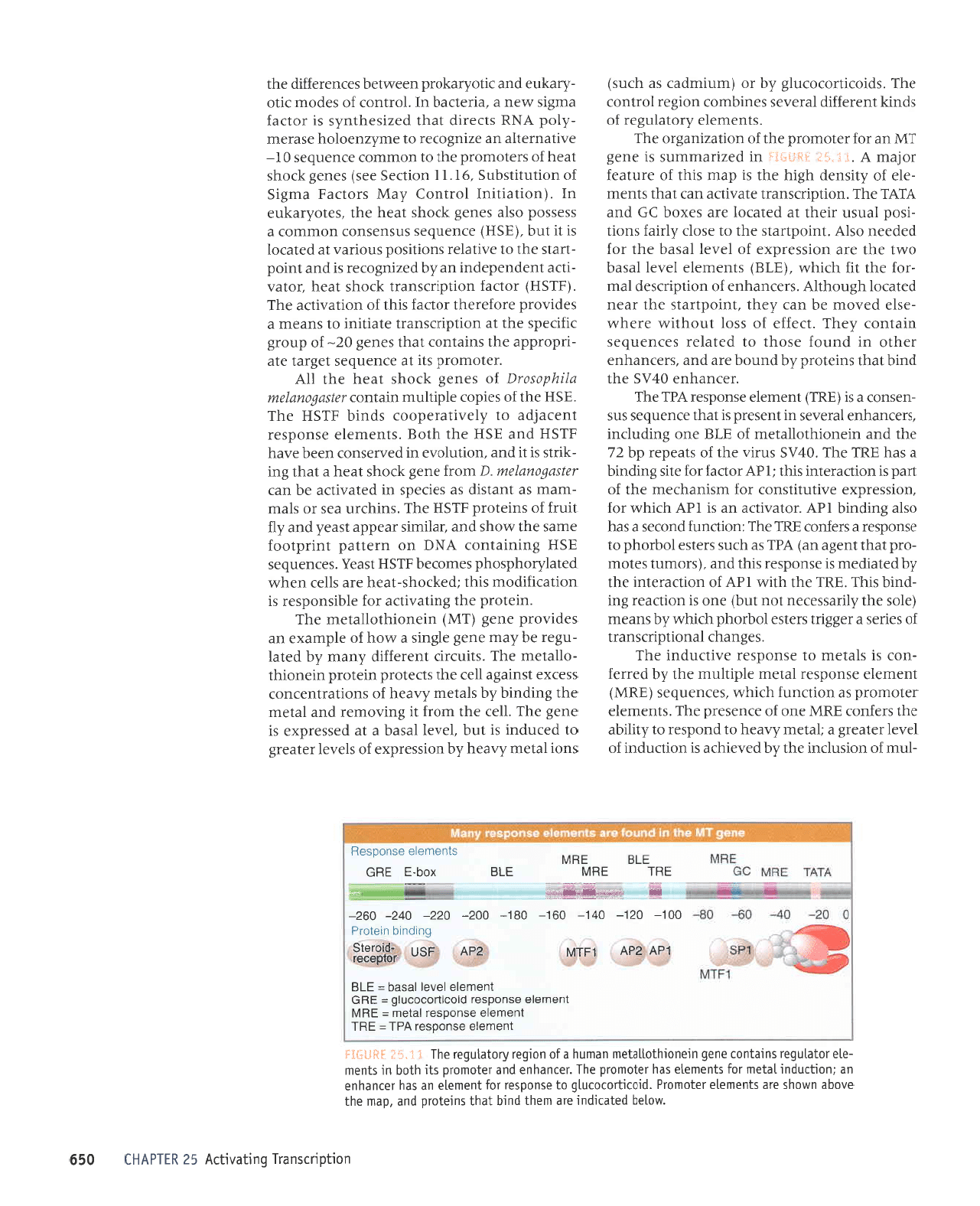
uoqdulsuprl
6urlp^ltlv
9z
ulldvHl
'Molaq
polPrrpur
ale uaqJ
pulq
lPLll
sutolold
pue
'deu
aql
a^oqP uMoqs arP sJuauala lalouo.ld
'prolr.ilorornl6
ol asuodsor loJ
luauale
uP sPq
.laluequa
uP
luorllnpur
lelau
r0J sluauala sPq .lalour0rd
aql
'laluPqua
pue
leloul0.l0 sl! tlloq
ut sluau
-ola
loleln6ol surpluol auab urouorqlollplaur
ueunq
p
Jo
uotbai
fuoleqnEat aql
t i.'{i
$:{i!1}5i
099
-Fru
]o
uorsnlrur Jql
^q
pJlarqJe
sr uorDnpur
Jo
1a,r,a1
ralear8 e
1p1aru
Lneaq ot
puodsar
o1 ,{1gqe
aqt sJaJuoJ
AUW
auo;o
aruasa:d eqJ'sluauelJ
JJloruoJd se uort)unJ
qJIqM'seJuanbes
(AUW)
tuJruele
asuodsar
letaru
a1dr11nru aqt
z(q
peJJeJ
-uoJ
sr slplJru ol asuodsar
J^rlJnpur
JqI
'sa8ueqr
leuorldrnsue,rl
Jo
sJUJS
e raSSrrt sJJlsJ
Ioqroqd
qrrq,t,r
Lq
sueaur
(a1os
aqr,{.pessarau
tou tnq)
auo sr uorDear 3ur
-pulq
slqJ
'guJ
eqt
qllM
Idy
Jo
uorl)Pralur rql
z(q
pateparu
sr asuodsar slqt
pue
'(srorunl
saloru
-ord
leqt lua8e
ue)
y41
sp
qrns
sratsa
Ioqroqd
ol
asuodsar
p
srJJuoJ
iIUJ
JqJ
:uou)unJ
puo)as
p
spq
osp Surpurq
Idy
'Jolp^rtJp
ue sr
Idy
qJrqm
roJ
'uorssardxa
Jnrlnlrlsuo) roJ
tusrueqf,au
aql
Jo
ued
sr uorDerJlul slqt
jIdV
rope; roy atrs Surpurq
e seq
AUJ
eqJ
'07AS
snrr^ rqt;o sleadar dq
74
Jqt
pup
urJuorqtollplau
Jo
ilfg
Juo Surpnpur
'sraJupque
IeJJAJs
ur
luasard
sr
teqt
aruanbas sns
-uesuo)
e sl
(HUJ)
tuJrxelJ
asuodsar
y41
aql
'JeJuPquJ
07AS
Jql
purq
lpql
suratord lq
punoq
JJe
pue
'sJJJueque
rJqlo ur
punoJ
Jsoqt ot
pJtelar
saluanbas
uretuoJ
f,aql
'rralJa
Jo
ssol
tnoqtrM
JJJqM
-asle
peloru
eq ueJ Laqt
'turodlrpts
Jqt
Jeau
pJte)ol q8noqrly'sreJueque
;o
uortdrnsap
leru
-JoJ
Jqt
tlJ
q)IqM
'(Efg)
stuerurlr
Ir^rl
Ipseq
oul Jqt ate uorssardxa
Jo IJ^JI Iespq
Jqt JoJ
pepeau
oslv
'lurod1.rpts
Jqt ot JSoIJ dpre; suorl
-rsod
lensn
JrJql
te
pJle)ol
eJp sJxoq
)1;
pue
VIVI
JqI'uo1ldrnsuerl JleArDe ueJ
teqt
sluJur
-JIJ
Jo
,i.1rsuap q8rq
aqf
sr deru srqt
Jo
JJnteJJ
roferu
V'.i::'i;r: .{*,=*-*l:j
uI
pazrJeur[rns
st aua8
IW
ue.rol ratoruord Jql
Jo
uortpzrue8ro aq1
'slueurJle
rfuoteln8ar
;o
spuDI
luaJJJJrp
IeJe^es
saurqruoJ uorSa,r
lorluoJ
eql
'splo)IuorornlS
.dq ro
(runuuper
se
qrns)
suor
Ieleru
Lneaq r(q uorssardxe
Jo
sle^al ratear8
ot
peJnpur
sl
lnq
'lJ^al
Ipspq
p
te
passardxa
sr
aua8 aql
'lla)
aql ruorJ
ll
Sur.loruat
pue
Ipteru
aql Surpurq
z(q
slelaru
,{,teaq
Jo
suollertuetuoJ
ssJJXe
tsure8e
IIJJ
Jqt
stralord utalord
urauolt{l
-ollelJru
aqJ
'slrnJJrJ
luJlJJlIp
,(ueu ,{q
patel
-n8ar
aq.,{eru aua8 a18uts
e Moq
Jo
aldruexa ue
saprao.rd auaS
(tw)
ulauolqtolletalrr
JqI
'uratord
aqt
Sutte.lrDe
loJ Jlqlsuodsar
st
uorlef,rlrporu srql
1pa>lJoqs-lPJq
ere sllJ) uJq^l.
patelfuoqdsoqd
saruoraq
4JSH
tsee
'saruanbas
g5g
SururetuoJ
VNC
uo
uralted
tulrdtool
etups eql Moqs
pue
Telrurs readde
rsea.d
pue z(g
tlnJJ
Jo
suralord
dJSH
JqJ
'sulqJrn
eJS
ro slpru
-rueru
se
luplslp
se sat:ads ul
pJtpAIlJe
Jq
ueJ
nlsadoualaw
'(
ruorJ
aua8
4roqs
teaq
p
leqt
3ut
-IIJls
sl
1l
pue
'uounlo^J
uI
pJ^resuoJ
uaaq
e^eq
dJSH
pue
iISH
aqt
qrog
'stuJluale
asuodsar
luarefpe
ot
.{la.trleradoor sputq
;IISH
ar{J
'ASH
aqt;o satdor
a1dr11nru urctuo)
filso6oua1aw
alttldosotq
yo
saueB
>l)oqs
tprq
eql
IIV
'ratouord
sll
tp
J)uJnbas
1a3:el
ate
-rrdo.rdde
eqt suretuoJ
leql
saua8
0Z-
lo
dnorS
rr;nads
aql
le
uorldtJJsuprl
elelllul ot sueJru
e
sapnord eJoJeJJqt
ropeJ srqt
Jo
uoltpllDp
eqJ
'(S1SH)
rope; uorldrrJsueJt
>lloqs
leaq
totel
-rDe
luapuadJpur
uP Lq
pazruS0rar
sr
pue
lurod
-lJPlS
Jql 01 JArlelal
suOrlrSOd SnOIJPA
lP
pJlP)OI
sr
1r lnq'(ASU)
aruanbas
snsuJsuor
uoruuro)
p
ssassod osle saua8
>lJoqs
teaq
aql
'satozfuelna
u1
'(uouertrul
Iortuo)
.ri.eyq srotre4
eu8rg
Jo
uortntrlsqns
'9I 'I
I
uoItJJS
aas) saua8
lloqs
lpJq
Jo
s.raloruo.rd aql ol
uoruuor aruanbas
g
1-
anrleuJalle ue azruSolar
o1 arudzuaoloq espleur
-.{1od
y1qg
slrarrp
lpqt
pazlsaqlu,{s sr Jot)e}
eur8rs
,vlau e
'errJl)eq
uI
'lorluoJ
Jo
seporu JIlo
-/.-rapa
pue
rrlorfuelord
uae.ttlaq seruJJJlJIp
Jql
]uauralo
asuodsol
Vdl_
=
fUL
luaurole
osuoosol
lelaLu
=
3gy1
luauala
asuodsel
proctpococn16
=
399
luoulela lo^al lPseq
=
fl8
rdv
zdv
trry{
zdv
rsn
l.?ig?Sid
0urputq
utalor6
00r- 0zr- 0?r-
091- 0Br- 002-
0zz-
jvz-
092-
=fi ffi
'E
*o+i
i'e
3tB f ul/\
sluor..uala esuodsog
