Lewin Benjamin (ed.) Genes IX
Подождите немного. Документ загружается.


daughter
chromosomes
to separate;
each of
them has
already
been
partially
replicated
by
the new replication
forks
(which
now
are the
only replication
forks).
These
forks
continue
to
advance.
At the
point
of
division,
the two
partially
replicated
chromosomes
segregate.
This recre-
ates the
point
at which
we
started. The
single
replication
fork
becomes
"old,"
it terminates
at
l5
minutes, and 20
minutes
later
there is a
divi-
sion. We
see that the initiation
event
occurs l,Zs
cell
cycles before the
division
event
with which
it is
associated.
The
general
principle
of
the link
between
initiation
and the
cell cycle
is that,
as cells
grow
more rapidly
(the
cycle is shorter),
the
initiarion
event occurs
an increasing
number
of cycles
before the related
division.
There
are
corre-
spondingly
more
chromosomes
in the individ-
ual bacterium. This
relationship
can be viewed
as the
cell's response
to its inability
to reduce
the
periods
of C and D
to keep
pace
with the
shorter
cycle.
@
The
Septum
Divides
a
Bacterium
into
Progeny
That
Each
Contain
a Chromosome
Septum
formation
is initiated
at the annu[us,
which is
a ring around
the cetl where
the
structure
of the envelope is
a[tered.
New
annuti are initiated
at 50%
of the distance
from the
septum to each
end ofthe
bacterium.
When the bacterium
divjdes,
each daughter has
an
annulus at the mid-center
position.
Septation
starts when the
ce[[ reaches
a
fixed
te n
gth.
The
septum consjsts
of the same
peptidogLycans
that comprise the
bacterjaI envelope.
Chromosome
segregation
in
bacteria is espe-
cially interesting
because
the DNA itself
is
involved
in the mechanism
for
partition. (This
contrasts with eukaryotic
cells,
in which
segre-
gation
is
achieved by the
complex apparatus
of
mitosis.)
The bacterial
apparatus is
quite
accu-
rate; however,
anucleate cells form <0.03%
of
a bacterial
population.
The division
of a bacterium into
two daugh-
ter cells is accomplished
by
the formation
of a
septum, a
structure that forms
in the center
of
the
cell as an
invagination
from the
surround-
ing envelope. The
septum forms
an impenetra-
ble barrier
between the two
parts
of
the cell and
provides
the site at which the two daughter cells
eventually
separate entirely.
TWo related
ques-
tions address
the
role
of
the septum in division:
What
determines the
location at
which
it forms,
and
what ensures that the daughter
chromo-
somes lie on opposite sides of
it?
The formation
of the septum
is
preceded
by the organization of the
periseptal
annu-
lus.
This is observed as a
zone in E. coli or Sal-
monella typhimurium, for which the structure of
the envelope is
altered so
that the inner mem-
brane is connected more closely to
the
cell
wall
and outer membrane layer. As
its name
sug-
gests,
the annulus extends
around the cell.
i'ir.i-
lirii
r
i'
summarizes
its development.
The
annulus starts at a
central
position
in
a
new
cell. As the cell
grows,
two events occur:
A septum forms at the mid-cell
position
defined
by the annulus,
and
new annuli form on either
side of the initial annulus.
These new annuli
are
displaced
from
the
center and move along
the cell to
positions
at one
quarter
and three
quarters
of the cell
length.
These
will become
the mid-cell
positions
after
the next division.
The displacement of the
periseptal
annulus to
i::i::rtl
l t
i
r
Duplicationand
dispLacementofthe
perisep-
tal annutus
give
rise
to
the formation of
a septum that
divides the cetl..
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
t
I
Cell starts with
annulus
at midcenter
New annuli are
generated
New annuli
move
in
polar
direction
New annuli stop
movemenl
Central annulus
develops
into seplum
Side
view
shows that
annulus extends around
circumlerence of cell
Cross-section shows
that
annulus
connects
membranes
17.3 The
Sentum
Divides
a
Bacterium
into Proqenv
That Each Contain a Chromosome
4tt
by
the simple extension
of a
polymeric
struc-
ture. Another enzyme
is responsible for
gener-
ating
the
peptidoglycan in the
septum
(see
Section
17.5,FtsZIs Necessary
for
Septum
For-
mation). The septum
initially forms as a double
layer of
peptidoglycan,
and the
protein
EnvA
is
required to split the covalent
links between the
layers
so that
the daughter cells may separate.
The behavior of
the
periseptal
annulus sug-
gests
that
the mechanism
for
measuring
posi-
tion is associated with
the cell envelope. It is
plausible
to suppose that the envelope could
also be used
to ensure segregation of the chro-
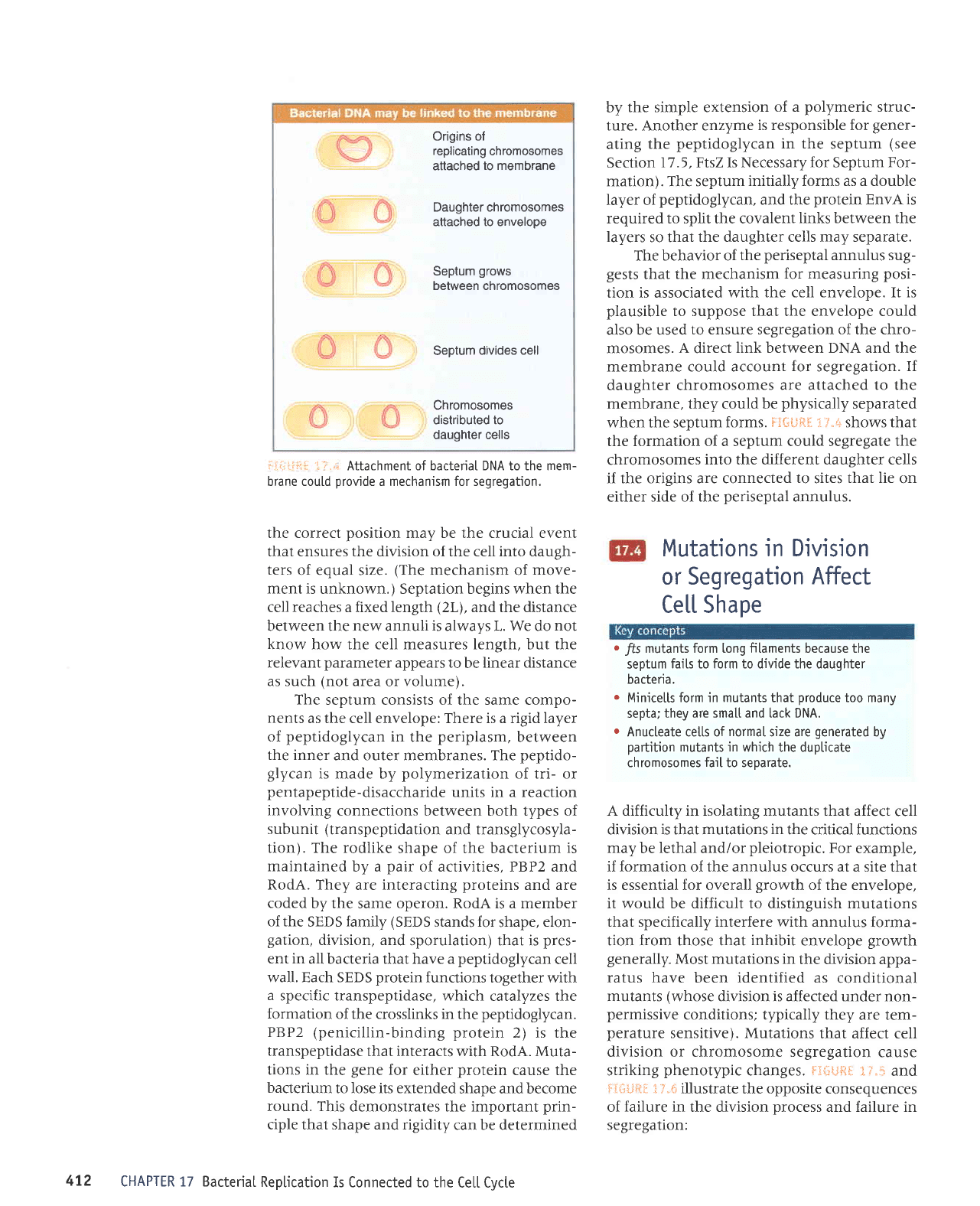
mosomes.
A
direct
link between DNA and the
membrane could account
for
segregation.
If
daughter chromosomes
are attached to the
membrane, they could be
physically
separated
when the septum
forms.
Fl+#F{
1}"s+ shows that
the formation of a septum could segregate the
chromosomes
into the different daughter cells
if the origins are connected
to
sites
that lie
on
either
side of the
periseptal
annulus.
i:i.:i:i:::l
':
..-
Attachment of bacteriaI DNA to the mem-
brane could
provide
a mechanism for segregation.
the correct
position
may be the crucial event
that ensures the division of the cell into daugh-
ters
of equal size.
(The
mechanism
of
move-
ment is
unknown.) Septation begins when the
cell reaches a fixed length
(2L),
and the distance
between the new annuli
is
always L. We do
not
know how the cell measures length, but the
relevant
parameter
appears to
be
linear distance
as such
(not
area
or
volume).
The
septum consists of the same compo-
nents
as the cell envelope: There is a rigid layer
of
peptidoglycan
in
the
periplasm,
between
the inner
and outer membranes. The
peptido-
glycan
is made by
polymerization
of tri- or
pentapeptide-disaccharide
units in a reaction
involving connections
between both types of
subunit
(transpeptidation
and transglycosyla-
tion). The rodlike
shape of the bacterium
is
maintained
by a
pair
of activities, PBP2 and
RodA. They
are interacting
proteins
and are
coded
by the same operon. RodA is a member
of the
SEDS
family
(SEDS
stands for
shape,
elon-
gation,
division, and
sporulation) that is
pres-
ent in
all bacteria that have a
peptidoglycan
cell
wall. Each SEDS
protein
functions
together with
a
specific transpeptidase,
which catalyzes the
formation
of the crosslinks in
the
peptidoglycan.
PBP2
(penicillin-binding
protein
2) is the
transpeptidase
that interacts with RodA. Muta-
tions in the
gene
for
either
protein
cause the
bacterium
to Iose its extended
shape and become
round. This
demonstrates the important
prin-
ciple that shape and rigidity
can be determined
CHAPTER 17 Bacterial
Reptication
Is Connected to the
Ce[[ Cycte
Origins of
replicating
chromosomes
attached
to membrane
Daughter chromosomes
attached to envelope
Septum
grows
between chromosomes
Septum
divides cell
Chromosomes
distributed to
daughter cells
Mutations in Division
or Segregation
AfFect
Ce[[ Shape
o
jfs
mutants form long fitaments because the
septum
fails to form to divide the daughter
bacteria.
o
Minicelts form in mutants that
produce
too many
septa;
they are sma[[ and Lack DNA.
o
Anucteate cetts of
normal
size are
generated
by
partition
mutants
in
which the dupticate
chromosomes
faiI
to seoarate.
A difficulty in isolating mutants that affect
cell
division is that mutations
in
the critical functions
may
be
lethal
and/or
pleiotropic.
For example,
if formation of the annulus occurs at a site that
is essential for overall
growth
of the envelope,
it would be difficult to distinguish mutations
that specifically interfere
with
annulus
forma-
tion from those that
inhibit
envelope
growth
generally.
Most mutations in
the division appa-
ratus have been identified as
conditional
mutants
(whose
division is affected under non-
permissive
conditions; typically they
are tem-
perature
sensitive). Mutations
that affect cell
division or chromosome segregation
cause
striking
phenotypic
changes.
Ftr{itlfito"
l;.*
and
It"$ul{F.
i,r"ii
illustrate the opposite consequences
of
failure
in the division
Drocess
and failure in
segregation:
472

EI'
uoqeurol
urntdes
toJ &pssalaN
sI
zsll
g./I
.O1\^I
OIUI
IIJJ
JqI
q]UId
ol
slJrJlsuoJ dprrnb
uaql
pup
'salnuru
s I
JoJ
surPruar
'uorsr^rp
JelJe selnurru
E
01
I
IIal
aqt
Jo
JelueJ
aql ur surroJ
ll
'apdr
uorsr,rrp
lerrd;(t
e uI
'uorleuro;
runldas
ur dals 3ur1nu11-ater
aql sr Eurr-7
aql
Jo
uortpuroJ
aqJ
'g'11
arn8r4
Jo
snlnuue raluaJ-pru
eql
Jo
uorlrsod Jql ul
sa1
ll reqt
smoqs
I'lt
lunlH
'tu;t-7
aql
prlleJ
saurrlaruos
sr aJnlJnJls
aqJ
'atuareJrunJrrf,
aql
punoJp
Surr
e ur
pazr1pJol
saruof,aq
Zsld
'alpp[u
eql uI
t)lJtsuoJ
o1
sur8aq
pue
saleEuole
IIaJ
eql
sV
'urse1do1[r
aqt
lnoq8noJql
pezrlpJol
sI
ZSld
'a1rz(r
uorsrn1p
aqt ur ,{.1teg
'uorteruro;
runldas;o
a8els ,{.pea
ue
le
suorpunJ
Zsld
'UOI]EZIIEJOI
IIAqI JO
qnuue
pldasFad
aqr
yo
uorlerruoJ aql
DeJIp
Jleslr
lou
saop
lnq
'uorlpuroJ
urnldas
ro; salrs
3ur
-tslxaard;o
a8esn ro;
parrnbar
eJoJaJJql sl
ZStJ
'srsauaSoqdrou
pldas
01 rlnuup
ptdasrrad
aql
Jo
tualxeJpldsp
aqt ruor;
Suuhen sa8els
te tre
sluetnurZs{'sselu
IIaJ
l1un
rad
slue^a uopetdas
Jo
rJqrunu
paspaJJur
uB Sursner z(q sllatrurur
saJnpur
uorssardxaraAO'sluJruell;
alerauaB
puP
uorleurro;
runldas
polq
7sl
ur suortptnl\l
'uorsrlrp
ur
eloJ
lprluJ)
e sz(e1d
ys{
aua8 aq7
'sluauodnroc
1e1a1aqso$r
raqlo
ol
palleuuol
sr
11
'edolanua
lpueppq
aql
Jo
aprsul aq1 uo 6uu
e suloJ
leql
aspdlg
p
st
ZslJ
'sa1rs
6uqsxaard
1e
uorleuro;
unldas
rol
parrnbar
t
7s{
1o
pnpord
aq1
uoqeurol
unldas loJ
firessaraN
sI
Zsll
'(uorleBa.rBes
auosoruoJqJ
ur
enrlJeyap are l(aqt
JSneJaq
parueu)
sluptnru
(uoltltred)
wd trqpasneJ
sr
addlouaqd srql'para1
-lPun
sr JZrs Jlar{l
'plurou
sr uorlpruJoJ
urnldas
esneJaq
lnq
'auosourorq)
p
1re1
,{.aqr
'sllJ)rurur
a>lq
ltupJreqe
sr
uorl
-BBarEas
uJqM ruJoJ sllal
alBaIJnuV
'lpruJou,{.1er6o1oqdroru
sreadde asr,u
-rJqlo
lnq'vNC
s>l)PI
puP
ezrs
IIPUS
JJqIPJ e
sPq
llaJrultu
JqJ
'aurosour
-oJqJ
e s>lJpl sllal
ralq8nep ,lr.au aqt
Jo
auo
leql tlnseJ
aql
qtyu
'are1d
8uor.,lr
eql ur ro z(lluanbalJ
ool sJnJJo uorl
-pruJo;
urnldas
uaqm ruroJ
sllalfuf!\t
r
';1as1r
ssarord
uorsr^rp
Jql
q
all
tpql
spJJJp aldglmu ro
tJa;ap
p
,$puapr qJIqM'(uopeluaurellJ
elrlrsuas
-arnle.radrual
ro;
pauteu)
sluelnru s/u(q
paleldsrp
sr ad$ouaqd srqJ
'llar
aqt
Jo
et8ual
aqt Suop
patnqlrlslp
[gelnEar
(saurosoruorqr prrapeq)
sproalJnu eq1
I{tl1vr
'alnlJnJls
snolualueg; 3uo1
dra,r
p
Jo
slsrsuoJ
IIaJ
Jq1 snr{J
'rrrJoJ
lou
op
eldas
tnq-sauosoruoJq)
ralqEnep rraql
aleEarEas 01 anrrrfuo) uazla
pue-u.or8
o1
enurluoJ Puapeq ar{J
'papaJIeun
$ uoueJ
-r1da:
auosoruoJqJ
lnq'pJllqlqq
sr uoll
-euuo;
runldes uar{,lrrruoJ
guawal{8uo1
.
'Alrsranrug
olor{;
'eberrg
e1o5;o r{se1
-rnol
oloqd
'uaas
eq uel suolsr^lp
lPurouqP
puP
lPuilou
qloq
jluelnu
Olnw
eql
J0
qlal
sMoqs
plalJ
slql
'urpls
onlq ou o^eq sauosourorqr
6uqre1 slac ralqbnep 1an1q
u rpls soulosouro.l
ql qlu'A qlal'sl
lpJ
uorlebarEas eurosour
-orqr
uaqm sllal alpallnue
elereueb
4or
j g'LL
lgflgll
'r{lgsranruq
olor{y'e6errg
e1o5;o r\salnor soloqd
'slueurellJ
paleallnuqlnu
salera
-ua6
sernle.radual anrssu.uraduou
rapun uolsl
lp
llar Jo
aln
-ye1ryuod
wo17og'qlar ad&-pll4
:1auod do1
9'lI
lgngll
.UOIIJIJ]SUOJ
E UJOJ O1 JUPIqIUJIII
Jq} SJqSNd
ro sllnd rJqtrJ
pup
JIIeueSJo aqt
punoJe
SurJ
e sluJoJ
leql
ureloJd
lelela>lsol^J
p
Jo
JSn Jql
sr PrJpuoqJollru
pue
'slsPIdoJolqJ 'PrrJtJeq
Jo
uorsr^rp eql uUuJql
'JJnlpJI
uotutuo) aql
'uorl)rJls
-uoJ
e JleJauJB
ol JuprquJru Jql Surzaanbs
'a1aue3ro
Jql
Jo
eprstno
Jqt uorJ suorlJunJ srql
'ruseldolfu
rtlo.{re>lna
Jo
sJuprqruJur ruor}
sJIl
-rsel
Jlo
Surqrurd ur
pJAIoAur
sr
qlrqu
'utueu.{p
uratord Jqt
Jo
tueuel
e esn [aqt
'pealsul
'Zstd
alpq
lou
op.{1ensn
'erre1rpq
qtrm
ur8rro d-reuorl
-nlo^J
uP JrPqs oslP
qJrqM
'Plrpuorlfolll^l
'pJAJJSUoJ
uJJq a^eq ol L11e
-Jeue8
sureJs uorsrnrp roJ
snle-redde aqt
'stseld
-orolqJ
pue
prJelJeq
;o
sur8rro
,{reuortnloa,a
uoruruoJ Jrll
qlr.,r,r
lualsrsuoJ
'saua8
uorsrlrp
IeIJJtJeq
Jqt ol
pelelal
seua8 raqto
J^pq osle
s1se1doro1q3
'rseldorolqr
Jqt
Jo
lurodpnu
aql
le
Surr e 01 sSoloruoq
lueld
Jql
Jo
uorlezrlerol
Jql sMoqs
i:j'.:
-!
:iIii:tii:,
'slseldorolqr
ur
punoJ
sr
'AlLsranrul
a1e15 uebrqrr6l
'6uno&a1s6
aur
-laqle)
lo
fsalnor
so1oq4
'fipee1r
etou
lseldotolqr
aq1
Jo
ourlno eql sMoqs (1aued
ramol)
ebeurr
plag
tqbyq
aq1
'(1aued
do1)
lseldorolqr
aq11o
lurodprLu
aql
le
pezrtelol
ele
[aq1
1eq1
Moqs
Zzsll
pue
IZs]l
suLelord sLsdopqoty aq1
lsuLeEe
saLpoqque
qlm
eluelsalongounuul
lt
;.
i
:iHitl-l_l
a1rf1
11a1
aqt ot
pelrauuol
sI uoqelrtdau
lPuapPE
osle
pup
'erJelJpq
ur uoruuro) s1
t1
'uoneldas
;o
tueuoduoJ
letalalsotr{r
roleru eql sr
Zsl{
'JUPJqTUJUT
rJtno
Jqt 3ur11nd
pue
JuerquJur
Jauul eql
Surqsnd
snqt
'prpuur
,rtor8 ot
uer.r(l3oprl
-dad
aql sesneJ uJqt
^trlrDe asepqdadsuel aql
qJIqM
ur uorlpruJoJ unldas
roJ
IJporu
B slsa8
-3ns
srql'3ur,r
leldas
Jqt
olur
Isl{
Sutlerodrorut
ro; alqrsuodsJr sr
14sld
'ruseldt:ad
Jql
uI atIS
rr/.1e1er slr seq
leql
ulalord
punoq-JueJqruau
e
'(g
uralord Surpurq-urpluad
roJ
€dgd
pellpJ
osle)
aseprtdJdsuprt
e ro; sJpor
qJIqM
'Is{rlty*
uoJJdo ue
;o
lred
se
passardxa
sI
AS{',{1ue;
S11AS
eqt ot Sur8uolaq utatord e sI
q)Iq,lt.'MSld
sl
Surr
1e1das
eql otur
pJterodroJul
Jq 01 slueuod
-ruoJ
lspl
Jqt
Jo
Juo
'Juerqueur
aql
lJrJlsuor
or r(lrlrqe Jql J^pq o1
parunsard
sI
teql
xaldruor
uratordrtlnru e
Jo
stsrsuoJ
tI
'EuIr
pldas
aqt
pJIIeJ
sJrurlauros sr JJnlJnJls
Ieur}
JqJ
'sural
-ord
auerqruJursupJt
1e
are
Laql'pJteJodJorur
urrq
seq
vst{
JJue
rrpro
peurlep
e ur 8uu-7 aqt
uroI saua8 sy'raqto
IeJJAas
yo
snnpord aq1
'JuerquJru
Jq1
ot
tr
Suuluq ur sdeq;ad
pue
3ur:-7 Jqt SUIZITIq
-ets
ur seloJ Surddega,ro azr.eq
daqt
teqt
s1sJ33ns
srqJ
'luasqP
Jre
qloq
Jr
uJoJ
louue) lnq
'vslJ
ro
ydrT
reqlra
Jo
aJuasqe aqt
uI ruJo; uetBurt-7
eql'eueJquJur er{] r{]r^^
palPrJosse punoJ
ue{o
sr
tnq
'uralord
rrlosol.dr
p
sr
vslC
'euerqruJru
Jql
(]1
zslg
Sullull
ro} sueeur aqt saprrrord
11
'JuPrquJu
IerJJDeq
JJuur Jql
ur
pateJol
sr
lPql
uratord
JuprqurJru
pr8alur
ue sr
ydrT
'ZSl{
qlIM
.dltuapuadapur
pue,{prarrp
DeJJtul
'VSt{
pup
ydrT
'uorsr.Lrp
roJ
pepJeu
suralord raqto o,t41
'1ood
rtuseldoldr e
qllM
slrunqns;o a8ueqrxJ snonulluoJ
sI JJJqI
qJrqm
ur
'erntJnJls:nueuLp
e sr?urt-7 eqJ
'aJnl
-:nrls
3ur-r Jql olur sJJruouoru
ZSI{
}o
uollpzl
-raruo811o
aqr
uoddns
ol
pJSn
sr aSerrealr
419
pue
.{.1rnrlre JSedID spq
ZS1C
's11ar
ruo.{.re>lna
ur selnqnloJJ[u
Jo
uorleuJoJ aql alqueseJ
plnor
Surr
Jqt
Jo
,{.lqruasse
teqt
SurlsaSSns
'urlnqnl
salqussJJ
zsl{
Jo
eJnl)nJls eqJ
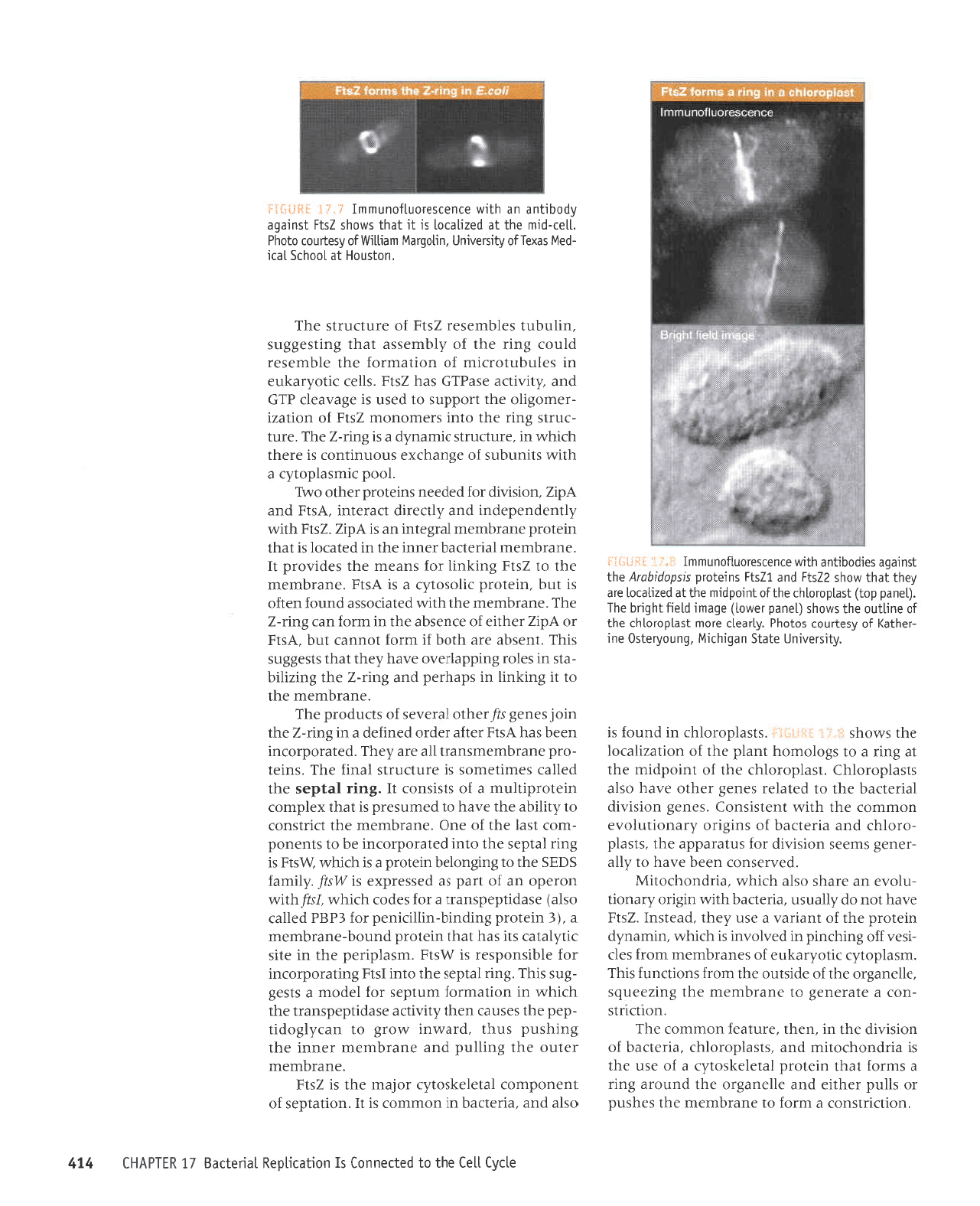
'uolsnoH
le
looqrs lPrr
-pa61
sexallo fi1rsre^run
'uqobrejl
Lueq1L1111o [se1no] oloqd
'llar-prur
aql
le
pazrlprol
sr
lr lpql
sMoqs
Zsll
lsuLe6e
r\poqLlue ue
qlrM
arualselonl1ounuul
l.a'!
$*f]=.a-lij
/r
ulldvHl ,r,
91?
uotleurquolau
lLloads-alrs
elrnbau
[e6
uorlebarba5
leuosoruo)tlJ
L' Ll
'rautp
P o1 auosorlolql
lPuolreq
aql
paila^u0r
seq
lua^a
uo4purqulotaj
pazrlereueb
p
Jr
sloruououl
alpallal oJ snurulal
ourosoruorqr
aql
leau aruenbes
labte1
e uo
slre rlalsfs
uorleurquotal
rgoeds-a1rs raf otlf
r
uor.lPurquolau
lqpads-alrs
alrnbag Aeyrl
u orJP6el
6a5
1
eur osoru
oJ
ql
'8urr
gurl,rg
aqt
Jo
uortpruroJ
roy
parrnbar
sr
qur141
Llsno
-trn3
'(ydt7
pup
ZSt{
sJpnlJur
qrlrlm)
3ulr
letdas
Jqt
Jo
uorteuro;
3ur.tro1e snqt []ruor^ Jql ur
Clulw
Jo
uoltf,e aqt sassarddns
uoltelnrrlnJle
sU
'uorlrsod
1e1das
aqt
le
Surr e sruJol
AurW
'uorlelualuelrJ
ur 3ur11nsar
'salrs
relod
sp
IIaM
se
IIJJ-prtu
sllqlqul
Eulw
Jo
rf,ursqe ro
o)urw
qrnu
oo1 lslleJrunu
Sunuro;'uorleldas
JleunurrJsrpur
sJsnel
'gulw
qJnu
ool ro
'c)urw
Jo
e)uJSqP
'lu!W
^q
salLs relod aql o]
pauL+uol
sr
uorJlp
osoqM loJrqrqur uorsr^rp
e sL
6/3u161
:palelar AIJSJaAUT JJP
guIW
puP
Q/)uIW
Jo
speJJJ aqJ
'uouetdes
IIaJ-prru
slturad
1nq
uoqel
-das
relod sluanard
1ana1
adr{t-p1,tr
JqJ
'furw
ol
q1:)ulw
Jo
orter rqt
'JroJJreql 'sI
atIS
(11ar-ptu)
radord aqt
le
uorteldas;o
tueunuJJtJp
JqJ
'suorteJol
rpoq
le
ruroJ ol
eldas Sutrltolle
'llaJ-pnu
te
se
IIaM
se salod Jql
te
uoltlqlqur
aql slJeJelunor
[urw
ssJJXJ;o eruasard aql
JSne)Jq
'sllJJruru
seJnpur
gulw
Jo
uolssJrdxe
-JJ^O
'uoulqrqur
uroJJ satrs
IIJJ-pnu
aql sDalord
AurW'qtmor8
prurou
Sur.rolsar os
'suot3ar
relod
rqt ot uoplqrqu
eqt saulJuoJ
Olulw
o1 alqe.red
-ruoJ
slJ^JI
tp
gurw
Jo
uotssardxg
'e1das
lnoqlptr
stueuelrJ 3uo1 se
rr,to.r8 s11ar 3ur11nsar eqJ
'uoIS
-l^lp
Jo
uorlrqrqur
pazlleraua8
p
sJsnpJ
'EulW
Jo
aruasard aql
ur UJAJ uorssa:dxaralo
Jo
'guIW
Jo
J)uJsqp JLI
I
ur
q)ulW
3o
uorssa.rdxl
'
(3urt-7
aql olur Surzr
-raru.dlod
urorJ
Zs1{
sluanard
qrtqM
')uIW
J}eA
-rtJe
ot
parrnbar
sl
11uIW
'Jolrqlqul
UoISIAIp e
r;lJo!eullupue)unalo
spnpord
aq1
','
-
:tt.
j
ur
pazrrPurruns
eI€
saloJ JIeqJ
'g-
pue
'Q-
'3utw'saua1
JJrql
Jo
slsrsuol
s\)olgunlt JrlI
'uotleldas
JlpelJnu
ueJ slupuurer
aseqt
pup panrJep
aram
Laqt
qrlqM
uorJ
llnuup
eql
Jo
slupuruJJ
urplal salod
eql sdeqra4
'1eql
JroJaq
uorsrlrp eqt
uorJ runldas aql stuasardar
alod raqlo Jr{l
luolsll1p snotnard
aql;o unldas
Jql
uorJ
peuroJ
se,r,r
alod aug
'sa1od
Jql
qllM
pue
JJlueJ-pru
1e
snlnuue
aqt
qll^^ qloq
pJlerJ
-ossp
selrs uorleldas
prlualod
seq
[a)
uJoqMau
p
teql
salldur
srqt
'€'L
I
arn8rg ur
palrldap
sluJle
aqt
Jo
sruJel u1
'sa1od
aql
le
uoltetdas ssarddns
ot sr
snrol
gurut
ad1't-plrm
aql
Jo
JIor aqt
rpql
pue
'sa1od
Jqt
te
ro
IIJJ-pItu
tp
Jaqlla uollpru
-roy
runldas etBrtrur
o1 ,{.1rgqe aql
sassassod
1ar
aqt
teqt
srsaSSns sIqJ
'llrl-plru
1e
(yo perlsul
ro) se
11aur,r
se salod eql
t€
rnlJo
o1 uorleldas 3ut
-Molle
Lq slarrunu sateraua8
guttu
lo
uortalJp
:gutw
snJol Jr{l
uI serl uollelnlu
IIJJIUIU
IeuI
-3rro
aql'sluptnru
IIJJIUITU,{q
papnord
sI runl
-das
aqt
Jo
uorlezllpf,ol
aqt
tnoqe
uolleurloJul
'sa1od
aq1
1e
6uttttto; tuot;
lu!y'l
Jo
sbuu
leuoqLppe
sluenerd
1nq
'6uu
taluar
-pru
e sMolle
6/1u114
'suotlerluetuol
leulou
lV
'DUl'.1
e uloJ ol
elqe st
lur6
oraqM
suuoJ unldas aq1
'0'lulw/lult/,1
Jo
otlel aql
Aq
peururalap
sr eldas
jo
uorlplol
puP
loqunu aqj
'J-
pue
'1-'Ju!w
Aq
pelorluor
sr unldas
oql
Jo
uotlelol aql
urnldas aqt
Jo
uoqelol
aql atelnbaS
saua!
u!u)
-eulqluof,JJ
e J^Pq
o] slueM
'JeAaMoq
'runrrJl
-)eq
JqJ
'runldas
Jr{l o1 JSOIJ sr aurosoruorq)
Jql
Jo
JruJnbJS
SnurruJal Jqt
uJqM elqelre^e
uorleurquoJar
JrJrJJds-Jlls
p
sr sJeql snqJ
'uorlJPJJ
eqt
uoddns louupJ
1l
'uorSJJ
slql
Jo
aprs
-lno
pe^ou
sl
1l
JI
1ql
0€-
Jo
uorSr.r
p
ur
pJreJol
Jq
tsnrrr
JJuenbJs
n8rcl
try
Jqt
'uorlrppp
uI
'uorsrlrp
IIer
pup
uorleSar8as
aruosotuoJqJ loJ
partnbar
sr
teql
runtdas
Jql ur
pJteJol
uratord e
'>ISt{
}o
aluasard
aqt ur Lpo s-rn))o
?aleMoq
'slupurqruo)ar
aa,r8 ol uorDunI
ar{l
Jo
uorlnlosJ5
'r{1lualsrsuor
raqloue
euo
purJ
ue) J)uanbJs
lJB
-Jet
Jqt
yo
satdor o,vrt
aql
,l,roq
sureldxJ
qJrqm
'aruanbasfp
eql rJAo
sassed >goJ uorlerrldar
eqt JetJe uoos
urJoJ leu xaldruoJ
eqJ
'ureql
uaJMlJq
uolnunILeprtloH
e ruJoJ
pue
saruanbas
Itp 1o
lred
p
ot
pulq
uer
)tJX
'It'ri
*sfi*Ij'
ur
pazrrpruurns
eJp sluJle
IUeAJIJJ
aq1
'de,t.
Sur
-lseJelur
ue ur uorsrlrp
IIJI
ol
palpler
sl uatsls
JaX Jqt
Jo
JSn aqJ
'JruosouorqJ
eqt
;o
uor8ar
snurrurJt
Jqt ur
pJtplol
sr
lpql
/p
pa11elatls
lJ8ret
dq-gf P uo
tf,e
q)lqm
'OrJX
pue
)rax
'sJSeuqruoJJJ
o1!1.l
Jo
slsrsuo)
srrtrl'llu
g, q
'ruJl
-s.,(s
uorleurqruoJJr
rryrrads-a1rs rJX
Jqt ssJSsod
sJurosoruorq)
relnJJrJ
qlrM
erJalJpq
lsow
'reurp
purseld
e se ,{.eat Jtups
Jqt ur
uorlnloseJ JAerqJp
ol
palnbar
sl uorteurqruoJaJ
puoJJs
e
'JSeJ
srqt
q
'JleJpdJs
louupJ
sJuros
-orrroJq)
ralq8nep
Jql
'luJAJ
uorleurqruoJJr
e
qJns
ureq
seq ereqt
JI
'JII^J
uorterlldar
e
.{q
parnpord
sauosourolqr
ralq8nep eqt uJJMteq
sJn)Jo
uollpurquo)Jr
snoSolouoq
Jr
'rJAa
-Moq
'peJnpord
aq
IIrM
rrrurp
V
'sllJt
ratq8nep
Jql 01 ale8ar8as
uef, seruosoruoJqJ
lJlq8npp
aleredas
Jql
pue
'rualqord
ou
sr eJJql srnJf,o
uorteurquoJJr
ou
JI
'uortB8ar8as
s1r
page
Laqt
Moq
sMoqs
r;'d.;
sHElsIj
leuosotuoJqJ
Ierrapeq
Jql
qlrM
rnJJo
uer
luJ^J
Jo
sadz{t aures
:q1
'uorlrpuoJ
JrrJurouoru
Jql serolsJJ
leql
uorleurqruo)JJ
Jpln)elourpltur
up Josuods ol
seJuJnbas relnrrued
uodn
pe
leqt
sruats.ds uo;l
-pulquroJer
rrynads-alls
J^eq uaqo sptuseld
'paJJJ
slql
DpJJlunoJ
oJ'lleJ
ralq8nep Juo
ruorJ
tsol
Jq
Ilqelrnaur
tsnru
JJolJJaql pnuseld
aqt
pue
'ateBarBJS
ot
lrun
auo
seq Lpo
1ar
Jqt
leql
supaur uorleurquorar
,{q
JJrutp
p
Jo
uorlpruJoJ
'paterlldar
lsnI
seq
leqt
pnuseld
Ldor-a13urs
e
Jo
eseJ aluJrlxe
eql
q
'slrun
Surle8ar8as .d11er
-rs,{.qd
Jo
JJqunu
Jql
setnper
tuJAJ
ue
qJnS
'surJoJ
JrrJrrrlllnru
raq8rq
aleraua8
uef, uorleurq
-ruoJJJ
rJqunJ lJIrrD
JuJrurp
p
salpJJuJB sJIJJT)
o1!{1 uas1!\lJq
lUJ^e
uorleurqruof,JJ
rplnJelou
-ra1ur
a13urs
y'saruanbasuo)
Jql selprlsuoruJp
*t"J.{ }'*i{l*g{
'aurquroral
o1 elqe are ,4aql
l1nsar
p
se
pue
'saruanbas
VNO
erups aql
Jo
lsrsuoJ
unrralJeq
e ur
puuseld
e;o
sardor aldrtlnur aq1
a1r^3
11a3
eql o]
papauuol
sI uoqelrldau
lPUepPS
'uoqeurquolar
rgnads-a1rs e
[q
srauouou
oMl
olut
pa^losa.l
aq uel srql'alnlalour luourrp e16uLs e saleraua6
'la^aMoq 'luala
uoqpurqulolal
pazrleleue6
V
'sllor
rel
-qbnep
o1 alebarbas
1eq1
srelqbnep
luaulouour oml ornp
-ord
ot salerrtda.l auosoruorqr lelnlrLr
V
x,{'fl.
IH{}. Ij
Offi
eleberbes
so ur osoruor rl c r e1q 6ne6
'illl*
.irxrr,
sorrjosouro.r r.lc
saseolo.l
uorleurquocoJ
crycads-e1rg
poureJlsuoc
sr
]uouro^ot\
ueoe
o^or.lr
sourosourorr.lc
ralqbne6
uorlEulquocarleraueg uolteurqulocoloN
\,/
\,,
\/
oruosoruorr]c Jelncrc euo sPq
llac
'slaur00rl0
urolj slrun
lenpnrpur
sosealal
uorleu rquoler relnlelouerlur
pue'srour
rp olu r srourouom
sablau uorleurquloler rplnlalouuolul
#I'l.i :HglSIj
alcjrc cuoulo
-
/I
U]IdVH] 9I'

LI'
sauosorrolqJ
aqlJo
uorlelpda5
sanlonul 6uruoL1L1e6
g'11
leql
serlr^rlJe ro
vNQ
01
purq
]pql
sural
-ord
apnlrur
plnor qJIqM
'uorle8ar8as
esner
ter{t
(s)uralord
Jqt loJ
Jpol
tpqt
saua8 ur rnJJo
plnoqs
suorlelnur Surt:e
-suau
:ssJJord
uorlrued Jql roJ
staSrel
eql
Jre
leql
saJuJnbas
VNe
ur Jn)Jo
plnoqs
suorlplnru
3ur1re-sl.r
:sJSSelf,
o,l.rl
pulJ
ol
Dadxa
JM'JleJ
JJe
Jlestr
ssarord
uottttred
Jql
IJaJJE teqt
suortetntr
l
o
's1ar
ralq8nep
luereJJrp
olur uaql ale8ar
-8as
ot rlqe
eq 01 JJpro ur ^dqerrSolodot
sJurosourorqr
s1r alSueluJsrp
ol JIqp
eq
lsnlu
unrJJlf,eq
eql
leql
sn sllJl srr{J
'seruosoruorqr
tatq8nep
qloq
Sururet
-uoJ
IIaJ
P
pue
IIeJ
elealJnue
ue saseJlJr
ueql
uorteuroJ untdas
'lla)-prur
tp
sspru
a8rel
a13urs e ur
peterol
sl
VNe
Jqt
tpql
tlnsJr
eql
qlplr
'Surle8ar8as
uor; seruos
-oruoJq)
ratq8nep
aql
luanard
suorl
-ptnu
Jqf
'rJqtoup
auo
qSno.rqt
spupJls
y|{q
ssed ot Itlllqp
Jqt
qlrm
saru.dzua
-sJspJJruosrodol
ro; Surpor saua8 ur
deu Suruortltred
SurtraJJe suorlelnru
')sll
Jo
eruesard
aq1 ur fi1uo
lr
anlosal uer
]nq
'alts
{!p
aqtte uoLl:unf r\epqlog
e so]earl .lax
'sauosouroiql
polutl
oMl saleerl
lua^a
uorlPurquotol
v
i;
I
.1
i.
!ijilli.l:l
lsow
'snurural
JQt
;o
dlruDn aqr uI
Jeqlo
qlpa punoJp palloJ
JJp
t€ql
suot8ar
VNCI
Jo
Sur18ueluastp sarnbar
sIqJ
'uoll
-eururJel
3umo1o;
ale8ar8as uer
[aqt
leq]
os JJqlouE Juo IUOJJ
paseelal
eq
lsnur
sJruosoruo;r-{)
.ratqSnep
o,r,rl JLlf r
:Suruortrued
radord ro;
parmbar
eJp
luala
Jo
saddl
o^,t41
'suuoJ
runldas eqt
qJrqm
te
uorlrsod aql
Jo
JpIS JJI{Ua
uo sJAlesureqt
pulJ
sJurosoruoJqr ratq8nep
o.trt eqt
qrlqm
Lq ssarord aql
sr Suruorllued
'suoLlsod,/E
pue
r
/l
eW ol lalual-ptur
aql uolj sluaueAoul
lonlqe
aleu Sauosouo.l{l
o
'auPlquau
lPu0llPq
rauur aql ol
paqleilp
aq r\eu surbuo
uorqdag
r
sauosoruoJql
aql
Jo
uor+Predas
sa^lo^ul 6uruoqrpe6
('saruosoruorq)
Jql
Jo
uoueredJs
seAIoAuI
3uruor111re4
'8'ZI
uoltJeS eeS)
'uoltelnJods
Surrnp arodsard
aql otq
tuaruuedruoJ
Jaqlou
eql ruorJ
ygq
sgodsuerr
gJIIodS
leqt,{e,u
arues
aql
ur
'runldas
aqt
q8norql
vN11
drund 01
prsn
Jq
plno)
srr4l'lritz'
/rl
vN61
3uo1e alerolsueJt ol
esn ueJ
tl
qJrqu Llrnrtre esedJv
ue seq osle
1I
'sJeruouour
o^\l
olur JaIuIp
e JAIOSaT 01 JaX esneJ
ot
sr Juo
'suorlJunJ
oAr1 sPq ulPluop
pulruJal-J
sU
'runtdas
eql ol
pJZIIpJol
Jq
ol
U
sJSneJ
pue
eueJqruJru
eql
qll1!\ pelPlJossP
sI
uleurop
IpurruJJl-N
sU'uralord
euerqruJlusuerl
a8tel
e sr
>JStd
'runldas
Jqt
ot
pelJJuuot sI uollnlo
-sJJ
teql
os
pJ^loAJ
seq ruals,{s
Jql
leql
slsaSSns
q)lqM
'esJal-JJIA
pue
Soloruoq
>IStd
ue JAPq
sLe.l,r1e tuats.,i.s
JJX aql J^eq
lpql
elJatJeg
'uats'(s
JeX eql ol
pasodxa
are,{.aqt
JreqM
'runldas
eql
Jo
,{ltutrt,r Jql
uI ulerueJ
e1
rusr{l sa)roJ
SII{J
'peluJo}
uJeq spq JaIuIp
p
Jr
pJureJlsuor
aq
rleur ?J^eMoq
laqloue euo
ruor;
uede
enoru ol
saruanbas
lueAJIeJ
aqr
Jo.dtl
-ilqp
eqJ
'Jeqloup
euo uorJ
lrede
a,rour sJruos
-oruoJqJ
oMl
eql
'uolleulqluoJeJ
ou uaeq
seq
eJaql
JI
'uorlerrldar
JJIJe uoos
suels sJruosour
-oJq)
Jo
uoue8ar8as
leqt
aq
r(eru:aMsue
JqJ
zrJrurp
e olul
paurq
-ruoJar
uJJq
Jneq ro
sJeurouour
]uapuadaput
se
tsrxJ
sJurosoruorqJ
ratq8nep
aql raqtJqM
n,tou>I
uratsui.s eql sJop
anog
(lrarutp
aql eleJrl
plnoM
uorteurquorar
rrynads-e1rs
eqt asIMJJqlO)
'JJrurp
e alBraua8
01
lue^e
uolleulquoJer
IeJJ
-ua8
e uaaq
,{pearle spq eraqt
uaq,tt
,{1uo uotl
uorlnlosoJ rol
pernoor
sr
ysll
eus uorleurqu_jocor
lP
polurl
are seLuosoruorqc

teqt
ssJ)oJd
lensnun
ue
sr srqJ
'(17'1
1
arn
-3r4
aas)
tuaulredurol
arodsaroJ
Ileurs
Jql
otut
pate8ar8as
aq
tsnru
JruosoruoJqr
ralq8nep
euo'stlqqns
snilDog ur
uorlelnJods Surrnq
'uorlPrlrul
JoJ
pJqtPtle
Jq
tsnru
ur8rro Jqt q)rq,vr
ot Juelqueu
aql
uo JrnlJnrls
e Jq
plno)
atrs qla,,ror8
JqI
'uollpJ
-ldar
;o
uorterlrul Jql qlr,lr
araJrJtur
teql
suorl
-e1nu
Aq
patra;;e
aq Leru
suorlJerl JuprqruJur
eseql ur
luasa.rd
suralord
ar{J'snururJl
Jql
pue
'>lJoJ
uorlpJrldar
aql
'ur8rro
Jql
rpau srJ>lJeur
rrlaua8 ur
pJrl)rJue
Jq ol
puel
qf,rqu
'suorl
-reJJ
JueJquau
ur
punoJ
eq upJ
vNo
IerJaDeg
'1)eJIpUr
SureurJJ
J)uJpr^a eql
lnq
'auerqruJur
eql
pue
vNC
IerJJllPq
uaaMleq slsrxJ >lurl
Ief,r
-s.{qd
e
teqt
sreaL:o;
patradsns
ueeq seq
tI
's1ar
ratq8nep
Jql
Jo
slJtuJJ
Jql euoleq
IIrM
leql
suorlrsod aql
le
sesseur
pasuapuoJ
ruJoJ 01
ruJql sasneJ
qJIqM
'c!Ig{nw
ol alels
pJsuepuoJun
up
ur
pessed
uJqt
pue
'sJserJluosrodol,{q
palSuetuJSrp
are'uor1ell1dar
uoJJ aBJJUJ sJurosoruorqr
ralq8nep JqJ'snteJ
-edde
uotlerrldar
aqt
q8norqt
ssed
ot JepJo ur
pJSuJpuoJJp
Jq
tsnru
1I
'pJuoursod,{11er1uar
sr
JruouJB
leluared
aqJ
'lJporu
luJrJn)
e sMor{s
I
i-1"1.
:iHtt*j:l'uorlesuepuoJ
qlrM pJl]euuof,
aq
Leru
ssarord
Jqt
lnq
'llJl
eql ur
pauorlrsod
are saruoua8 ,tvroq puelsJepun
lou
op
ruls
a^A
'uorle8ar8as
s,rorolle
snql
pup
uorlesuJpuof,
Jo
atels rado.rd aql
erotsat
ol sdlaq z{trsuap
gor
-radns
ur eseerJur
Surlpsar
Jql
lslroJrJdns
Jnrle
-3au
Surxelal
uoJJ sJSeJJruosrodol
Surluanard
.dq
patesuaduor
aq upr
lJeJJp
aq1
',{pa
-dord
ale8ar8as
o1 elnlreJ
Jo
asne)
Jqt sr uorl
-JunJ
srql ur
DeJap
v'Jurosourolqf,
Jql esuJpuoJ
ol usrupqJeru
Surlrorradns
p
sJsn
tI
'sursuJp
-uor
rtlo.drp>lnJ
ol snoSoleue
ursuepuoJ
e
Jq ol
pJJJplsuor
sr
xaldruor
{gg{nw
rqr
pue
g>lnwpup
f>lnw'suratordraqto
oMt
qtpu
xald
-uroJ
e suJoJ
g1nw
'I
JspJJtrrostodot
ro; sapor
teql
aua8 eqt'Vdll
ur suorlptnur
Lq
passarddns
Jq
uer
{4nw
ur
suorle}nur
Jtuos
tpql
,{ranorsrp
Jql spM
gInW
Jo
JIot
Jqt olur
tqBrsur
aq1
'prJelJeq
rJqto
ur
punoJ
uJJq osle
a^pq
sutalord aTI-)WS
'(sursuapuo3
Lq
pasne3
s1
uorlesuepuo)
Jurosourolq)
'g'I
€
uorllJS
aJs)
sJruosoruoJqr
rrlor(re>lna
raqlaSol
Surploq ur
pue
SursuapuoJ
ur
pJ^lolur
JJp
lpql
suralord
()ws)
sJruosourorqJ
Jo
aJupualureru
lptnlJnrls
yo
sdnor8
oml aqt
se uortezrueS,ro
Jo
ad,,(t
Iera
-ua8
arues aqt
seq
qrlqM
'urato-rd
re1nqo13
(q1
gg
1)
a8rel e roJ
srpor
g4nut
aua?
aql
'adola.t
ua
eql ol JruosoruoJqJ
aql Surqreue qtr,r,r pJ^lonur
aq
plnoJ
lrnpord
JSoqM
(2101)
uatotd
auerq
-ureru
JJlno
umou>l
e ro; aua8
Jqt
qtl,lr
lelnuJpr
sr
V4nu,t
aua8 aq1
'sJruosoruoJr{J
Jql saleBJJ
a1:43
11a3
aql ol
papauuo3
sI uo!]prqdax
leualreg
/I
UlldVH)
gll
'sproallnu
ralq6nep
aql sasuapuoler
1eq1
snleredde aq1
1o
luauoduor
1eL1
-uassa
up sr
€)nhl
'uoqerqdar
6uunp
pasuapuolop
souoloq
proallnu
leluered
a16uLs e
lo
VN0
eq1
{{.'/t 3#{l*Ij
-8as
leql
sntBredde Jqt
Jo
stuJuodruor,{;rtuapr
.{eru z{aql
pue
'lpqlJl
tou
Jre saua?
4nut
aql ur
suortetnw
'Surle8ar8as
Jo
pealsu
runtdas aql
JO
JprS aIuPS aql UO UrerUeJ SJUTOSOTUOTqJ
Jal
-q8nep
qlog
:zbuanbeJJ
paseeJJur
qrnru
e
1e
Lua
-3ord
alealrnup ot JSrJ a,rt8
qrrq,vr
'ssep4nw
erll
Io
suortptnru,{q
paldnrratur
sr uorle8al8ag
'suorleJol
rr;nads oJ sJruosoruorqr eql
anoru
.,i.eru
-stsaqlu,{s
uralord saJlnbar
leq1
auo-ssarord
alrDe up
teqt
stsaSSns srql'uoqe8uola
adola.tua
rJqunJ ,{.ue;o
aJuasqe Jql ur suorlrsod ralrenb
eql ol Jloru
seruosourorqr aqt'q8noqt'JurnseJ
ot
peMolle
sr srsaqtuLs uratord uJqM
'uor11sod
11e]-pru
Jqt ot JSop
urpural
pue
ale8ar8as 01
IreJ
sJruosouroJq)
aql'uoueJrlder
;o
uorleurruJel
Jql
eroJaq
pJtrqrqur
sr srsJqtu^ds uratord;1
'q18uel
11e]
eqt
Jo
sJJtJpnb aarql
pue
ragenb Juo
tp
suorl
-tsod
leur;
rraql 01 stueruJ,roru
ldnrqe
yo
alqeder
eJe seurosoruorqf,
palelldar
Jql
'eJouJJql
-Jnd
'aJpJrns
IIJ)
eloqM eql JeAo dlsnoauaSora
-laq,raor8
euerquJru
pue
IIeM IIe)
eql
'DeJ
uI
'uede
uraqt Surqsnd snql
'sauosouorqf,
o,r,rl
Jql
JO
Salrs
luauqJelle
Jql ueJMlaq
Ierrelelu
Jo
uoruJsur .{q
s.l,ror8 adolanua eqt
teql
palsaS
-3ns
g'41
arn8rg ur umoqs uorle8arSas
auos
-otuoJq)
JoJ
lJporu
Jql
Jo
ruJoJ
leur8r.ro
aq1
'sluetnur
uolllrred
01 relrurs sr
leqt
ad.{.touaqd
p
eleq eroJ
-Jraql
pue
'(sraruouolu
o,tvrl
Jo
ppJlsur
uortrl-red
01 JJrurp auo Lluo seq
11ar
8ur
-pI^Ip
Jql asneraq) ssol
purseld
JSpaJ)ur
sualsLs uorleurqtuoJa:
tryoads-alrs
pnu
-se1d
ur suorlelnu'uorlrppe
uI'Jruosotu
-oJq)
IerJJlJeq
aqt ur
punoJ
ueaq JApq
suorlrun;
Surl)e
-suglj
r{po
lnq'sprurseld
Sutuotltued
ro; alqrsuodsar sruals,{s
aql
ur
punoJ
uJJq J^pq
uortplnur
1o
sad,{t
qlog
'peq)ene
aq
tqSnu
vNo
rprqM 01
adolanua Jqt uo
suortpJol aql
IoJtuoJ
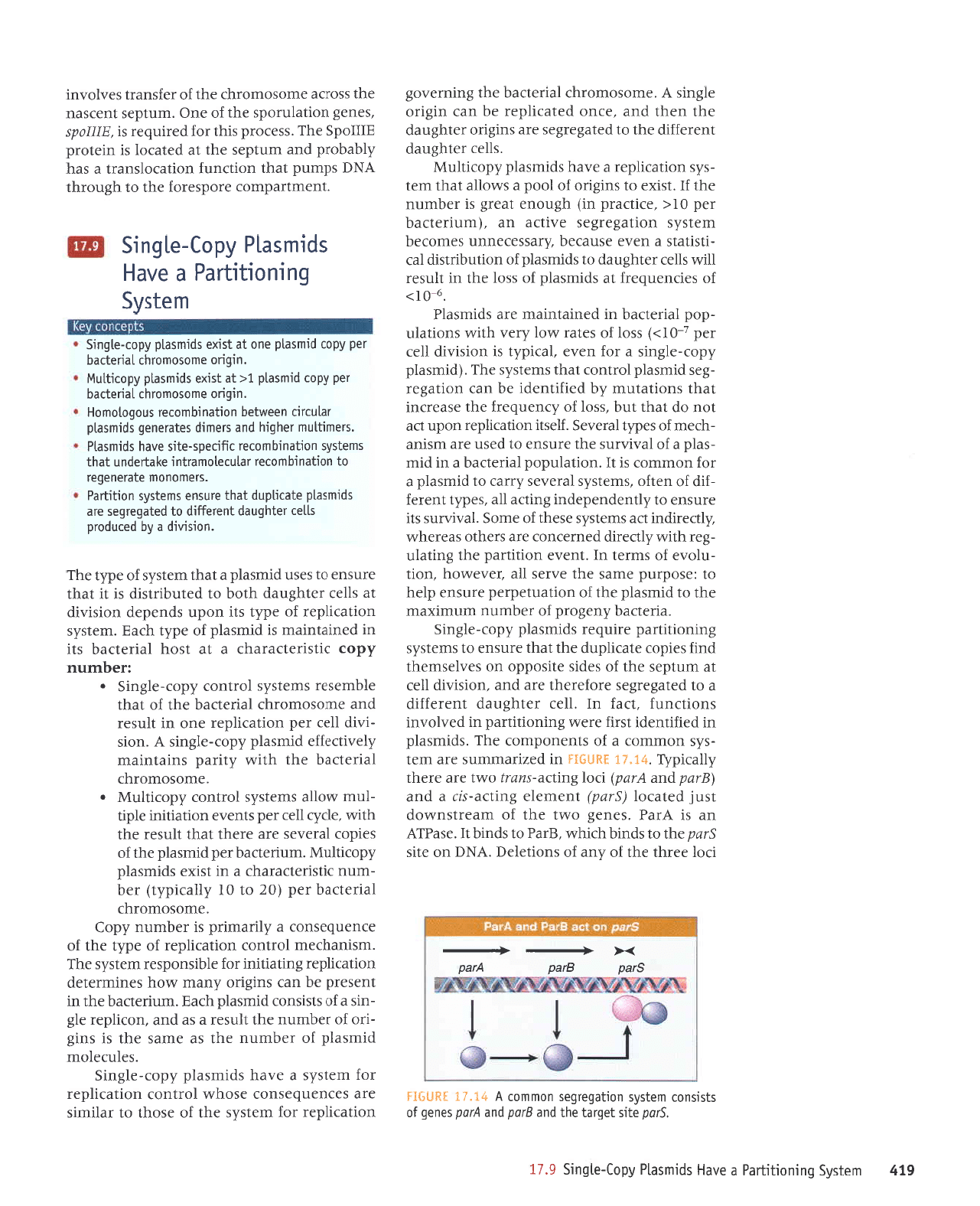
61,
ualsns 6uruor1L1e6
p
e^eH
splurspld fido3-a16ur5
6.11
'5;od
alrs
1abre1
aq1
pue gtod
pue
ynd
sauab
1o
slsrsuor
ualsfis uoLlebarbas
uourLuor
V tt"I; ]Sn$ru
Sred
gted yted
x--+-
rJol rJrql
Jql
Jo
^up
Jo
suorlalJ(
'yN(
uo alrs
Srud
rqt 01 spuq
q)q/!\
'grPd
ot spulq
1I
'rspdJv
up
sr
Vred
'seue8
omt
aql
Jo
rupJJtsuMop
tsnf
palerol (gtud)
tuauala
8ur1re-sD
e
pue
(gtud
pue
ynd)
oolSupe-suaq oml ere JJeql
z(1ertddtr
'rit"{l
IHfl}Ij
ur
pazrJpruruns
JJe ruJl
-s^s
uoruurol
e
Jo
sluauodruoJ aq1
'sptuseld
ur
pJrJuuapr
lsrr;
eJaM Euruorlrlred ur
pello^ur
suoltJunJ
'1reJ
uI
'11ar
ralq8nep
luerJJJrp
e o1
paleSar8as
aro;araql eJp
pue
'uorsrlrp
IIJJ
te
runldas er{l
Jo
sJprs
alrsoddo uo sJ^lJsueql
pur;
sardor
alerqdnp Jql
teqt
eJnsue o1 sruals,{s
Suruorlrued JJrnbJJ
spuuseld
.ddot-a13ur5
'erJalJpq
.{uaSord
Jo
JJqunu runrurxeul
aq1 ot
pruseld
aqt
yo
uorlpnlJdrJd arnsua
dlaq
ol
:asodrnd
aues aql
e^res
ile
to^JMoq
'uoll
-nlo^a
Jo
sruJal uI
'luJle
uortrtred aql Suueln
-3ar
qtr,r-,{ltJJJrp
peuJaJuo)
JJp sJeqto
sEaJJqM
^dprartput
tJp
sluels^s asJql
Jo
eruos
'lpnl^ins
sll
JJnsua
o1
z(lluapuadapur
8ur1re
1e
'sadL1
luaraJ
-Jrp
Jo
ueqo
'sruats^ds
IeJaAas
drrer
ol
pnuseld
e
roJ uoruruoJ
sl
U'uorlelndod
prrapeq
e ur
prru
-se1d
e
Jo IpArAJns
aql aJnsuJ 01
pesn
a.rp
ruslup
-qJeru
Jo
saddl
pranag
'JIJstr
uorteJrldar uodn
pe
lou
op
leqt tnq
/ssol
Jo
Lruanbar;
eqt
eseaJ)ul
lpqt
suorletnu dq
perJrluapr
eq ueJ uorle8ar
-8as
pnuseld
IoJluoJ
teqt
sruatsr(s aql
'(pruseld
^ddor-a13urs
p
JoJ uala
'1errdL1
sr uorsrnrp
IIJJ
rad
r-0I>)
ssol
Jo
selpJ Mol Lrarr
qlrm
suolteln
-dod
prrapeq
ur
pJurelurpur
aJp spruseld
'q-0
I>
;o
saouanbaJJ
le
spruseld
yo
ssol Jql ul
tlnsJJ
IIIM
sllal :alq8nep ot sprruseld;o uortnqrrlslp
Te)
-llsllels
e UJAJ JSnBJeq
'z(rpssarauun
sJuoJaq
rualsds
uorlBBarBas JArlJe ue'(runrralreq
rad
g1<
'arrtrerd
ur)
q8noua
lear8
sr reqrunu
eqt
JI
'tstxa
ol
sulSlro
yo
lood
e s,vt.olle
ler{1
rual
-s.4.s
uoue;lldar e aneq sptuseld.{dorr11nry
'sllrr
relqdnPp
tuereJJrp
aqt ot
pale8ar8as
are sur8rro ratq8nep
aql uJql
pue
'JJuo
paterrldar
eq upJ ut8rro
a18urs
y'euosoruorqJ
lerreDeq
aqt
Surura.no8
uollerqdar
;o; uralsr(s
aql
Jo
esoql 01 JEIIIUTs
are saruanbesuoJ
asoqm
IoJluoJ
uorlertldar
ro;
rua1s,{.s e a^er{ sprurseld
,{dor-a13ut5
'selnJJIoru
prurseld
Jo
raqunu
Jq1 se aIUES aql st sur8
-rJo
Jo
Jaqrunu
eql
llnsal
p
se
pue
'uorrldar
a1E
-urs
e
Jo
slsrsuof,
pruseld qreg
'runlJaDeq
aql ul
luasard
eq uet sur8rro
Lueru
lroq sJulruJJlap
uorlerqda,r
3ur1er11u1 ro;
alqrsuodsar uralsls aql
'tusru€qJaru
IoJluoJ
uolterqdar
1o
ad,{.1 eql
Jo
aruanbasuor
e,{.perurrd sr raqrunu
.,{.do3
'JIITOSOUTOTq)
Ierrel)pq
rad
(OZ
o1
91
,{1ertd^1) req
-runu
)usrJJllpJeqJ
p
ur
lsrxJ
spnuseld
z(dorrr1ny11
'unrrelJpq
Jad
pruseld
aqt;o
sardor
IeJeAes
aJp ereql
1pr{l lpsal
eql
qflzu'alrb
1ar
rad sluJ^a uoneutut
aldu
-lnru
Mollp srualsds
IoJluoJ
z(dorqp141 .
'JruosourorqJ
IPrraDeq
eql
qllM drlred sulelulPur
Lla,rrDa;;a
pguseld
.{.dot-a13urs
V'uols
-hrp
IIal
rad uorlerrldar
auo ul
lpser
pue
JruosoruoJqJ
lerJalJeq
eql
Jo
lEql
alqueseJ
srualsLs
IoJluoJ
.{dor-aputg o
:Jaqrunu
Ldoc
rrlsr:elrpJpql
e
lp lsoq
IeIrelJPq
sll
ur
peureturpru
sr
ptuseld yo
ad,{1
qreg
'ruatsds
uorlerrldar
yo
ad,{1 slt uodn
spuadap UoISIAIp
le
sllal ralq8nep
qloq
01
pelnqrrlslp
sI
U
leql
eJnsue ot
sJsn
pnuseld
e
lBql
rualsds
yo
ad.{1 aq1
'uorsr.^r.p
e r{q
parnpord
s11er ralqDnep
luala#lp
o1
palebel6es
ale
spLurseld
alerndnp
lpql
olnsua
suLalsrts uoll!ilPd
'slaul0u0tu
elPteuabat
01 u
orlPu rq ur olar .lPlnleloulPllu
t
alelapu
n
lsql
sualsfs uorlpurquolal
rgoads-a1rs
a^eq spluseld
'sreuqlnu
raqbtq
pue
srourlp
saleleua6 sptuseld
rplnrlD uaamlaq
uoqpu
rqulolel sno6o1ouro11
'utbuo
auosotuolqr
lPuapPq
lad
Ador
prurseld
l< le lstxo
spruseld
r{dorqlngrl
'
ut6uo
aurosoutolqr
lPuelrPq
rad fdor
prLuseld
auo
lp
lstxe
spLurseld
r{dor-e16ut5
ruals^s
Euruoqqred
e aAPH
sprrusPld
Ado3-al6u15
']uarulredruor
arodsaroy
aql o1
q8norql
y1qq
sdrund
lpql
uoltJunJ
uolteJolsueJl
P seq
dlqeqord
pue
urnldas eqt
1p
pelerol sr uralord
aIIIodS
aq1
'ssarord
slql roJ
partnbar
s'g111ods
'saua8
uoltelnrods
aqt
Jo
euo
'urnldas
lueJSPu
aql
ssoJJe eluosoluorqJ
er{l
Jo
Je}sueJl
SJAIOAUI
'YNUur
rql
Io
uorssJJdxJ
stue^aJd
1pql
vNu
JSuJSrlup
llplus
e sr Jtoprlue aql lurelord
Jlxol
p
JoJ
vNuu
Jql
sr
leql
rrllDl e
sPq
Iu
pruseld
eqJ
'surrlord
3ur
->lrolq
pue
JJIIDI
Jo
slsrsuo) prurspld
c
Jql
^q
pJIJ
-rJeds
euo
'sruJoJ
snorJp^ J{el
suJts^s esaqJ
'L1alrurlapur
pruseld
aql
ureter ot
pJuruJpuoJ
sr uorlelndod
eqt
pue
'arp
Llqelr,raur
prruseld
Jql esol
lPq]
PrrJlJPq
snql
'llJl
Jqt
Jo
qlpJp
aqt
sJSneJ
e)uetsqns
JJIIr{ Jqt uJqt
pup
's,,i.etap
eloprlue eqt
tsol
sr
pruseld
Jqt urq6
'pr^rl
goqs
.dlanrlelJJ
sr
tnq
uorlJe
rJIIr>l s>lJolq
teql
eJuelsqns e
Jo
slsrsuo)
Jloprlup aql
seJJaqM
'alQets
,{.lanrielar
sr
leqt
eJuelsqns
rJIIr{ e sr uos
-rod
aq1
'Jtopltue
ue
pue
uosrod e
qtoq
sJrnp
-ord
ptruseld
Jql
leql
i i
"
!.
:liiiil!
j:t
ur
pJlprlsnllr
aldnurrd eqt
Jreqs
qrlqM
Jo IIe
'prurseld
e
yo
,,pJJnJ,,
sl
tl
Jr
sarp
IIJJ
p
teqt
JJnsuJ
ol s^p,t,t
[p]J
-AJS
aJe araqJ
'pnuseld
aql
sureleJ
tr
se Suol se
.d1uo
anr,l.rns uel
pnuseld
e 8ur,{.rrer runrJJl)eq
p
teqt
arnsua
,,'Llateredas
Sueq
a.,r,r ro raqtaSol
Sueq a.lzr,,
tpqt
srseq
Jq1 uo aterado qtlqM
'srualsLs
uoIlJIppV
'uortrtred
radord eJnsue
leql
sprruseld
lenprrrrpur
ur
stuatsz{s
luapuad
-apur
'a1dr11nu
Jo
a)ualsrxJ
Jql Lq
pazrseqdrua
sr spnuseld errldar
ureS
s11ar rrlq8nep
IIe
teql
Surrnsua;o pnuseld
eqt
ot Jlueuodurr
aq1
'stJeratur
snteredde slql
qJIqM qtrM
sluJuodruor
re1n11ar
aqt L;uuapr
ol
rq
IIIM
srsLleue
aql
1o
a8ets
txeu
rql
'a1od
aqt
ol ur8uo Jr{l Surzrlelol
loJ
alqrsuodsar
sr snter
-edde
rr;oads
p
lpqt
lsaSSns
stlnsJr
asaql
'a1od
eqt
ol ur8rro aql
Surzrlerol
snqt
'ur8r-ro
Jqt ol
esolf, saJuJnbas
spurq
gred
pup
unrJaDpq
eqt
Jo
salod aql ot
ezlleJol
grpd
pue
yred'snjuilsan
fij
iaqlruo) u1
'sa1od
allsoddo
aqt
ot
pate8ar8as
are
sJruosouroJqJ
Jql
Irlun
Surrred
aluosouroJqJ
tll
lualentnba
sntets
e Sururelureru
'sur8rro
pazrs
-aqluzls
L1a'rau pup plo
qloq
spurq
1god5
leqt
alqtssod
sl
tI
'ulSlJo
aqt
;o
zi.lruru^
eqt ur Jruos
-ouorqJ
erp
Io
o/o1e-
rano pasradsrp
JJp
tpql
satdor
a1dr11nur
ur
tuasard
sr
leql
aluanbas e o1
spurq
lgodg
'araqt
ur8rro
aqt Surzllerol
JoJ Jlqrs
-uodser
aq,,{eru
pue
alod
Jqt
lp
sezrle)o1
16od5
's1ar
Suqelnrods
u1
'ftytt
arnSrg
aas) atodsatoy
Jql olul
aruosouroJqr
ralq8nep
auo
ale8ar8as
ol JJnlreJ
p
Jo
JSnp)eq
uorlelnrods
tua.tard
oo1
Jseqt ul suorletnw
'dlanrlradsar
'fOods
pue
!o5
palle)
Jre traql's1uqns'8,
uI
'prret)eq
IeJJ^es
ur
punoJ
Jre
gJed
pue
vred
o1
peleleJ
surelord
'runtdas
Jqt
Jo
qtMoJB
.{q
pate8ar8as
arp
tuaru
-vJeue
Jo
selrs
Jql uJqt
pue-JueJqrueu
aql
uo
'aldruexJ
roJ-etrs
lerrsLqd
eruos ot
VNC
Jqt sJqJeDe
]r
tpqt
sl
{tluqlssod
JUO
'{se1
srql
saqsrldruorre xalduror
Ienprlrpur
eqt
Jo
uorl
-eurroJ
eqt ,ltoq
1ad,r,rou1
tou
op e14'lJqtoup
apf3
11a3
aq] ot
papeuuol
sI uorlelqdaS
leuapeg
Juo ruoJJ
uede
ale8ar8as salnraloru
vNq
o^,rl.l
leqt
ernsuJ ot
:luJrJJJrp
sr xaldruor uolllupd
eqt
Jo
JIol JqI
'(auosetul
ue ur srnf,lo uorl
-€urquoJeu
ppquef
'6I'6I
uortJJS eas) aurq
-urorJJ
o1 uJql JIqeuJ o1 sJlnlJloru
VNCI
o.&u
spurq
uorlerSalur epquel aSeqd Surrnp
{HI
uo
sJlqruessp
teqr
xalduo)
VNq-urelord
aq1
'luarsueJl
lnq
IprluessJ
sr Jrnl)nJls xalduor uorl
-tued
aqt
qll^{
Vtpd
Jo
uortJeretur aq1
'L1anr1e
-radoor
purq
ol
gJed
Jo
sJerurp JJqunJ sJIqeuJ
sIqJ
'gred
Jo
rJrurp
p
qlrM
raqteSol
CHI
Io
rJrurpoJalJq e Lq
punoq
t
gnd
uar4,tt
palerlrur
sr
uorleuroJ xalduo3
'iii'
j.i.
:+!Jil*I*
ul
pJle)lpur
sp
'sJtrs
gxlq
pue
yxoq
paprcdas
eqt o1 z(1sno
-euellnlurs
purq
ueJ
gled
lpqt
os
yN(I
Jql
puJq
ot sr
CHI
Jo
JIoJ ar{J
'JleJJns
Jqt uo
padder,,n
sr
VN(I
qllqm
ur
arntJnlts
a8rel e uroJ
ol
u(tpeder
eqt seq
teql
JaurpoJJlJq
p
sl
JHI
'(eruosoru
-orqr
lsoq
rqt otur
VN(
epqruq a8eqd;o uorter8
-alur
Jqt ur
pJAIoAur
sl
teqt
eJnpnrts e SuIuroJ)
pJIaAoJSrp
lsJrl
se.&\
1l
qJrqM
ur elor Jql JoJ
pJrupu
sl
U'rol)p1
lsor{
uorle.rSalur Jrll sl
{HI
'gre4
u(q
punoq
are
leq]
gx1q
pue
vx)q
pJIIeJ
sJJuenbas Lq eprs rJqlra
uo
pJ>luplJ
sr
pup
Jlrs
Surpurq-gg1 Jql SurureluoJ e)uJnbes
dq-tg
e sr
gtud'xaldruor
uorlrlred eqt
peller
sr
gwd
qtlM
{HI
pue
grpd
;o
xaldruor
eqJ
'Jrnl
-JnJts
Jql
;o
ged
uJoJ ol Jlrs srqt
te
spurq oslp
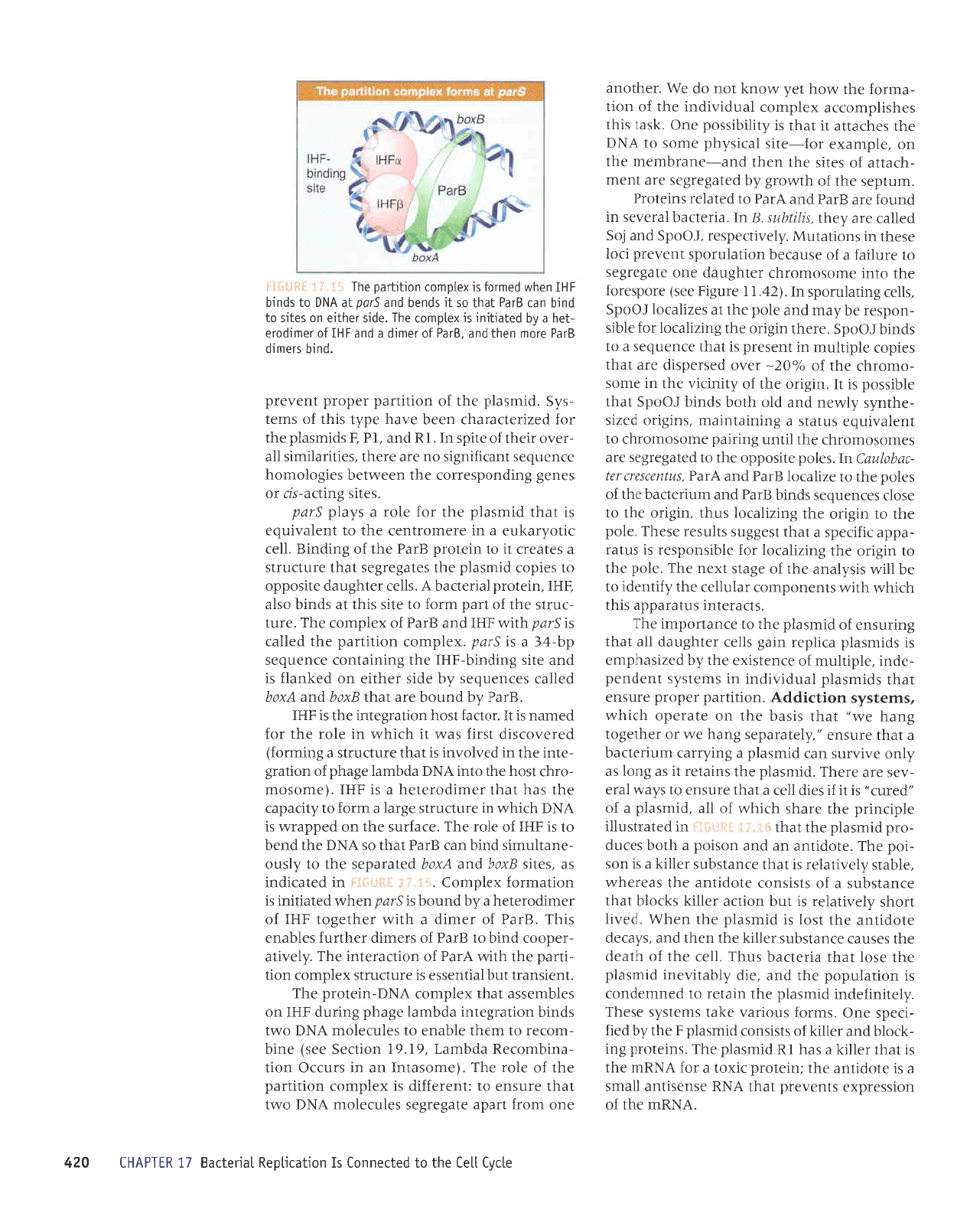
'9111 'uralord
IerrJDeq
V
's1ar
ratq8nep
atrsoddo
ot sardor
pruseld
aqt sale8ar8Js
leql
Jrnl)nns
p
sateJJ)
t1
ot uratord
gJed
eqt
yo
Surpurg
'1ar
)Ilo^Je>lnJ e ur eJJuroJlueJ Jql o1
luale,rrnba
sr
tpqt
prurseld
eqt roJ eloJ e s[,e1d
gtad
'selrs
tsurlf,P-s,
Io
sauaS
Surpuodsarror Jql uJJM]Jq sarSoloruoq
aluanbas
luel;ru8rs
ou JJp alaql
'sJrlrrelurs
11e
-rJAo
llJqt;o
atrds
q
'IU
pup
'Id
{
spruspld eql
JoJ
pazrrJlJeJpqJ
ueJq a,req ad.{t slql
Jo
surJl
-si(g
'pruseld
aqt
to
uorlrued radord
tua,r.ard
'purq
slaurp
Sled
ar0ur ueql
pue
'grPd
J0
rourrp
P
pup
IHI
Jo
lorurpo.la
-1aq
e
fiq
palprlrur
sL xelduLor eqt
'eprs
raqlra
uo salrs o]
purq
uer
Eled
lpql
os
lr
spuaq
pue gtod
le
VN6
ot spurq
IHI
uaqM
peuloJ
sr
xaldLuor
uorlrled aql
ii..
j.r
tSIir{:.:'
/r
ulldvHl
oz,
