Lefebvre A.H., Ballal D.R. Gas Turbine Combustion: Alternative Fuels and Emissions
Подождите немного. Документ загружается.

60 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
Equations 2.35 and 2.36 provide simple dimensionless relationships
between the quenching distance and the fuel drop size in the spray.
Essentially, they state that quenching distance is directly proportional to
drop size and is inversely proportional to the square root of gas pressure.
An increase in ϕ and a reduction in ρ
F
both reduce d
q
because they promote
evaporation by increasing the surface area of the fuel. Similarly, an increase
in B also accelerates evaporation, thereby decreasing d
q
.
Values of E
min
may be obtained for quiescent or low-turbulence mixtures
by inserting the calculated values of d
q
from Equation 2.35 or Equation 2.36
into the expression
Ec Td
min
.=
(
)
pA Astq
ρπ∆ 6
3
(2.37)
The results of such calculations are shown as solid curves in Figures 2.11
and 2.12. The very satisfactory level of agreement between theory and exper-
iment, as demonstrated in these gures, suggests that the model can predict
0.4
0.1
1
10
E
min
, mJ
100
0.6 0.8
Equivalence ratio
20
40
100
150
Quiescent mixtures
P = 100 kPa
B
st
= 1.5
eory
SMD, µm
1 1.2
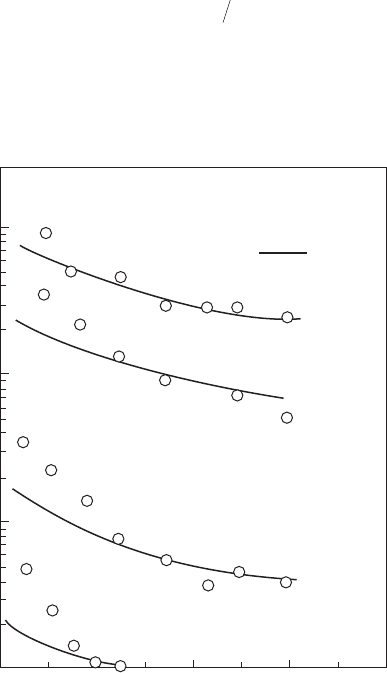
Figure 2.11
Minimum ignition energies of quiescent heavy fuel oil and air mixtures for various mean drop
sizes. P = 100 kPa, T
A
= 290 K. (From Ballal, D.R. and Lefebvre, A.H., Proceedings of the Royal
Society, London Series A, 364 (1717), 277–94, 1978.)
Combustion Fundamentals 61
with fair accuracy the effect of variations in fuel volatility, mean drop size,
and air pressure on minimum ignition energy. It also supports the basic
assumption of the model, namely, that over a wide range of test conditions
fuel evaporation is the rate-controlling step.
Although the above equations for d
q
in heterogeneous fuel–air mixtures
were derived for quiescent mixtures, they may be applied to owing mix-
tures in combustion systems without much loss of accuracy. This is because,
except for the very largest drops, most of the fuel spray is airborne, and the
relative velocity between the fuel drops and the surrounding air or gas is too
small to appreciably enhance either the rate of fuel evaporation or the rate of
heat loss from the spark kernel.
In a later study, Ballal and Lefebvre [45] extended the model described
above to include (1) the effects of nite chemical reaction rates, which are
10
4
1
10
E
min
, mJ
100
10
5
Pressure, Pa
φ = 0.65
U = 0
SMD = 60 µm
eory
Heavy fuel oil
Iso-octane
Gas oil
Diesel oil
2×10
5
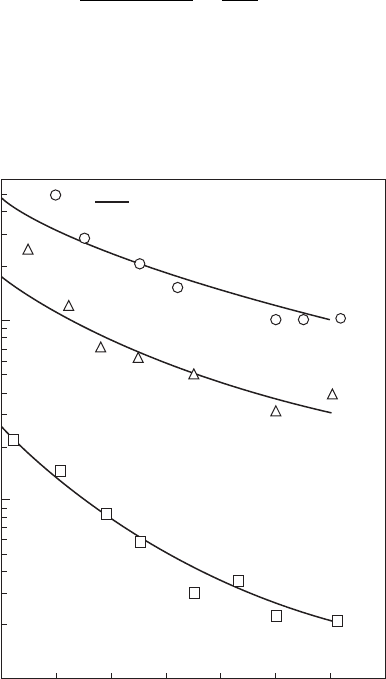
Figure 2.12
Effect of air pressure on minimum ignition energy. ϕ = 0.65, SMD = 60 µm, T
a
= 290 K, U = 0.
(From Ballal, D.R. and Lefebvre, A.H., Proceedings of the Royal Society, London Series A, 364 (1717),
277–94, 1978.)
62 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
known to be signicant for very well atomized fuels at low pressures and
low equivalence ratios; and (2) the presence of fuel vapor in the mixture
owing into the ignition zone. Thus, the model has general application to
both quiescent and owing mixtures of air with either gaseous, liquid, or
evaporated fuel or any combination of these fuels. Equations for quenching
distance were derived to cover all the conditions likely to be encountered
in practical combustion systems. Thus, for example, with chemical effects
included, Equation 2.35 becomes
d
D
BS
q
F
AstL
ln 1+
=
(
)
+
ρ
ρφ
α
32
2
2
0
10
..
.
5
(2.38)
The validity of the general model was tested experimentally for both qui-
escent and owing mixtures. Figure 2.13 shows measured values of E
min
plotted against equivalence ratio ϕ for quiescent mixtures of heavy fuel oil
0.4
1
10
100
E
min
, mJ
0.5 0.6 0.7
Equivalence ratio
P = 100 kPa
B
st
= 1.5
40
100
150
SMD,µm
Chemical effects included
0.8 0.9 1.0 1.1
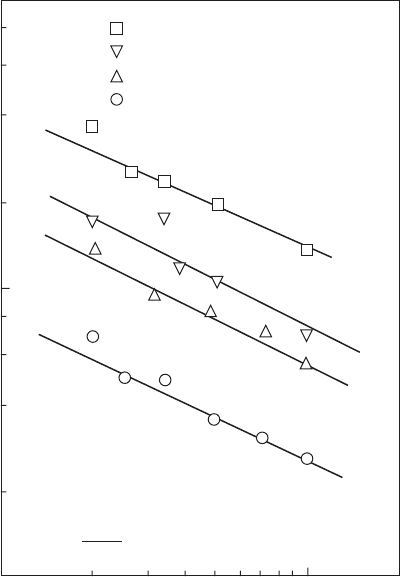
Figure 2.13
Improvements in correlation resulting from inclusion of chemical effects. Heavy fuel oil, U = 0.
(From Ballal, D.R. and Lefebvre, A.H., Eighteenth Symposium (International) on Combustion, The
Combustion Institute, Pittsburgh, PA, 1737–46, 1981.)
Combustion Fundamentals 63
and air. The gure shows that the general model provides a good t to the
data over a range of SMDs from 40 to 150 µm.
Figure 2.14 shows the inuence of pressure on E
min
for four different fuels
with a mean drop size of 60 µm when sprayed into a owing airstream.
Again, the excellent correlation achieved by including both chemical and
evaporation effects is apparent in this gure.
In general, where evaporative effects are dominant, Equations 2.36 and 2.37
indicate for both quiescent and owing mixtures that E
min
αP
–0.5
. However,
when chemical effects govern, Equations 2.30 and 2.32 show that E
min
αP
–2.0
for the extreme case where ame speed is independent of pressure. Thus,
for both stagnant and owing heterogeneous mixtures, the pressure expo-
nent of E
min
always lies between –0.5 and –2.0 and depends on the relative
Heavy fuel
Chemical effects included
400
200
E
min
, mJ
100
10
1
0.15 0.2 0.3 0.4
Pressure, Pa × 10
–5
0.6 0.8 1.0
i-octane
Gas oil
Diesel oil
Figure 2.14
Improvements in correlation resulting from inclusion of chemical effects. U = 15 m/s, ϕ = 0.65,
SMD = 60 µm. (From Ballal, D.R. and Lefebvre, A.H., Eighteenth Symposium (International) on
Combustion, The Combustion Institute, Pittsburgh, PA, 1737–46, 1981.)

64 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
importance of evaporative and chemical effects. In general, any change that
enhances the role of reaction kinetics, such as a reduction in pressure and/or
equivalence ratio, tends to increase the dependency of E
min
on pressure.
2.12 Spontaneous Ignition
Spontaneous ignition, or autoignition, is a process whereby a combustible
mixture undergoes a chemical reaction that leads to the rapid evolution of
heat in the absence of any concentrated source of ignition, such as a ame
or spark. In the lean-premix combustor, and other types of low-emissions
combustors where fuel and air are premixed before combustion, spontane-
ous ignition must be avoided at all costs because it could damage combustor
components and produce unacceptably high levels of pollutant emissions.
Spontaneous ignition delay may be dened as the time interval between
the creation of a combustible mixture, say by injecting fuel into a owing
airstream at high temperature, and the onset of ame. Ignition delay times
are often correlated using the Wolfer equation [46].
tP T
im
=
(
)
−
0434650
119
.exp ,
.
(2.39)
where t
i
is the ignition delay time in ms, P is the pressure in bars, and T
m
is
the initial mixture temperature in degrees K. To accommodate the effects
of equivalence ratio on ignition delay times, Equation 2.39 may be modied
and expressed in a more general form as
tAPERT
nm
im
=
(
)
−−
φ exp,
(2.40)
where A, n, and m are constants that are determined experimentally, P is
the pressure (usually expressed in atmospheres or bars), E is the activation
energy in cal/g mol, R is the gas constant (1.986 cal/g mol), and T
m
is the ini-
tial temperature of the fuel–air mixture in degrees K.
In view of their practical importance, measurements of spontaneous
ignition delay time have been conducted for many fuels over wide ranges
of ambient conditions and in a variety of test vehicles, including rapid-
compression machines, shock tubes, and continuous ow devices. The test
methods employed and the results obtained are described in reviews by
Mullins [47], Spadaccini and Te Velde [48], Goodger and Eissa [49], and
Lundberg [50].
Freeman, Cowell, and Lefebvre [51,52] used a continuous ow apparatus
to measure autoignition delay times. Twenty-ve equispaced fuel-injection
points ensured rapid mixing of gaseous fuel or fuel vapor with heated air at
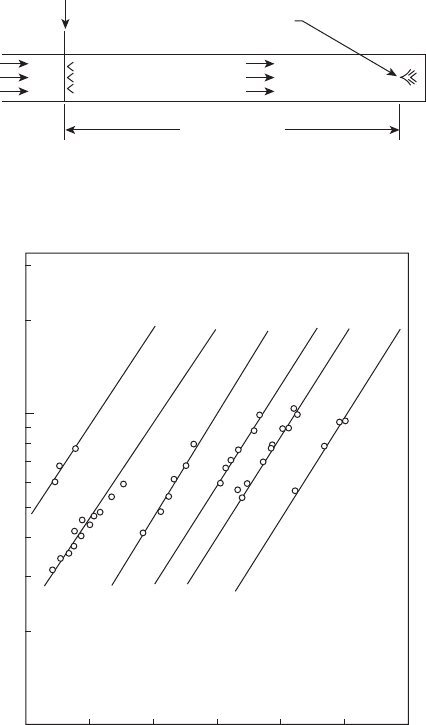
Combustion Fundamentals 65
entry to the test section. The concept is shown schematically in Figure 2.15,
where the ignition delay time is dened as the length L divided by the gas
velocity U. This method has the advantage that when spontaneous ignition
occurs, it does so under conditions that closely simulate those prevailing
in the premixing passages of advanced combustors. Some of the results
obtained for propane– and methane–air mixtures are shown in Figures 2.16
through 2.18.
Hot
air
U
Fuel/air
mixture
S. I. flame front
Delay length, L
Fuel
Figure 2.15
Basis of ignition delay time measurement technique. (Reprinted from Freeman, G. and
Lefebvre, A.H., Combustion and Flame, 58(2), 153–62, 1984. With permission from Elsevier, Inc.)
1.00
10
20
50
100
200
300
Delay time, ms
1.04 1.08 1.12
1000/T
m
, K
–1
1.16 1.20 1.24
Propane-air mixtures
φ = 0.4
2
1
35
7
10
Pressure, atmos
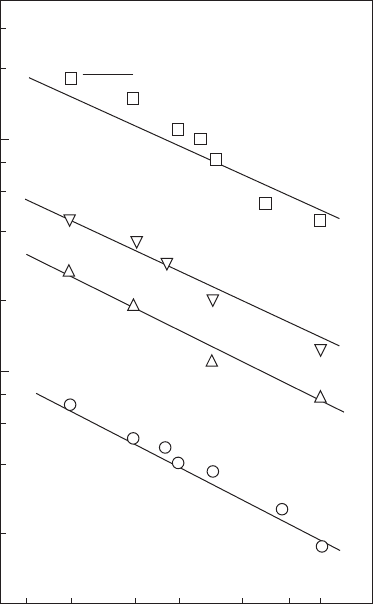
Figure 2.16
Inuence of pressure and temperature on ignition delay times of propane-air mixtures. (From
Cowell, L.H. and Lefebvre, A.H., SAE Paper 860068, 1986. With permission.)
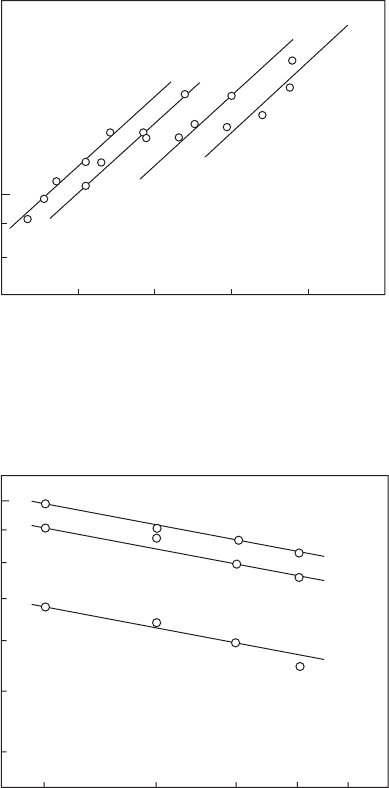
66 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
The form of Equation 2.40 suggests that a plot of ln t
i
vs. 1/T
m
should yield
a straight line with a positive slope, and this is borne out by the results pre-
sented in Figures 2.16 and 2.17. The values of E given by the slopes of the lines
in these gures are 38.2 kcal/g mol for propane and 25.0 kcal/g mol for meth-
ane. For kerosine (Jet A), the value of E was found to be 29.6 kcal/g mol.
1.00
70
100
Delay time, ms
200
Pressure, atm
78
9
10
Methane – air mixtures
φ = 0.4
1.02 1.04
1000/T
m
, K
–1
1.081.06 1.10
Figure 2.17
Inuence of pressure and temperature on ignition delay times of methane–air mixtures. (From
Cowell, L.H. and Lefebvre, A.H. SAE Paper 860068, 1986. With permission.)
0.2
40
50
60
70
80
Delay time, ms
90
100
0.3 0.4
Equivalence ratio, φ
Methane – air mixtures
T
m
= 1000 K
τα φ
–0.19
Pressure, atm
7
8
10
0.5 0.7
Figure 2.18
Inuence of mixture strength on ignition delay times of methane–air mixtures. (From Cowell,
L.H. and Lefebvre, A.H., SAE Paper 860068, 1986.)

Combustion Fundamentals 67
The inuence of pressure on t
i
is of great practical interest in view of the
continuing trend toward engines of higher pressure ratio. Its importance is
apparent from inspection of the experimental data plotted in Figures 2.16
and 2.17, which show a pronounced effect of pressure on t
i
. Analysis of these
and other data [53] led to values for n in Equation 2.40 of 1.2 for propane and
1.0 for both methane and kerosine (see Table 2.1).
There appears to be little agreement between different workers in regard
to the inuence of equivalence ratio on ignition delay time. Mullins [47]
observed no effect, whereas Ducourneau [54] and Spadaccini and Te Velde
[48] both found strong effects. Lefebvre et al. [53] examined the inuence of ϕ
on t
i
for several fuels and found in all cases that delay times were reduced by
an increase in equivalence ratio. Figure 2.18 is typical of the results obtained.
It shows for methane that t
i
αϕ
–0.19
. For propane and aviation kerosine, the
measured values for m were higher at 0.30 and 0.37, respectively.
The explanation for the marked lack of consistency between different
workers in regard to the inuence of ϕ on t
i
probably lies in the mode of
fuel injection. With liquid fuels, there is always the potential for stoichio-
metric combustion in regions close to the evaporating spray. Thus, measured
ignition delay times may be close to those for stoichiometric mixtures, even
though the average equivalence ratio of the mixture differs appreciably from
the stoichiometric value. Just how close will depend on the drop-size distri-
bution in the spray, because this governs the initial rate of fuel evaporation
and also the length of time that stoichiometric “streaks” of fuel–air mixture
can survive. The number of fuel-injection points is also important. In this
context, it is of interest to note that Tacina [55] obtained much more consis-
tent autoignition data with a single orice injector than with a 41-hole injec-
tor, which ostensibly should have provided a more uniform fuel–air mixture.
Presumably, this was because with a single injector the rate of fuel–air mix-
ing was so slow that the bulk of the prereactions leading up to the onset of
ignition took place in near-stoichiometric mixtures, regardless of the aver-
age equivalence ratio. With gaseous fuels, the inconsistencies associated
with slow fuel evaporation are no longer present, but the measured ignition
delay times are still very dependent on the time required for the fuel and air
to form a combustible mixture. As with liquid fuels, the longer the mixing
time, the closer the measured ignition delay times will approach stoichio-
metric values.
TABLe 2.1
Experimental values of constants in
Equation 2.40
Fuel E (kcal/g mol) n m
Propane 38.2 1.2 0.30
Methane 25.0 1.0 0.19
Kerosine 29.6 1.0 0.37
68 Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition
Another probable reason for the conicting evidence of the effect of
equivalence ratio on ignition delay time is that in continuous ow experi-
ments the fuel is almost invariably at a much lower temperature than the
hot airstream into which it is injected. This has the advantage of closely
simulating the actual engine situation but, from a fundamental viewpoint,
it has the drawback that any change in ϕ must also change the temperature
in the initial fuel–air mixing zone(s). As ignition delay time is exponentially
dependent on temperature, the effect of a small change in temperature in
these localized mixing zone(s) could be very pronounced and could largely
offset the effect of the corresponding change in ϕ on ignition delay time.
Note that the effect of this change in temperature on t
i
, will always be such
as to oppose the change in t
i
caused by the change in ϕ. The net result is
that measurements of t
i
, carried out in continuous ow devices will always
underpredict the effect of a change in ϕ on t
i
by an amount that depends on
the difference in temperature between the hot airstream and the injected
fuel gas or vapor.
Most analyses and equations for ignition delay time ignore the effects
of fuel vaporization, which is reasonable under conditions where the fuel
evaporation time is appreciably shorter than the mixing and reaction times.
However, it is important to bear in mind that spontaneous ignition delay
times are affected by both physical and chemical processes. For liquid fuels,
the physical delay is the time required to heat and vaporize the fuel drops
and to mix the fuel vapor in ammable proportions with the surrounding
air. The chemical delay is the time interval between the formation of a am-
mable mixture and the appearance of ame. Thus, the physical processes
are important in the early stages of spontaneous ignition, while in the later
stages the chemical processes become over-riding.
Rao and Lefebvre [56] have proposed a model for spontaneous ignition
that takes both chemical and physical effects into account and has general
application to both homogeneous and heterogeneous mixtures, including
situations where both fuel drops and fuel vapor are initially present. This
model leads to an equation where the ignition delay time, t
i
, is derived as the
sum of the times required for evaporation and chemical reaction. Calculated
values of t
i
from this equation show that fuel evaporation times are negligibly
small in comparison with chemical reaction times for well atomized, highly
volatile fuels, especially at conditions of low pressure and temperature. They
also show that fuel evaporation times become increasingly signicant with
increases in pressure and temperature.
When calculating ignition delay times for liquid fuels, it is customary to
disregard the fuel evaporation time. As discussed above, this is permissible
for well-atomized volatile fuels injected into airstreams at relatively low pres-
sures. However, it is important to recognize that for certain ultralow NO
x
com-
bustors (e.g., catalytic or lean premixed prevaporized (LPP)) when operating at
high-power conditions corresponding to high air pressures and temperatures,
the fuel evaporation time could be so long in relation to the chemical delay

Combustion Fundamentals 69
time that spontaneous ignition of the initial fuel vapor might occur before the
remainder of the fuel spray has had time to evaporate. Very ne fuel atomiza-
tion and rapid vapor–air mixing will be required to combat this problem.
In summary, autoignition data are very apparatus-dependent and, in par-
ticular, very fuel injector-dependent. Considerable caution should be exer-
cised in comparing and selecting autoignition data and in no circumstances
should experimentally derived equations for t
i
, be extrapolated to pressures
and temperatures outside the range of their experimental verication. Such
extrapolations could lead to erroneous results because differences in reaction
routes may occur over different levels of temperature and pressure. When
liquid fuels are injected into air at high pressures and temperatures, very
ne atomization is needed to promote rapid vaporization, thereby reducing
the risk of spontaneous ignition in the fuel preparation zone.
2.13 Flashback
An intrinsic feature of all premixed-fuel combustion systems is a tendency
toward ashback. Flashback occurs when the ame travels upstream from
the combustion zone into the premixing sections of the combustor. This
upstream propagation of ame takes place whenever the ame speed exceeds
the approach ow velocity.
Three main types of ashback have been identied: (1) ashback occur-
ring in the free stream, (2) ashback occurring through the low-velocity
ow in the boundary layer along the walls of the premixing section, and
(3) ashback driven by combustion-induced vortex breakdown (CIVB) in
swirl-stabilized combustors. Any mechanism may involve homogeneous
and/or heterogeneous reactions.
The most obvious free-stream mechanism would be the occurrence of
ashback due to a ow reversal in the bulk ow through the combustor.
This ow reversal could be a result of compressor surge or combustion insta-
bility. Flashback can also occur in the absence of ow reversal if the tur-
bulent ame speed through the gas in a premixing section is greater than
the local bulk velocity. Lean combustion tends to reduce ame speeds, but
other factors associated with the engine cycle, such as high temperatures,
pressures, and turbulence levels, and preignition reactions in the gas due
to appreciable residence times at high temperature levels, cause increased
ame speed. Therefore, ame speeds may be sufciently high to necessitate
increasing the minimum allowable velocity in premix-prevaporize sections
to fairly high levels to avoid disturbances to the combustion process.
The boundary-layer mechanism involves ashback through retarded ow
in a boundary layer. Important relevant parameters include the wall tem-
perature and temperature distribution, and the boundary-layer structure,
