Lallart M. Ferroelectrics: Characterization and Modeling
Подождите немного. Документ загружается.

First-principles Study of ABO
3
: Role of the B–O Coulomb Repulsions for Ferroelectricity and Piezoelectricity 5
a. Note that the fully optimized sturucture of BaTiO
3
is tetragonal with the Ti3spd4s PP,
whereas it is cubic (Pm
¯
3m) with the Ti3d4s PP. As shown in Fig. 1(a) and 1(b), c/a and δ
Ti
show significantly different results for a 3.7 whereas they show almost the same results for
a
3.7 , for both Ti PPs. This result suggests that the optimized results of ABO
3
with smaller
lattice parameters, e.g., under high pressure (Bévillon et al., 2007), are almost independent of
the choice of PP.
㻔㼍㻕 䠄㼎䠅
㻻
㼦
㻻
㼦
㼀㼕
㻻
㼤
㼀㼕 㻻
㼤
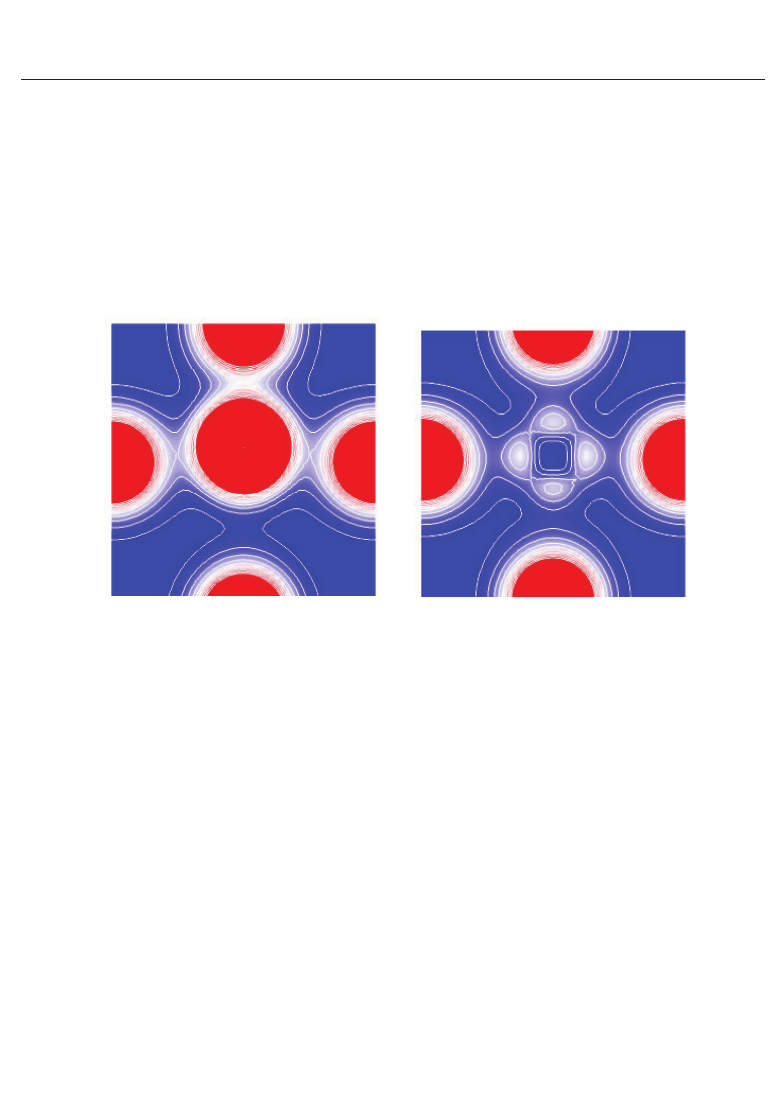
Fig. 3. Two-dimentional electron-density contour map on the xz-plane for tetragonal BaTiO
3
:
(a) with the Ti3spd4s PP, and (b) with the Ti3d4s PP. The optimized calculated results with a
fixed to be 3.8 are shown in both figures. The electron density increases as color changes from
blue to red via white. Contour curves are drawn from 0.4 to 2.0 e/
3
with increments of
0.2 e/
3
(Miura et al., 2010a).
The calculated results shown in Fig. 1 suggest that the explicit treatment of T i 3s and 3p
semicore states is essential to the appearance of ferroelectric states in BaTiO
3
. In the following,
the author investigates the role of Ti 3s and 3p states for ferroelectricity from two viewpoints.
One viwpoint concerns hybridizations between Ti 3s and 3p states and other states. Figure 2
shows the total density of states (DOS) of tetragonal BaT iO
3
with two Ti PPs. Both results are
in good agreement with previous calculated results (Chen et al., 2004; Khenata et al., 2005)
by the full-potential linear augmented plane wave (FLAPW) method. In the DOS with the
Ti3spd4s PP, the energy “levels", not bands, of Ti 3s and 3p states, are located at
−2.0 Hr
and
−1.2 Hr, respectively. This result suggests that the Ti 3s and 3p orbitals do not make any
hybridizations but only give Coulomb repulsions with the O orbitals as well as the Ba orbitals.
In the DOS with the Ti3d4s PP, on the other hand, the energy levels of Ti 3s and 3p states are
not shown because Ti 3s and 3p states were treated as the core charges. This result means that
the Ti 3s and 3p orbitals cannot even give Coulomb repulsions with the O orbitals as well as
the Ba orbitals.
Another viwpoint is about the Coulomb repulsions between Ti 3s and 3p
x (y)
states and
O
x (y)
2s and 2p
x (y)
states in tetragonal BaTiO
3
. Figures 3(a) and 3(b) show two-dimentional
electron-density contour map on the xz-plane for tetragonal BaTiO
3
with the Ti3spd4s PP, and
that with the Ti3d4s PP, respectively. These are the optimized calculated results with a fixed
399
First-Principles Study of ABO
3
: Role of the
B–O Coulomb Repulsions for
Ferroelectricity and Piezoelectricity
6 Ferroelectrics
㻔㼍㻕㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻌㻔㼎㻕
㼦
㻻
㼦
㻻
㼦
㻻
㼦
㻻
㼦
㻞㼟
㻞㼜
㼦
㼤
㻻
㼤
㻻
㼦
㻟㼜
㼤
㻞㼜
㼤
㻟㼟
㼀㼕
㻟㼜
㼦
㻞㼟
㻯㼛㼡㼘㼛㼙㼎
㼞㼑㼜㼡㼘㼟㼕㼛㼚
㼤
㻻
㼤
㻻
㼦
㻟㼜
㼤
㻞㼜
㼤
㻟㼟
㼀㼕
㻟㼜
㼦
㻞㼟
㻯㼛㼡㼘㼛㼙㼎
㼞㼑㼜㼡㼘㼟㼕㼛㼚
㼤
㼀㼕
㻻
㼤
㼦
㻟㼜
㼤
㻞㼜
㼤
㻟㼟
㻯㼛㼡㼘㼛㼙㼎㻌㼞㼑㼜㼡㼘㼟㼕㼛㼚
㻞㼟
㼤
㼀㼕
㻻
㼤
㼦
㻟㼜
㼤
㻞
㼤
㻞㼜
㼦
㻟㼜
㼦
㼞㼑㼜㼡㼘㼟㼕㼛㼚㼞㼑㼜㼡㼘㼟㼕㼛㼚
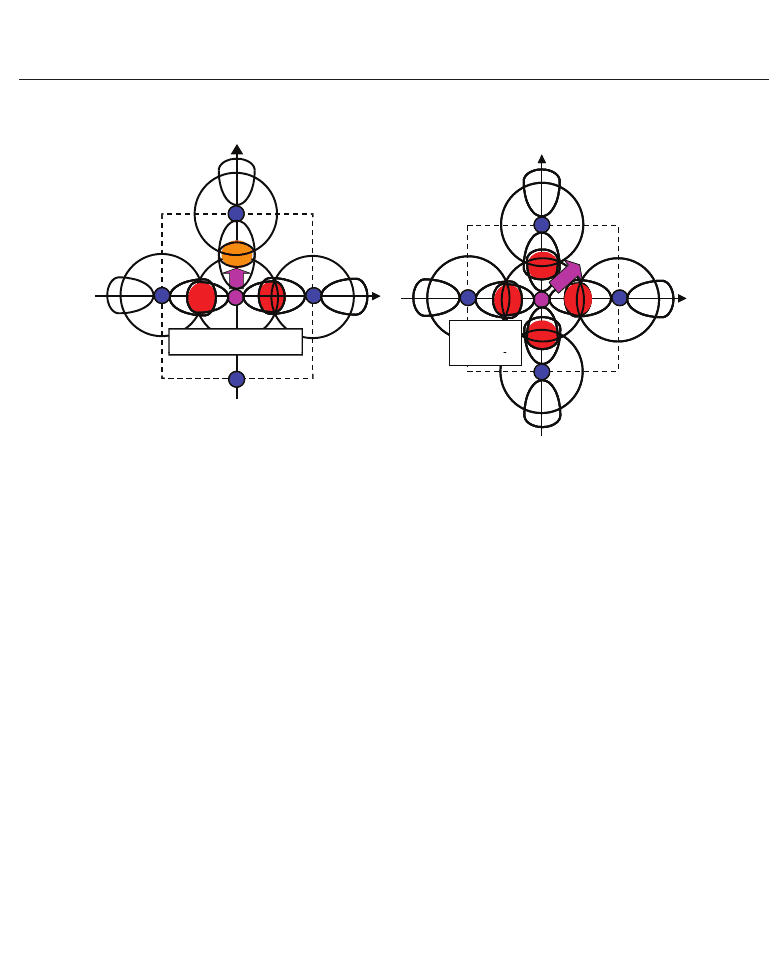
Fig. 4. Illustrations of the proposed mechanisms for the Coulomb repulsions between Ti 3s
and 3p states and O 2s and 2p states in BaTiO
3
: (a) anisotropic Coulomb repulsions between
Ti 3s and 3p
x (y)
states and O
x (y)
2s and 2p
x (y)
states, and between Ti 3s and 3p
z
states and
O
z
2s and 2p
z
states, in the tetragonal structure. (b) isotropic Coulomb repulsions between
Ti 3s and 3p
x (y)(z)
states and O
x (y)(z)
2s and 2p
x (y)(z)
states, in the rhombohedral
structure (Miura et al., 2010a).
to be 3.8 , and the electron density in Fig. 3(a) is quantitatively in good agreement with the
experimental result (Kuroiwa et al., 2001). The electron density between Ti and O
x
ions in
Fig. 3(a) is larger than that in Fig. 3(b), which suggests that Ti ion displacement is closely
related to the Coulomb repulsions between Ti 3s and 3p states and O 2s and 2p states along
the [001] axis (the z-axis in this case).
The present discussion of the Coulomb repulsions is consistent with the previous reports. A
recent soft mode investigation (Oguchi et al., 2009) of BaTiO
3
shows that Ba ions contribute
little to the appearance of T i ion displacement along the [001] axis. This result suggests that Ti
ion displacement is closely related to the structural distortion of TiO
6
octahedra. In the present
calculations, on the other hand, the only difference between BaTiO
3
with the Ti3spd4s PP
and with the Ti3d4s PP is the difference in the expression for the Ti 3s and 3p states, i.e.,
the explicit treatment and including core charges. However, our previous calculation (Miura
& Tanaka, 1998) shows that the strong Coulomb repulsions between Ti 3s and 3p
z
states and
O
z
2s and 2p
z
states do not favour Ti ion displacement along the [001] axis. This result suggests
that the Coulomb repulsions between T i 3s and 3p
x (y)
states and O
x (y)
2s and 2p
x (y)
states
would contribute to Ti ion displacement along the [001] axis, and the suggestion is consistent
with a recent calculation (Uratani et al., 2008) for PbTiO
3
indicating that the tetragonal and
ferroelectric structure appears more favourable as the a lattice parameter decreases.
Considering the above investigations, the author proposes the mechanism of Ti ion
displacement as follows: Ti ion displacement along the z-axis appears when the Coulomb
repulsions between Ti 3s and 3p
x (y)
states and O
x (y)
2s and 2p
x (y)
states, in addition to the
dipole-dipole interaction, overcome the Coulomb repulsions between Ti 3s and 3p
z
states and
O
z
2s and 2p
z
states (Miura & Tanaka, 1998). An illustration of the Coulomb repulsions is
shown in Fig. 4(a). In fully optimized BaTiO
3
with the Ti3spd4s PP, the Ti ion can be displaced
due to the above mechanism. In fully optimized BaT iO
3
with the Ti3d4s PP, on the other
㻔㼍㻕 㻔㼎㻕
400
Ferroelectrics - Characterization and Modeling
First-principles Study of ABO
3
: Role of the B–O Coulomb Repulsions for Ferroelectricity and Piezoelectricity 7
hand, the Ti ion cannot be displaced due to the weaker Coulomb repulsions between Ti and
O
x (y)
ions. However, since the Coulomb repulsion between Ti and O
z
ions in BaTiO
3
with the
Ti3d4s PP is also weaker than that in BaTiO
3
with the Ti3spd4s PP, the Coulomb repulsions
between between Ti and O
x (y)
ions in addition to the log-range force become comparable
to the Coulomb repulsions between Ti and O
z
ions both in Ti PPs, as the lattice parameter
a becomes smaller. The above discussion suggests that the hybridization between Ti 3d and
O
z
2s and 2p
z
stabilizes Ti ion displacement, but contribute little to a driving force for the
appearance of Ti ion displacement.
It seems that the above proposed mechanism for tetragonal BaTiO
3
can be applied to the
mechanism of Ti ion displacement in rhombohedral BaTiO
3
, as illustrated in Fig. 4(b). The
strong isotropic Coulomb repulsions between Ti 3s and 3p
x (y)(z)
states and O
x (y)(z)
2s and
2p
x (y)(z)
states yield T i ion displacement along the [111] axis. On the other hand, when
the isotropic Coulomb repulsions are weaker or stronger, the Ti ion cannot be displaced and
therefore it is favoured for the crystal structure to be cubic.
㻜㻚㻟
㻜㻚㻟㻡
㻝
㻝㻚㻞
㻝㻚㻠
㻔㼍㻕 㻔㼎㻕
㻾㻟㼙㻌㻮㼍㼀㼕㻻
㻟
㻦㻌㻥㻜㻙䃐㻌㼢㻚㼟㻚㻌㼂㻛㼂
㼞㼔㼛㼙㼎㼛
㻾㻟㼙㻌㻮㼍㼀㼕㻻
㻟
㻦㻌䃓
㼀㼕
㼢㻚㼟㻚㻌㼂㻛㼂
㼞㼔㼛㼙㼎㼛
㼓
㻚㻕
㻜
㻜㻚㻜㻡
㻜㻚㻝
㻜㻚㻝㻡
㻜㻚㻞
㻜㻚㻞㻡
㻜㻤
㻜㻥
㻝
㻝㻝
㻝㻞
㻝㻟
㻝㻠
㻜
㻜㻚㻞
㻜㻚㻠
㻜㻚㻢
㻜㻚㻤
㻝
㻜㻤
㻜㻥
㻝
㻝㻝
㻝㻞
㻝㻟
㻝㻠
䃓
㼀㼕
㻔䊅㻕
㻥㻜㻌䇵 䃐㻌㻔㼐㼑
㼓
㻜
㻚
㻤
㻜
㻚
㻥
㻝
㻝
㻚
㻝
㻝
㻚
㻞
㻝
㻚
㻟
㻝
㻚
㻠
㻜
㻚
㻤
㻜
㻚
㻥
㻝
㻝
㻚
㻝
㻝
㻚
㻞
㻝
㻚
㻟
㻝
㻚
㻠
㼂㻛㼂
㼞㼔㼛㼙㼎㼛
㼂㻛㼂
㼞㼔㼛㼙㼎㼛
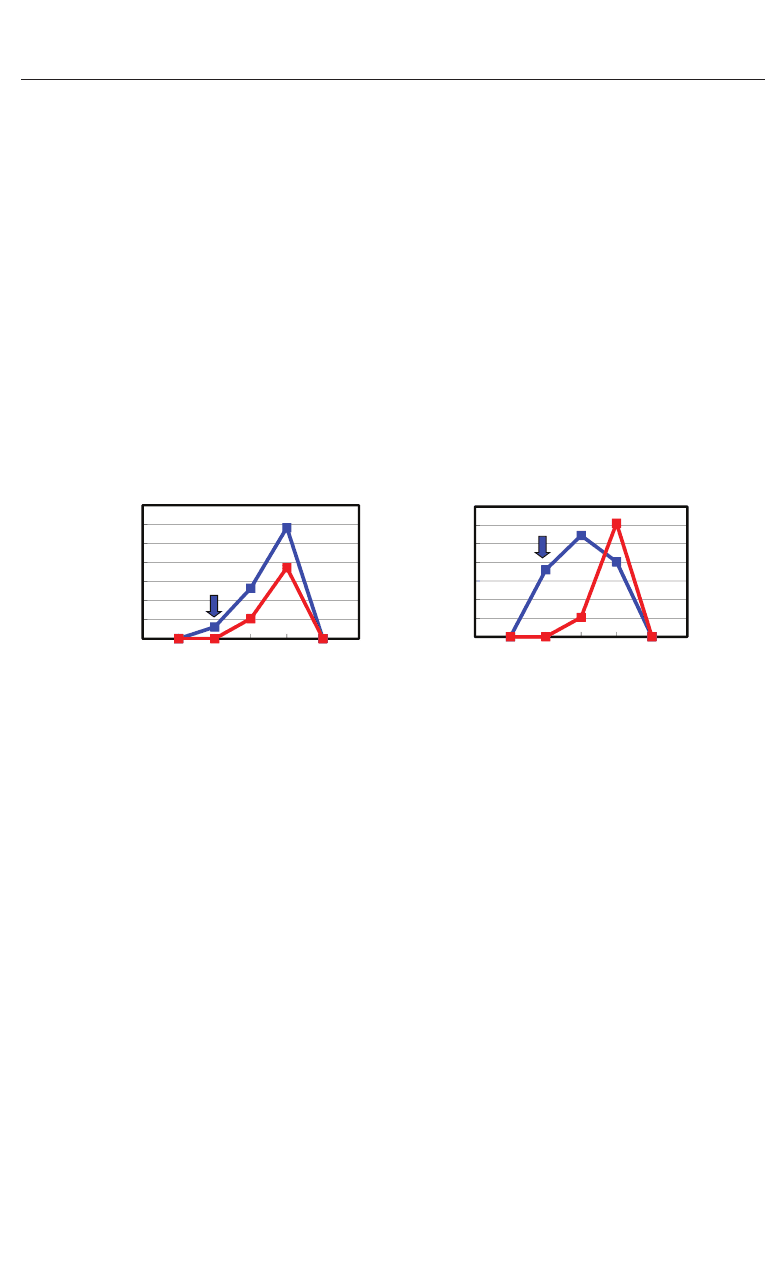
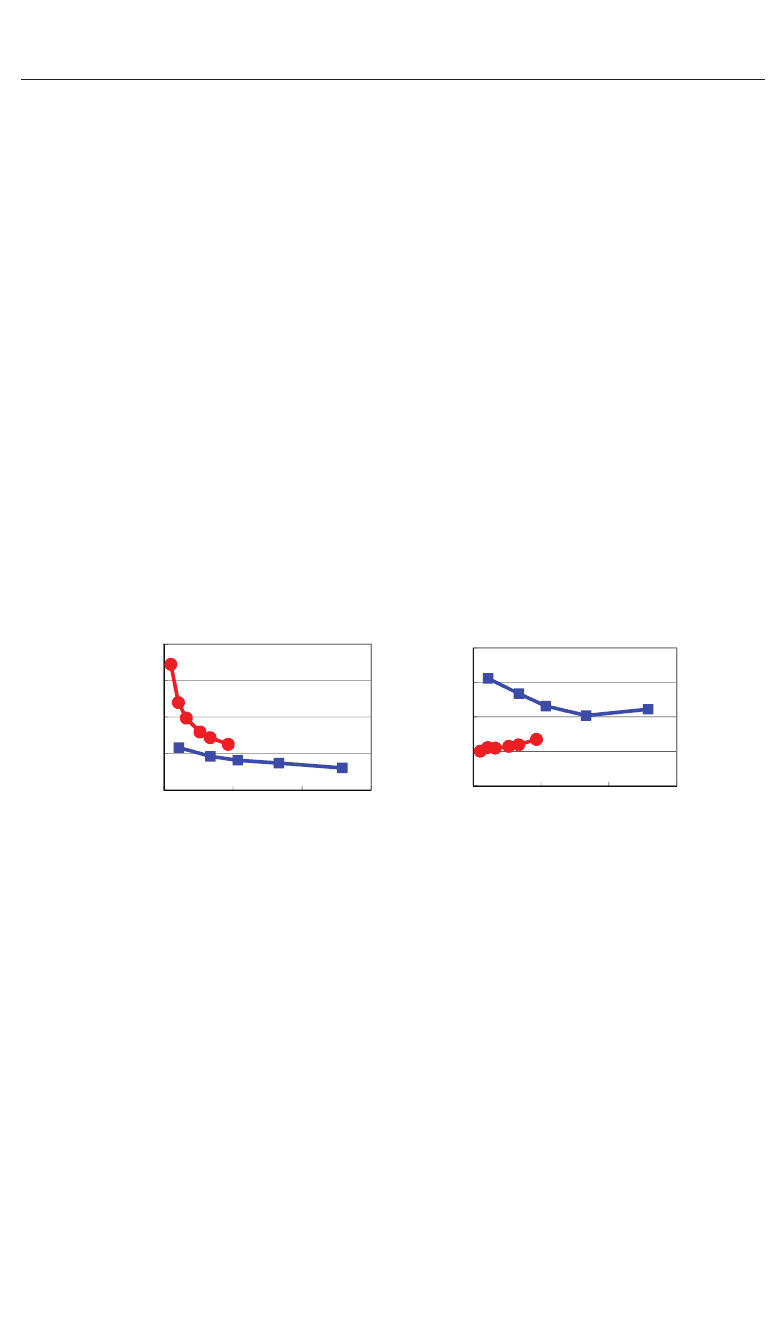
Fig. 5. Optimized calculated results as a function of the fixed volumes of the unit cells in
rhombohedral BaTiO
3
: (a) 90−α degree and (b) δ
Ti
to the [111] axis. Blue lines correspond to
the results with the Ti3spd4s PP, and red lines correspond to those with the T i3d4s PP.
V
rhombo
denote the volume of the fully optimized unit cell with the Ti 3spd4s PP. Results with
arrows are the fully optimized results, and the other results are those with all the inner
coordinations optimized for fixed volumes of the unit cells (Miura et al., 2010a).
Let us investigate the structural properties of rhombohedral BaTiO
3
. Figures 5(a) and 5(b)
show the optimized results of the 90
−α degree and δ
Ti
as a function of fixed volumes of
the unit cells in rhombohedral BaTiO
3
, respectively, where α denotes the angle between
two lattice vectors. In these figures, α denotes the angle between two crystal axes of
rhombohedral BaTiO
3
,andδ
Ti
denotes the value of the Ti ion displacement along the [111]
axis. Results with arrows are the fully optimized results; V
rhombo
denote the volume of the
fully optimized unit cell with the Ti 3spd4s PP. The other results are those with all the inner
coordinations optimized with fixed volumes of the unit cells. The proposal mechanisms about
the Coulomb repulsions seem to be consistent with the calculated results shown in Fig. 5: For
V/V
rhombo
0.9 or 1.3, the isotropic Coulomb repulsions are weaker or stronger, and the
Ti ion cannot be displaced along the [111] axis and therefore the crystal structure is cubic for
both Ti PPs. For 0.9
V/V
rhombo
1.3, on the other hand, the isotropic Coulomb repulsions
401
First-Principles Study of ABO
3
: Role of the
B–O Coulomb Repulsions for
Ferroelectricity and Piezoelectricity
8 Ferroelectrics
are strong enough to yield Ti ion displacement for both Ti PPs. However, since the magnitude
of the isotropic Coulomb repulsion is different in the two Ti PPs, the properties of the 90
−α
degree and δ
Ti
are different quantitatively.
3.2 Role of the Ti–O Coulomb repulsions for piezoelectric SrTiO
3
and BaTiO
3
As discussed in the previous subsection, the Coulomb repulsions between Ti 3s and 3p
x (y)
states and O
x (y)
2s and 2p
x (y)
states have an important role in the appearance of the
ferroelectric state in tetragonal BaTiO
3
. In this subsection, the author discusses the role of
the Ti–O Coulomb repulsions for piezoelectric SrTiO
3
and BaTiO
3
.
㻜 㻢
㻜㻚㻤㻌
㻝㻚㻜㻌
㼙
㼊㻞㻕
㻔㼍㻕 㻔㼎㻕
㻝㻚㻞㻜㻌
㻝㻚㻟㻜㻌
㼙
㻞
㻕
㼏㻛㼍㻌㼢㻚㼟㻚㻌㼍
㻼
㻟
㼢㻚㼟㻚㻌㼍
㻮㼍㼀㼕㻻
㻟
㻮㼍㼀㼕㻻
㻟
㻜㻚㻜㻌
㻜㻚㻞㻌
㻜㻚㻠㻌
㻜
㻚
㻢
㻌
㻟㻚㻢㻌 㻟㻚㻣㻌 㻟㻚㻤㻌 㻟㻚㻥㻌 㻠㻚㻜㻌
㻼㻟㻌㻔㻯㻛
㼙
㻔㻭㻕
㻝㻚㻜㻜㻌
㻝㻚㻝㻜㻌
㻟㻚㻢㻌 㻟㻚㻣㻌 㻟㻚㻤㻌 㻟㻚㻥㻌 㻠㻚㻜㻌
㼏㻛㼍
㼍 㻔㻭㻕
㻼
㻟
㻔㻯㻛
㼙
㻿㼞㼀㼕㻻
㻟
㻿㼞㼀㼕㻻
㻟
㼏㻛㼍㻌
㼍㻌
㻔㻭㻕
㼍
㻌
㻔㻭㻕
㼍㻌㻔䊅㻕 㼍㻌㻔䊅㻕
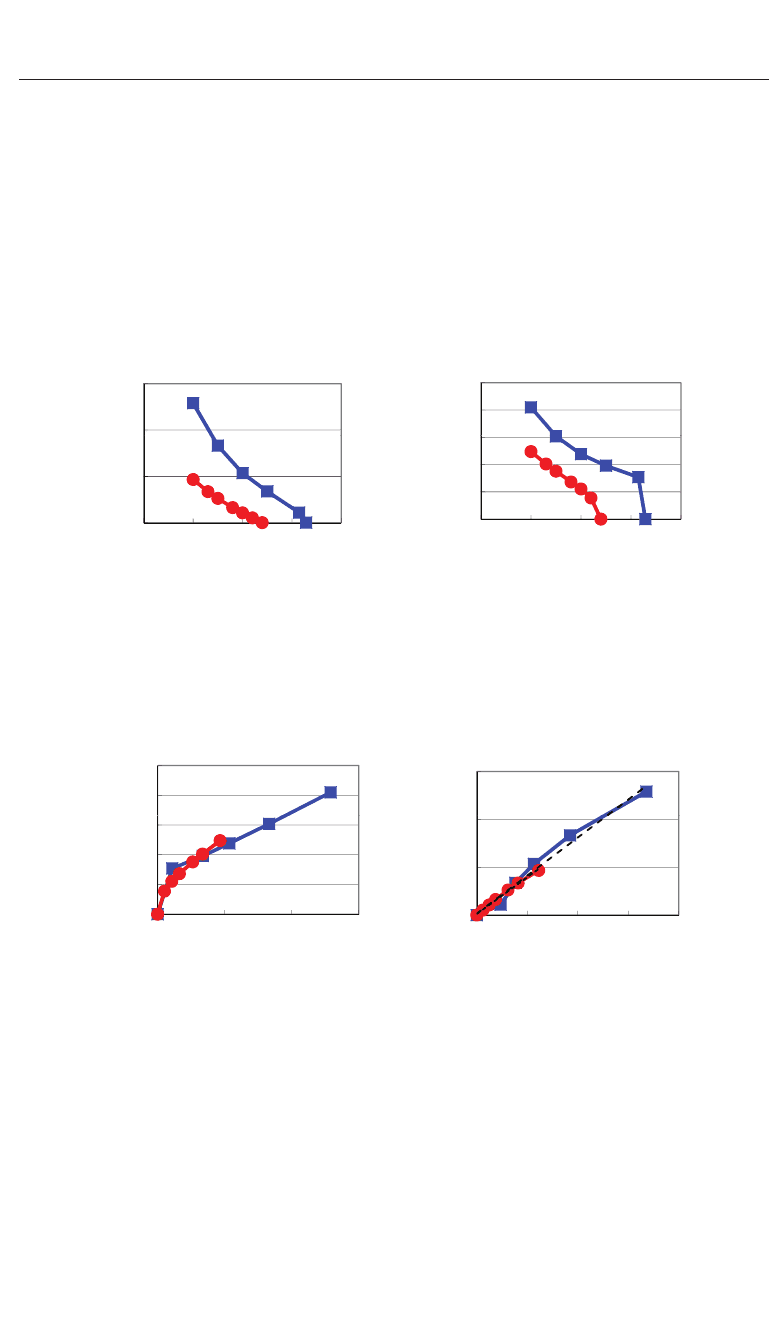
Fig. 6. Optimized calculated results as a function of a lattice parameters in compressive
tetragonal SrTiO
3
and BaTiO
3
:(a)c/a ratio and (b) P
3
, i.e., spontaneous polarization along
the [001] axis (Furuta & Miura, 2010).
㻜㻚㻤㻌
㻝㻚㻜㻌
㻕
㻜 㻞㻜
㻜㻚㻟㻜㻌
㻔㼍㻕 㻔㼎㻕
㻼
㻟
㼢㻚㼟㻚㻌㼏㻛㼍
㻕
㻮㼍㼀㼕㻻
㻟
㻮㼍㼀㼕㻻
㻟
㼏㻛㼍㻌䇵 㻝㻌㼢㻚㼟㻚㻌㻼
㻟
㻞
㻜㻚㻜㻌
㻜㻚㻞㻌
㻜㻚㻠㻌
㻜㻚㻢㻌
㻝㻚㻜㻜
㻝㻚㻝㻜
㻝㻚㻞㻜
㻝㻚㻟㻜
㻼㻟㻌㻔㻯㻛㼙㼊㻞
㻕
㻜㻚㻜㻜㻌
㻜㻚㻝㻜㻌
㻜
㻚
㻞㻜
㻌
㻜 㻜
㻜 㻞
㻜 㻠
㻜 㻢
㻜 㻤
㼏㻛㼍㻌㻙 㻝
㻼
㻟
㻔㻯㻛㼙
㻞
㻕
㻿㼞㼀㼕㻻
㻟
㻟
㻿㼞㼀㼕㻻
㻟
㼏㻛㼍㻌㻙 㻝
㻝㻚㻜㻜
㻌
㻝㻚㻝㻜
㻌
㻝㻚㻞㻜
㻌
㻝㻚㻟㻜
㻌
㼏㻛㼍
㻜
㻚
㻜
㻌
㻜
㻚
㻞
㻌
㻜
㻚
㻠
㻌
㻜
㻚
㻢
㻌
㻜
㻚
㻤
㻌
㻼㻟㼊㻞㻌㻔㻯㼊㻞㻛㼙㼊㻠㻕
㻼
㻟
㻞
㻔㻯
㻞
㻛㼙
㻠
㻕
Fig. 7. (a) P
3
as a function of c/a ratios, and (b) c/a ratio as a function of P
2
3
.Thesevaluesare
derived from the calculated results as shown in Figs. 6(a) and (b). Dotted and dashed lines in
Fig. 7(b) serve as visual guides for SrTiO
3
and BaTiO
3
, respectively (Furuta & Miura, 2010).
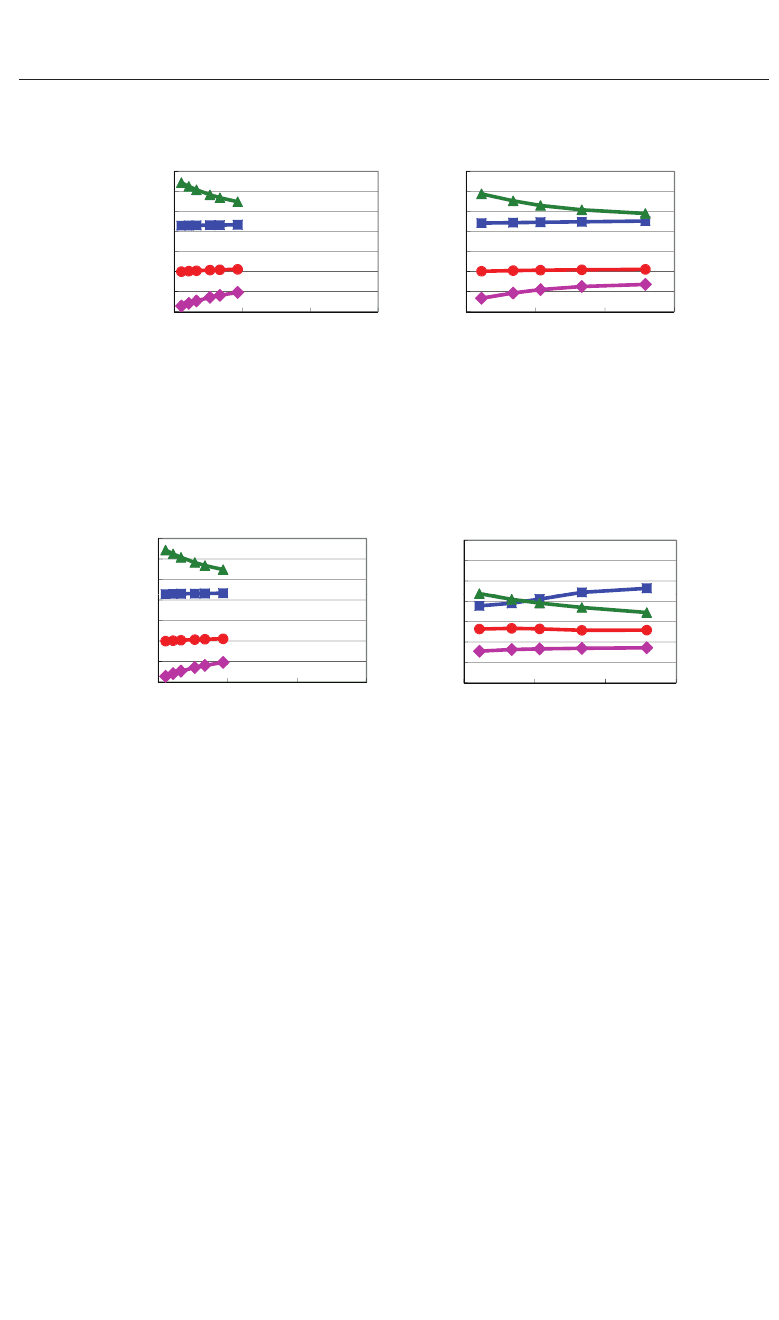
Figures 6(a) shows the optimized results for the ratio c/a as a function of the a lattice
parameters in tetragonal SrTiO
3
and BaTiO
3
. These results are the fully optimized results
and the results with the c lattice parameters and all the inner coordinations optimized for
㻜㻚㻡㻌
㻝㻚㻜㻌
㻕
㻔㼍㻕 㻔㼎㻕
㻮㼍㼀㼕㻻
㻟
㻞
㻕
㼑
㻟㻝
㼢㻚㼟㻚㻌㼏㻛㼍
㻝㻞㻚㻜㻌
㻝㻢㻚㻜㻌
㻞㻕
㻞
㻕
㼑
㻟㻟
㼢㻚㼟㻚㻌㼏㻛㼍
㻿㼞㼀㼕㻻
㻟
㻙㻝㻚㻜㻌
㻙㻜㻚㻡㻌
㻜㻚㻜㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㼑㻟㻝㻌㻔㻯㻛㼙㼊
㻿㼞㼀㼕㻻
㻟
㼑
㻟㻝
㻔㻯㻛
㻜㻚㻜㻌
㻠㻚㻜㻌
㻤㻚㻜㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㼑㻟㻟㻌㻔㻯㻛㼙㼊
㼑
㻟㻟
㻔㻯㻛
㻮㼍㼀㼕㻻
㻟
㼏㻛㼍
㼏㻛㼍
402
Ferroelectrics - Characterization and Modeling
First-principles Study of ABO
3
: Role of the B–O Coulomb Repulsions for Ferroelectricity and Piezoelectricity 9
fixed a. The fully optimized parameters of SrTiO
3
( a = 3.84 : cubic) and BaTiO
3
( a =3.91
and c = 4.00 : tetragonal) are within 2.0% in agreement with the experimental results in room
temperature. Figures 6(b) shows the evaluated results for P
3
as a function of the a lattice
parameters in tetragonal SrTiO
3
and BaTiO
3
,whereP
3
, which is evaluated by eq. (2), denotes
the spontaneous polarization along the [001] axis. Note that the tetragonal and ferroelectric
structures appear even in SrTiO
3
when the fixed a lattice parameter is compressed to be
smaller than the fully-optimized a lattice parameter. As shown in Figs. 6(a) and 6(b), the
tetragonal and ferroelectric structure appear more favorable as the fixed a lattice parameter
decreases, which is consistent with previous calculated results (Miura et al., 2010a; Ricinschi
et al., 2006; Uratani et al., 2008). The results would be due to the suggestion discussed in the
previous section that the large Coulomb repulsion of Ti–O bondings along the [100] axis (and
the [010] axis) is a driving force of the displacement of Ti ions along the [001] axis, i.e., the large
Coulomb repulsion along the [100] axis (and the [010] axis) is essential for the appearance of
the tetragonal structure. Figure 7(a) shows the relationship between P
3
and the ratio c/a,
where P
3
and c/a are derived from the calculated results shown in Figs. 6(a) and 6(b). The
property of BaTiO
3
in Fig. 7(a) is in qualitatively agreement with a previous calculational
result (Ricinschi et al., 2006). Figure 7(b) shows the relationship between the ratio c/a and
P
2
3
.Notethatc/a − 1isproportionaltoP
2
3
with alomost the same coefficients in both SrTiO
3
and BaTiO
3
. Clearly, the ratio c/a is a good parameter in both tetragonal SrTiO
3
and BaTiO
3
with in-plane compressive stress. Therefore, in the following, the author uses the ratio c/a as
a parameter for the investigations of the piezoelectric properties.
㻜㻚㻡㻌
㻝㻚㻜㻌
㻞
㻕
㻔㼍㻕 㻔㼎㻕
㻮㼍㼀㼕㻻
㻟
㼙
㻞
㻕
㼑
㻟㻝
㼢㻚㼟㻚㻌㼏㻛㼍
㻝㻞㻚㻜㻌
㻝㻢㻚㻜㻌
㻞㻕
㼙
㻞
㻕
㼑
㻟㻟
㼢㻚㼟㻚㻌㼏㻛㼍
㻿㼞㼀㼕㻻
㻟
㻙㻝㻚㻜㻌
㻙㻜㻚㻡㻌
㻜㻚㻜㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㼑㻟㻝㻌㻔㻯㻛㼙㼊
㻞
㻿㼞㼀㼕㻻
㻟
㼑
㻟㻝
㻔㻯㻛
㼙
㻜㻚㻜㻌
㻠㻚㻜㻌
㻤㻚㻜㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㼑㻟㻟㻌㻔㻯㻛㼙㼊
㼑
㻟㻟
㻔㻯㻛
㼙
㻮㼍㼀㼕㻻
㻟
㼏㻛㼍
㼏㻛㼍
Fig. 8. Evaluated piezoelectric constants as a function of c/a ratios in optimized tetragonal
SrTiO
3
and BaTiO
3
:(a)e
33
and (b) e
31
(Furuta & Miura, 2010).
Figures 8(a) and 8(b) shows the piezoelectric properties of e
33
and e
31
as a function of the
ratio c/a in tetragonal SrTiO
3
and BaTiO
3
. The ratio c/a is optimized value as shown in
Fig. 6(a) and e
33
and e
31
are evaluated values in their optimized structures. Note that e
33
become larger at c/a ≈ 1, especially in SrTiO
3
. These properties seem to be similar to the
properties arond the Curie temperatures in piezoelectric ABO
3
; Damjanovic emphasized the
importance of the polarization extension as a mechanism of larger piezoelectric constants in
a recent paper (Damjanovic, 2010). Contrary to e
33
, on the other hand, the changes in e
31
are much smaller than the changes in e
33
, but note that e
31
shows negative in SrTiO
3
while
positive in BaTiO
3
.
403
First-Principles Study of ABO
3
: Role of the
B–O Coulomb Repulsions for
Ferroelectricity and Piezoelectricity
10 Ferroelectrics
㻞 㻜
㻠㻚㻜㻌
㻢㻚㻜㻌
㻤㻚㻜㻌
㻟
㻮㼀㻻
㻞 㻜
㻠㻚㻜㻌
㻢㻚㻜㻌
㻤㻚㻜㻌
㻟
㻿㼀㻻
㻔㼍㻕 㻔㼎㻕
㻟
㼆
㻖
㻟㻟
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻿㼞㼀㼕㻻
㻟
㻟
㼆
㻖
㻟㻟
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻮㼍㼀㼕㻻
㻟
㼀㼕
㻿㼞
㼀㼕
㻙㻢㻚㻜㻌
㻙㻠㻚㻜㻌
㻙㻞㻚㻜㻌
㻜㻚㻜㻌
㻞
㻚
㻜
㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㼆㻖㻟
㻟
㻛
㻙㻢㻚㻜㻌
㻙㻠㻚㻜㻌
㻙㻞㻚㻜㻌
㻜㻚㻜㻌
㻞
㻚
㻜
㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㼆㻖㻟
㻟
㻛
㼆
㻖
㻟
㼆
㻖
㻟
㻟
㻻
㼤
㻻
㼦
㻮㼍
㻻
㼤
㻻
㼦
㼏
㻛
㼍㼏
㻛
㼍
Fig. 9. Evaluated Born effective charges Z
∗
33
(k) as a function of c/a ratios: (a) SrTiO
3
and
(b) BaTiO
3
.O
x
and O
z
denote oxygen atoms along the [100] axis and the [001] axis,
respectively (Furuta & Miura, 2010).
㻠㻚㻜㻌
㻢㻚㻜㻌
㻤㻚㻜㻌
䃖㻟
㻮㼀㻻
㻠㻚㻜㻌
㻢㻚㻜㻌
㻤㻚㻜㻌
㻟
㻿㼀㻻
㻔㼍㻕 㻔㼎㻕
㻔㼍㻚㼡㻚㻕
䌖㼡
㻟
㻛䌖䃖
㻟
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻿㼞㼀㼕㻻
㻟
㼀㼕 㻮㼍
㻿㼞
㼀㼕
䌖㼡
㻟
㻛䌖䃖
㻟
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻮㼍㼀㼕㻻
㻟
㻔㼍㻚㼡㻚㻕
㻙㻢㻚㻜㻌
㻙㻠㻚㻜㻌
㻙㻞㻚㻜㻌
㻜㻚㻜㻌
㻞㻚㻜㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
䌖㼡㻟㻛䌖
㻙㻢㻚㻜㻌
㻙㻠㻚㻜㻌
㻙㻞㻚㻜㻌
㻜㻚㻜㻌
㻞㻚㻜㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㼆㻖㻟
㻟
䌖㼡
㻟
㻛䌖䃖
㻟
㻻
㼤
㻻
㼦
㻿㼞
㻻
㼤
㻻
㼦
䌖㼡
㻟
㻛䌖䃖
㻟
㼏㻛㼍
㼏㻛㼍
Fig. 10. Evaluated values of ∂u
3
(k)/∂η
3
as a function of c /a ratios: (a) SrT iO
3
and (b) BaT iO
3
.
“a.u." denotes the atomic unit (
≈ 0.53) (Furuta & Miura, 2010).
As expressed in eq. (4), e
3j
is the sum of the contributions from the clamped term and the
relaxed term. However, it has been generally known that the contribution to e
3j
from the
clamped term is much smaller than that from the relaxed term; in fact, the absolute values of
the e
33
clamped terms are less than 1 C/m
2
in both SrTiO
3
and BaTiO
3
. The author therefore
investigates the contributions to the relaxed term of e
33
and e
31
in detail. As expressed in
eq. (4), the relaxed terms of e
3j
are proportional to the sum of the products between the Z
∗
33
(k)
and ∂u
3
(k)/∂η
j
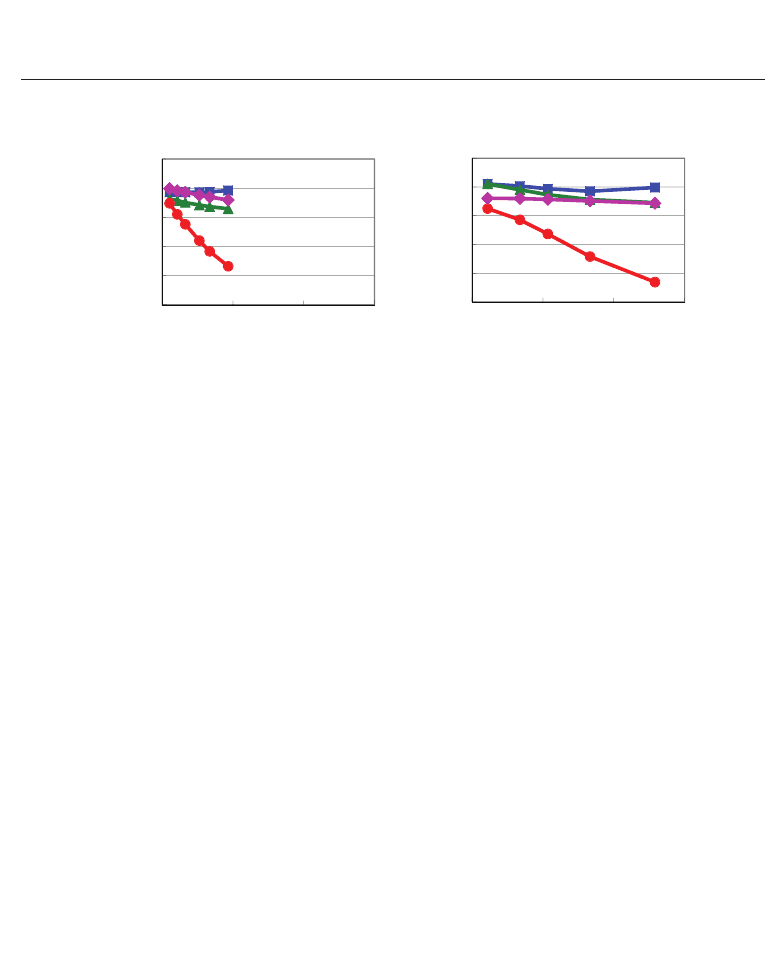
(j = 3 or 1) values. Let us show the evaluated results of Z
∗
33
(k), ∂u
3
(k)/∂η
3
,
and ∂u
3
(k)/∂η
1
in the following. Figures 9(a) and 9(b) show the Z
∗
33
(k) values in SrTiO
3
and BaTiO
3
, respectively. Properties of the Z
∗
33
(k) values are quantitatively similar in both
SrTiO
3
and BaTiO
3
. Therefore, the difference in the properties of e
33
and e
31
between SrTiO
3
and BaTiO
3
must be due to the difference in the properties of ∂u
3
(k)/∂η
j
. Figures 10(a) and
10(b) show the ∂u
3
(k)/∂η
3
values in SrTiO
3
and BaTiO
3
, respectively. In these figures, O
x
and O
z
denote oxygen atoms along the [100] and [001] axes, respectively, and η
3
is defined as
η
3
≡ (c − c
0
)/c
0
,wherec
0
denotes the c lattice parameter with fully optimized structure.
Clearly, the absolute values of ∂u
3
(k)/∂η
3
are d ifferent in between SrTiO
3
and BaTiO
3
.On
㻙
㻜㻚㻜㻌
㻝㻚㻜㻌
䃖㻝
㻜㻚㻜㻌
㻝㻚㻜㻌
䃖㻝
㻔㼍㻕 㻔㼎㻕
㻔㼍㻚㼡㻚㻕
䌖㼡
㻟
㻛䌖䃖
㻝
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻿㼞㼀㼕㻻
㻟
㼀㼕 㻮㼍
㻿㼞
㻻
㼦
䌖㼡
㻟
㻛䌖䃖
㻝
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻮㼍㼀㼕㻻
㻟
㻔㼍㻚㼡㻚㻕
㻙㻠㻚㻜㻌
㻙㻟㻚㻜㻌
㻙㻞㻚㻜㻌
㻚㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
䌖㼡㻟㻛䌖
㻙㻠㻚㻜㻌
㻙㻟㻚㻜㻌
㻙㻞㻚㻜㻌
㻚㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
䌖㼡㻟㻛䌖
䌖㼡
㻟
㻛䌖䃖
㻝
㻻
㼤
㼦
㼀㼕
㻻
㼤
䌖㼡
㻟
㻛䌖䃖
㻝
㼏㻛㼍
㼏㻛㼍
404
Ferroelectrics - Characterization and Modeling
First-principles Study of ABO
3
: Role of the B–O Coulomb Repulsions for Ferroelectricity and Piezoelectricity 11
㻙
㻝 㻜
㻜㻚㻜㻌
㻝㻚㻜㻌
䃖㻝
㻮㼀㻻
㻙
㻝 㻜
㻜㻚㻜㻌
㻝㻚㻜㻌
䃖㻝
㻿㼀㻻
㻔㼍㻕 㻔㼎㻕
㻔㼍㻚㼡㻚㻕
䌖㼡
㻟
㻛䌖䃖
㻝
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻿㼞㼀㼕㻻
㻟
㼀㼕
㻻
㻮㼍
㻿㼞
㻻
㼦
䌖㼡
㻟
㻛䌖䃖
㻝
㼢㻚㼟㻚㻌㼏㻛㼍㻌㼕㼚㻌㻮㼍㼀㼕㻻
㻟
㻔㼍㻚㼡㻚㻕
㻙㻠㻚㻜㻌
㻙㻟㻚㻜㻌
㻙㻞㻚㻜㻌
㻝
㻚
㻜
㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
䌖㼡㻟㻛䌖
㻙㻠㻚㻜㻌
㻙㻟㻚㻜㻌
㻙㻞㻚㻜㻌
㻙
㻝
㻚
㻜
㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
䌖㼡㻟㻛䌖
䌖㼡
㻟
㻛䌖䃖
㻝
㻻
㼤
㻻
㼦
㼀㼕
㻻
㼤
䌖㼡
㻟
㻛䌖䃖
㻝
㼏㻛㼍
㼏㻛㼍
Fig. 11. Evaluated values of ∂u
3
(k)/∂η
1
as a function of c /a ratios: (a) SrT iO
3
and
(b) BaTiO
3
(Furuta & Miura, 2010).
the other hand, Figs. 11(a) and 11(b) show the ∂u
3
(k)/∂η
1
values in SrTiO
3
and BaTiO
3
,
respectively; η
1
is defined as η
1
≡ (a − a
0
)/a
0
,wherea
0
denotes the a lattice parameter
with fully optimized structure. The absolute values of ∂u
3
(k)/∂η
1
, especially for Ti, O
x
,and
O
z
, are different in between SrTiO
3
and BaTiO
3
. As a result, the quantitative differences in
e
33
and e
31
in between SrTiO
3
and BaTiO
3
are due to the differences in the contribution of
the ∂u
3
(k)/∂η
j
values. In the following, the author would like to discuss the reasons of the
quantitative differences in e
33
and e
31
in between SrTiO
3
and BaTiO
3
.
Figure 12(a) shows the difference between the A–O
x
distance (R
A−O
x
)andthesumofr
A
and r
O
x
(r
A
+ r
O
x
) on the (100) plane as a function of the ratio c/a, where the values of the
ionic radii are defined as Shannon’s ones (Shannon, 1976). Note that R
A−O
x
is smaller than
r
A
+ r
O
x
in both SrTiO
3
and BaTiO
3
. However, the difference in absolute value between R
A−O
x
and r
A
+ r
O
x
in SrTiO
3
is much smaller than the difference in BaTiO
3
for 1.00 c/a 1.10.
This result suggests that the Sr–O
x
Coulomb repulsion on the (100) plane in SrTiO
3
is much
smaller than the Ba–O
x
Coulomb repulsion in BaTiO
3
and that therefore Sr and O
x
ions of
SrTiO
3
can be displaced more easily along the [001] axis than Ba and O
x
ions of BaTiO
3
.This
would be a reason why the absolute values of ∂u
3
(k)/∂η
3
of Sr and O
x
ions in SrTiO
3
are
larger than those of Ba and O
x
ions in BaTiO
3
. Figure 12(b) shows the difference between the
Ti–O
z
distance (R
Ti−O
z
)andr
Ti
+ r
O
z
along the [001] axis as a function of the ratio c/a.Note
that R
Ti−O
z
is smaller than r
Ti
+ r
O
z
in both SrTiO
3
and BaTiO
3
. However, the difference in
absolute value between R
Ti−O
z
and r
Ti
+ r
O
z
in SrTiO
3
is smaller than the difference in BaTiO
3
for 1.00 c/a 1.10. This result suggests that the Ti–O
z
Coulomb repulsion along the [001]
axis in SrTiO
3
is smaller than that in BaTiO
3
and that therefore the Ti ion of SrTiO
3
can be
displaced more easily along the [001] axis than that of BaTiO
3
. This would be a reason why
the absolute values of ∂ u
3
(k)/∂η
3
of Ti and O
z
ions in SrTiO
3
are larger than that in BaTiO
3
.
In the following, the author discusses the relationship between ∂u
3
(Ti)/∂η
3
and the ratio
c/a in detail. Figure 13(a) shows the properties of the diffrences in the total energy (ΔE
total
)
as a function of u
Ti
. In this figure, the properties of SrTiO
3
with c/a = 1.021 (η = 0.011),
SrTiO
3
with c/a = 1.093 (η = 0.053) and BaTiO
3
with c/a = 1.022 as a reference, are shown.
Calculations of E
total
were performed with the fixed crystal structures of previously optimized
structures except Ti ions. Figure 13(b) shows illustrations of ΔE
total
curves with deviations at
the minimum points of the ΔE
total
values, corresponding to the ΔE
total
curves of SrTiO
3
in
Fig. 13(a). Clearly, as η
3
becomes smaller, the deviated value at the minimum point of the
405
First-Principles Study of ABO
3
: Role of the
B–O Coulomb Repulsions for
Ferroelectricity and Piezoelectricity
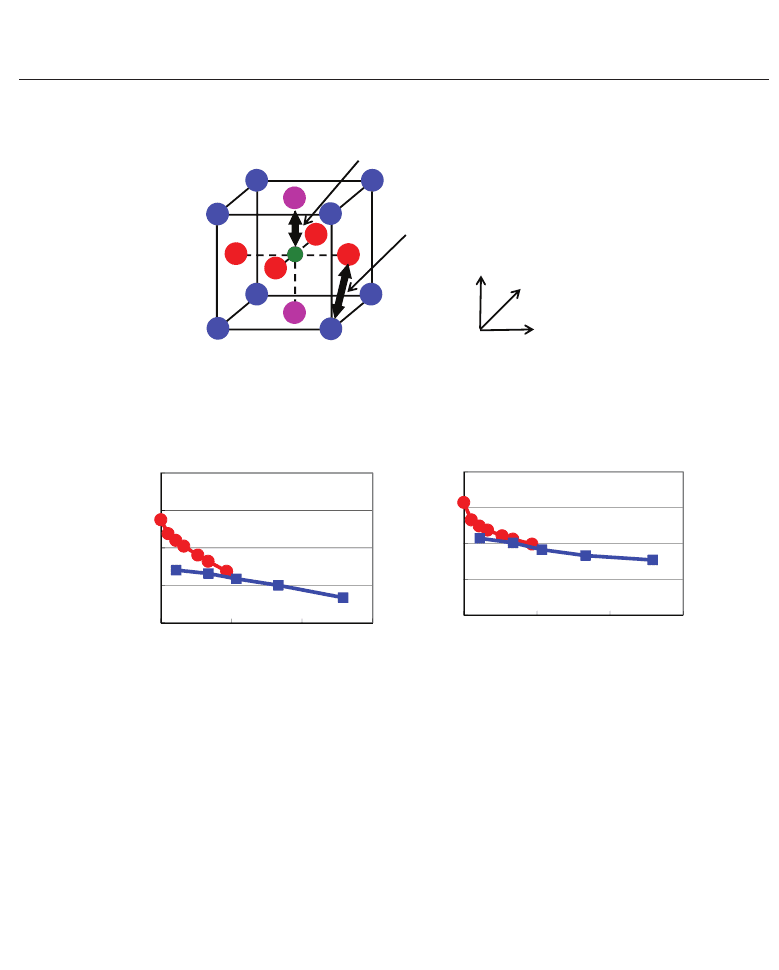
12 Ferroelectrics
㻻
㻻
㼦
㻾
㼀㼕㻙㻻㼦
㻾
㻭
㻙
㻻㼤
㻻
㼤
㼀㼕
㼇㻝㻜㻜㼉
㼇㻜㻝㻜㼉
㼇㻜㻜㻝㼉
㻔 㻕
㻭
㻻㼤
㻜㻚㻜
㻜 㻜
㻔㼍㻕 㻔㼎㻕
㻾
㻭㻙㻻㼤
䇵 㻔㼞
㻭
㻗㻌㼞
㻻㼤
㻕㻌㼢㻚㼟㻚㻌㼏㻛㼍㻌 㻾
㼀㼕㻙㻻㼦
䇵 㻔㼞
㼀㼕
㻗㻌㼞
㻻㼦
㻕㻌㼢㻚㼟㻚㻌㼏㻛㼍㻌
㻕
㻌
㻭㻌
㻔
㻿㼞㻌㼛㼞㻌㻮㼍
㻕
㻙㻜㻚㻟㻌
㻙㻜㻚㻞㻌
㻙㻜㻚㻝㻌
㻜㻚㻜
㻌
㼀
㼕㻙㻻㻟 㻙 㼞㼋㼀㼕㻻㻟 㻔㻭㻕
㻙㻜㻚㻟㻌
㻙㻜㻚㻞㻌
㻙㻜㻚㻝㻌
㻜
㻚
㻜
㻌
㻭
㻙㻻㻝㻌㻙 㼞㼋㻭㻻㻌㻔㻭㻕
㻭
㻙㻻㼤
䇵 㻔㼞
㻭
㻗㻌㼞
㻻㼤
㻕㻌 㻔䊅㻕㻌
㻿㼞㼀㼕㻻
㻟
㼀
㼕㻙㻻㼦
䇵 㻔㼞
㼀㼕
㻗㻌㼞
㻻㼦
㻕㻌 㻔䊅
㻕
㻿㼞㼀㼕㻻
㻟
㻮㼍㼀㼕㻻
㻟
㻙㻜㻚㻠㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㻾㼋
㼀
㼏㻛㼍
㻙㻜㻚㻠㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㻾㼋
㻭
㼏㻛㼍
㻾
㻭
㻮㼍㼀㼕㻻
㻟
㻾
㼀
Fig. 12. Evaluated values as a function of c /a ratios in optimized tetragonal SrT iO
3
and
BaTiO
3
: (a) difference between the A–O
x
distance (R
A−O
x
)andr
A
+ r
O
x
, and (b) difference
between the Ti–O
z
distance (R
Ti−O
z
)andr
Ti
+ r
O
z
, as a function of the ratio c/a. R
A−O
x
and
R
Ti−O
z
in ATiO
3
are also illustrated; all the ionic radii are much larger, and A and Ti ions are
displaced along the [001] axis in real ATiO
3
(Furuta & Miura, 2010).
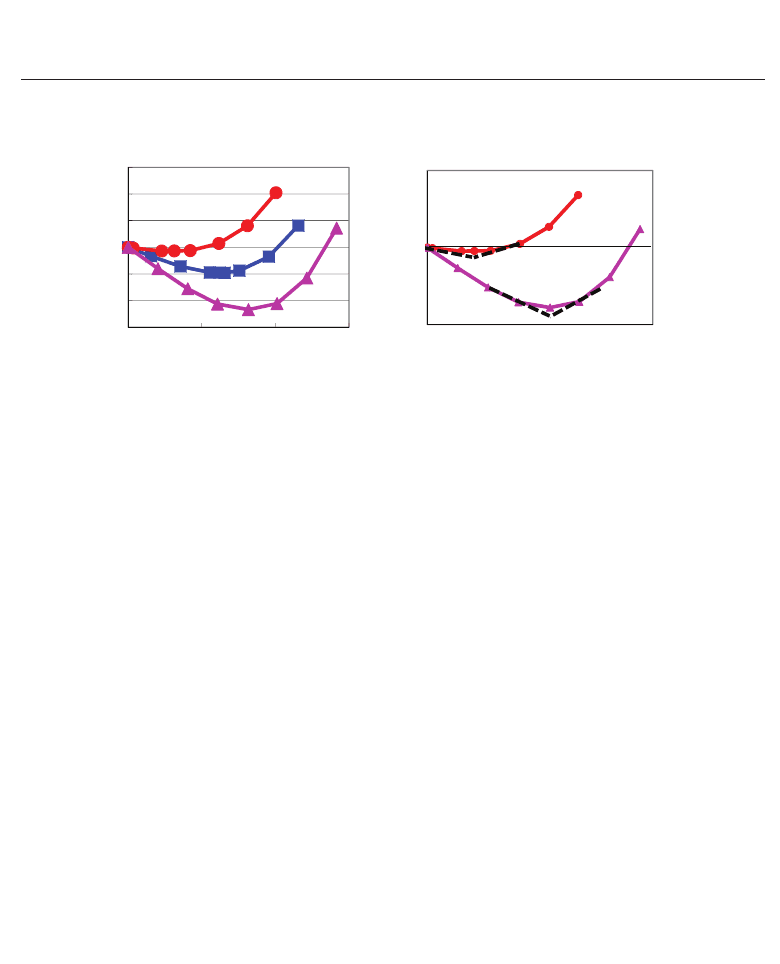
ΔE
total
values becomes smaller, i.e., the Ti ion can be displaced more favourably. On the other
hand, as shown in Fig. 10(a), the absolute value of ∂u
3
(Ti)/∂η
3
becomes larger as η
3
becomes
smaller. Therefore, the Ti ion can be displaced more favourably as the deviated value at the
minimum point of the ΔE
total
values becomes smaller.
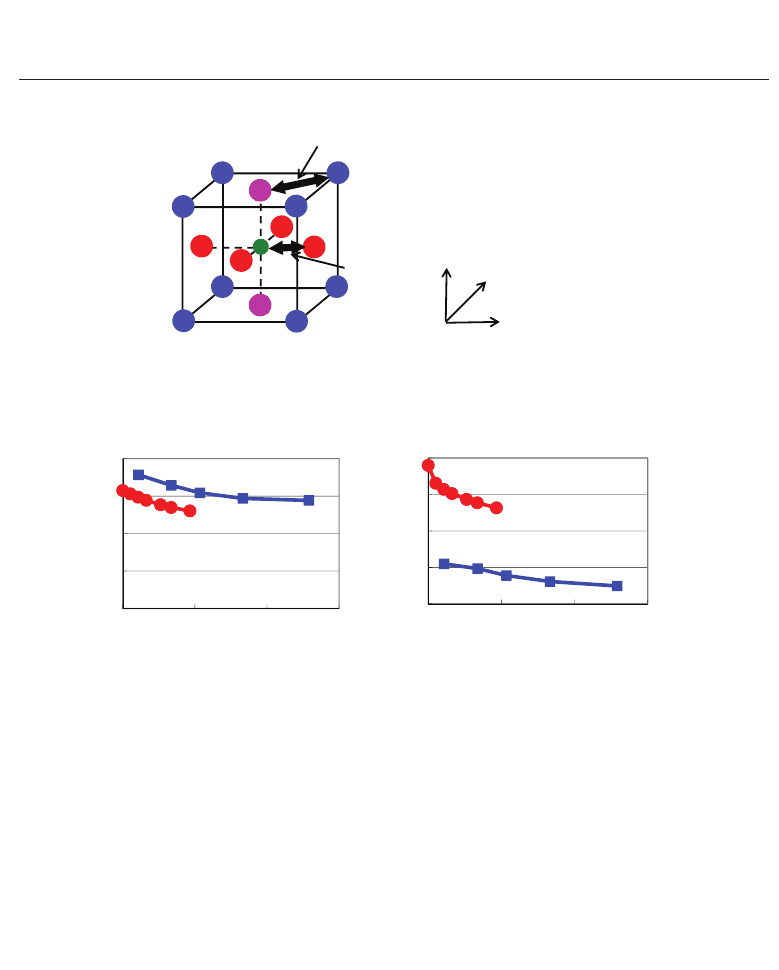
Next, let us discuss quantitative properties of e
31
, especially the reason why e
31
in SrTiO
3
shows negative while positive in BaTiO
3
. Figure 14(a) shows the difference between the
Ti–O
x
distance (R
Ti−O
x
)andr
Ti
+ r
O
x
along the [100] axis as a function of the ratio c/a.Note
that R
Ti−O
x
is smaller than r
Ti
+ r
O
x
in both SrTiO
3
and BaTiO
3
. However, the difference in
absolute value between R
Ti−O
x
and r
Ti
+ r
O
x
in SrTiO
3
is larger than that in BaT iO
3
, i.e., R
Ti−O
x
in SrTiO
3
is smaller than R
Ti−O
x
in BaTiO
3
. This result suggests that the Ti–O
x
Coulomb
repulsion along the [100] axis in SrTiO
3
is larger than that in BaT iO
3
and that therefore Ti and
O
x
ions of SrTiO
3
can be displaced along the [001] axis more easily than those of BaTiO
3
,
as discussed in previous subsection. This would be a reason why the absolute values of
∂u
3
(k)/∂η
1
of Ti and O
x
ions in SrTiO
3
are larger than those in BaTiO
3
. Therefore, each
㼂㻕
㻜㻚㻜㻜㻠㻌
㻜㻚㻜㻜㻢㻌
㼂㻕
㻔㼍㻕 㻔㼎㻕
䏓㻱
㼠㼛㼠㼍㼘
㼢㻚㼟㻚㻌䏓㼡
㼀㼕
㼂㻕
㻿㼞㼀㼕㻻
㻟
㻔䃖
㻟
㻩㻌㻜㻚㻜㻝㻝㻕
㻮㼍㼀㼕㻻
㻟
㼡㼚㼕㼠㻕
䏓㻱
㼠㼛㼠㼍㼘
㼢㻚㼟㻚㻌䏓㼡
㻟
㻔㼀㼕㻕 䏓㻱
㼠㼛㼠㼍㼘
㼢㻚㼟㻚㻌䏓㼡
㻟
㻔㼀㼕㻕
㻙㻜㻚㻜㻜㻢㻌
䏓㻱㼠㼛㼠 㻔㼑
㻙㻜㻚㻜㻜㻢㻌
㻙㻜㻚㻜㻜㻠㻌
㻙㻜㻚㻜㻜㻞㻌
㻜㻚㻜㻜㻜㻌
㻚㻌
䏓㻱㼠㼛㼠 㻔
䏓㻱
㼠㼛㼠㼍㼘
㻔㼑
㻜㻌
䏓㻱
㼠㼛㼠㼍㼘
㻔㼍㼞㼎㻚㻌
㻿㼞㼀㼕㻻
㻟
㻔䃖
㻟
㻩㻌㻜㻚㻜㻡㻟㻕
㻚㻌
㼡㼋㼀㼕㻌 㻔㻭㻕
㻚㻌 㻚㻌 㻚㻌 㻚㻌
㼡㼋㼀㼕㻌 㻔㻭㻕
㻌
䏓㼡
㻟
㻔㼀㼕㻌㻕㻌㻌㻔䊅㻕
䏓㼡
㻟
㻔㼀㼕㻌㻕㻌㻌㻔㼍㼞㼎㻚㻌㼡㼚㼕㼠㻕
406
Ferroelectrics - Characterization and Modeling
First-principles Study of ABO
3
: Role of the B–O Coulomb Repulsions for Ferroelectricity and Piezoelectricity 13
㼂㻕
㻜 㻜㻜㻞
㻜㻚㻜㻜㻠㻌
㻜㻚㻜㻜㻢㻌
㼑
㼂㻕
㻔㼍㻕 㻔㼎㻕
䏓㻱
㼠㼛㼠㼍㼘
㼢㻚㼟㻚㻌䏓㼡
㼀㼕
㼂㻕
㻿㼞㼀㼕㻻
㻟
㻔䃖
㻟
㻩㻌㻜㻚㻜㻝㻝㻕
㻮㼍㼀㼕㻻
㻟
㼡㼚㼕㼠㻕
䏓㻱
㼠㼛㼠㼍㼘
㼢㻚㼟㻚㻌䏓㼡
㻟
㻔㼀㼕㻕 䏓㻱
㼠㼛㼠㼍㼘
㼢㻚㼟㻚㻌䏓㼡
㻟
㻔㼀㼕㻕
㻙㻜㻚㻜㻜㻢㻌
㻜 㻜
䏓㻱㼠㼛㼠 㻔㼑
㻙㻜㻚㻜㻜㻢㻌
㻙㻜㻚㻜㻜㻠㻌
㻙㻜㻚㻜㻜㻞㻌
㻜㻚㻜㻜㻜㻌
㻜
㻚
㻜㻜㻞
㻌
㻜 㻜
㻜 㻝
㻜 㻞
㻜 㻟
䏓㻱㼠㼛㼠 㻔
㼑
䏓㻱
㼠㼛㼠㼍㼘
㻔㼑
㻜
㻜㻌
䏓㻱
㼠㼛㼠㼍㼘
㻔㼍㼞㼎㻚㻌
㻿㼞㼀㼕㻻
㻟
㻔䃖
㻟
㻩㻌㻜㻚㻜㻡㻟㻕
㻜
㻚
㻜
㻌
㼡㼋㼀㼕㻌 㻔㻭㻕
㻜
㻚
㻜
㻌
㻜
㻚
㻝
㻌
㻜
㻚
㻞
㻌
㻜
㻚
㻟
㻌
㼡㼋㼀㼕㻌 㻔㻭㻕
㻜
㻌
䏓㼡
㻟
㻔㼀㼕㻌㻕㻌㻌㻔䊅㻕
䏓㼡
㻟
㻔㼀㼕㻌㻕㻌㻌㻔㼍㼞㼎㻚㻌㼡㼚㼕㼠㻕
Fig. 13. (a) ΔE
total
as a function of u
Ti
in tetragonal SrTiO
3
and BaTiO
3
. (b) Illustration of the
ΔE
total
curves in tetragonal SrTiO
3
(η = 0.011) and SrTiO
3
(η = 0.053) with deviations at the
minimum point of ΔE
total
.
absolute value of Z
∗
Ti
× ∂u
3
(Ti)/∂η
1
( < 0) and Z
∗
O
x
× ∂u
3
(O
x
)/∂η
1
( > 0) in SrTiO
3
is larger
than that in BaT iO
3
. Figure 14(b) shows the difference between the A–O
z
distance (R
A−O
z
)
and r
A
+ r
O
z
on the (001) plane as a function of the ratio c/a.NotethatR
A−O
z
is smaller
than r
A
+ r
O
z
in both SrTiO
3
and BaTiO
3
. However, the difference in absolute value between
R
A−O
z
and r
A
+ r
O
z
in BaTiO
3
is larger than that in SrTiO
3
. This result suggests that the
Ba–O
z
Coulomb repulsion on the (001) plane in BaTiO
3
is larger than that in SrTiO
3
and
that therefore O
z
ion of BaTiO
3
can be displaced along the [001] axis more easily than that of
SrTiO
3
, as discussed in previous subsection. This would be a reason why the absolute value of
∂u
3
(k)/∂η
1
of O
z
ion in BaTiO
3
is larger than that in SrTiO
3
. Therefore, the absolute value of
Z
∗
O
z
×∂u
3
(O
z
)/∂η
1
( > 0) in BaTiO
3
is larger than that in SrT iO
3
. Finally, as a result, the above
investigations suggest that the signature of e
31
in SrTiO
3
or BaTiO
3
is closely related to the
difference in absolute values between Z
∗
Ti
× ∂u
3
(Ti)/∂η
1
and the sum of Z
∗
O
x
× ∂u
3
(O
x
)/∂η
1
and Z
∗
O
z
×∂u
3
(O
z
)/∂η
1
.
4. Summary
Using a first-principles calculation with optimized structures, the author has investigated
the role of the Coulomb repulsions between Ti 3s and 3p states and O 2s and 2p states
in ferroelectric BaTiO
3
. It has been found that the Coulomb repulsions between Ti 3s
and 3p
x (y)
states and O
x (y)
2s and 2p
x (y)
states are closely related to the appearance of
Ti ion displacement in tetragonal BaTiO
3
. This mechanism seems to be consistent with
the appearance of Ti ion displacement in rhombohedral BaTiO
3
. The present investigation
suggests that the Coulomb repulsions between Ti 3s and 3p states and O 2p states have
an important role in ferroelectricity. In addition to this suggestion, the author believes that
the present investigation will show a g uideline for the choice of PPs when first-principles
calculations with PP methods are performed. The author has also investigated the ferroelectric
and piezoelectric properties of SrTiO
3
and BaTiO
3
with in-plane compressive tetragonal
structures using a first-principles calculation. It has been found that the ferroelectric structure
even in SrTiO
3
appears with in-plane compressive structures. The piezoelectric constant e
33
drastically increases in SrTiO
3
rather than that in BaTiO
3
as the tetragonal ratio c/a (> 1) is
407
First-Principles Study of ABO
3
: Role of the
B–O Coulomb Repulsions for
Ferroelectricity and Piezoelectricity
14 Ferroelectrics
㻻
㼤
㼀㼕
㻻
㼦
㻾
㻭㻙㻻㼦
㻻
㼤
㼀㼕
㼇㻝㻜㻜㼉
㼇㻜㻝㻜㼉
㼇㻜㻜㻝㼉
㻭 㻔㻿㼞 㼛㼞 㻮㼍㻕
㻾
㼀㼕㻙㻻㼤
㻙㻜㻚㻥㻌
㻜㻚㻜㻌
㻭
㻕
㻔㼍㻕 㻔㼎㻕
䊅
㻕㻌
㻾
㼀㼕㻙㻻㼤
䇵 㻔㼞
㼀㼕
㻗㻌㼞
㻻㼤
㻕㻌㼢㻚㼟㻚㻌㼏㻛㼍㻌
㻮 㼀㼕㻻
㻾
㻭㻙㻻㼦
䇵 㻔㼞
㻭
㻗㻌㼞
㻻㼦
㻕㻌㼢㻚㼟㻚㻌㼏㻛㼍㻌
䊅
㻕㻌
㻿 㼀㼕㻻
㻭
㻌
㻔㻿㼞
㻌
㼛㼞
㻌
㻮㼍㻕
㻝 㻟
㻙㻝㻚㻞㻌
㻙㻝㻚㻝㻌
㻙㻝㻚㻜㻌
㻾
㼋㻭㻙㻻㻟㻌㻙 㼞㼋㻭㻻㻌㻔㻭㻕
㻙㻜㻚㻟㻌
㻙㻜㻚㻞㻌
㻙㻜㻚㻝㻌
㻾
㼋㼀㼕㻙㻻㻝 㻙 㼞㼋㼀㼕㻻㻝 㻔
㻭
㻾
㼀㼕㻙㻻㼤
䇵 㻔㼞
㼀㼕
㻗㻌㼞
㻻㼤
㻕㻌 㻔
䊅
㻿㼞㼀㼕㻻
㻟
㻮
㼍
㼀㼕㻻
㻟
㻾
㻭㻙㻻㼦
䇵 㻔㼞
㻭
㻗㻌㼞
㻻㼦
㻕㻌 㻔
䊅
㻿
㼞
㼀㼕㻻
㻟
㻮㼍㼀㼕㻻
㻟
㻙
㻝
㻚
㻟
㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㻾
㼏㻛㼍
㻙㻜㻚㻠㻌
㻝㻚㻜㻜㻌 㻝㻚㻝㻜㻌 㻝㻚㻞㻜㻌 㻝㻚㻟㻜㻌
㻾
㼏㻛㼍
㻾
㻾
Fig. 14. Evaluated values as a function of c /a ratios in optimized tetragonal SrT iO
3
and
BaTiO
3
: (a) difference between the Ti–O
x
distance (R
Ti−O
x
)andr
Ti
+ r
O
x
, as a function of the
ratio c/a , and (b) difference between the A–O
z
distance (R
A−O
z
)andr
A
+ r
O
z
. R
Ti−O
x
and
R
A−O
z
in ATiO
3
are also illustrated (Furuta & Miura, 2010).
close to 1. On the other hand, e
31
shows negative in SrTiO
3
while positive in BaTiO
3
,although
the changes in their absolute values are very small. The author has found that these properties
of e
33
and e
31
in SrTiO
3
and BaTiO
3
are closely related to the ionic distances.
5. Acknowledgements
The author thanks Professor H. Funakubo, Professor M. Azuma, M. Kubota and T. Furuta
for useful discussion. The present work was partly supported by the Elements Science and
Technology Project from the Ministry of Education, Culture, Sports, Science and Technology,
Japan. Calculations for the present work were partly performed by the supercomputing grid
cluster machine “TSUBAME" in Tokyo Institute of Technology.
6. References
Ahart, M., Somayazulu, M., Cohen, R. E., Ganesh, P., Dera, P., Mao, H., Hemley, R.,
Ren, Y., Liermann, P. & Wu, Z. (2008). Origin of morphotropic phase boundaries in
408
Ferroelectrics - Characterization and Modeling
