Jung Han. Innovations in Food Packaging
Подождите немного. Документ загружается.

Quality of packaged foods 27
100-
^ 90-
^
o 80-
3
i 70-
tJ
CO
.-g'
60-
CO
D- 50-
*o
^ 40-
3
o
E 30-
<
20-
10-
0-
vv
\S.
\ \^
V\
^ \
V >v
VV
^L
\m
\ " -© ^
N. "^«,
\ " >^ ^ ^ ^ First order
Zero order \ ~^^"~~----^--
1 1 1 n 1 1
50
100
150 200
Time (days)
250 300
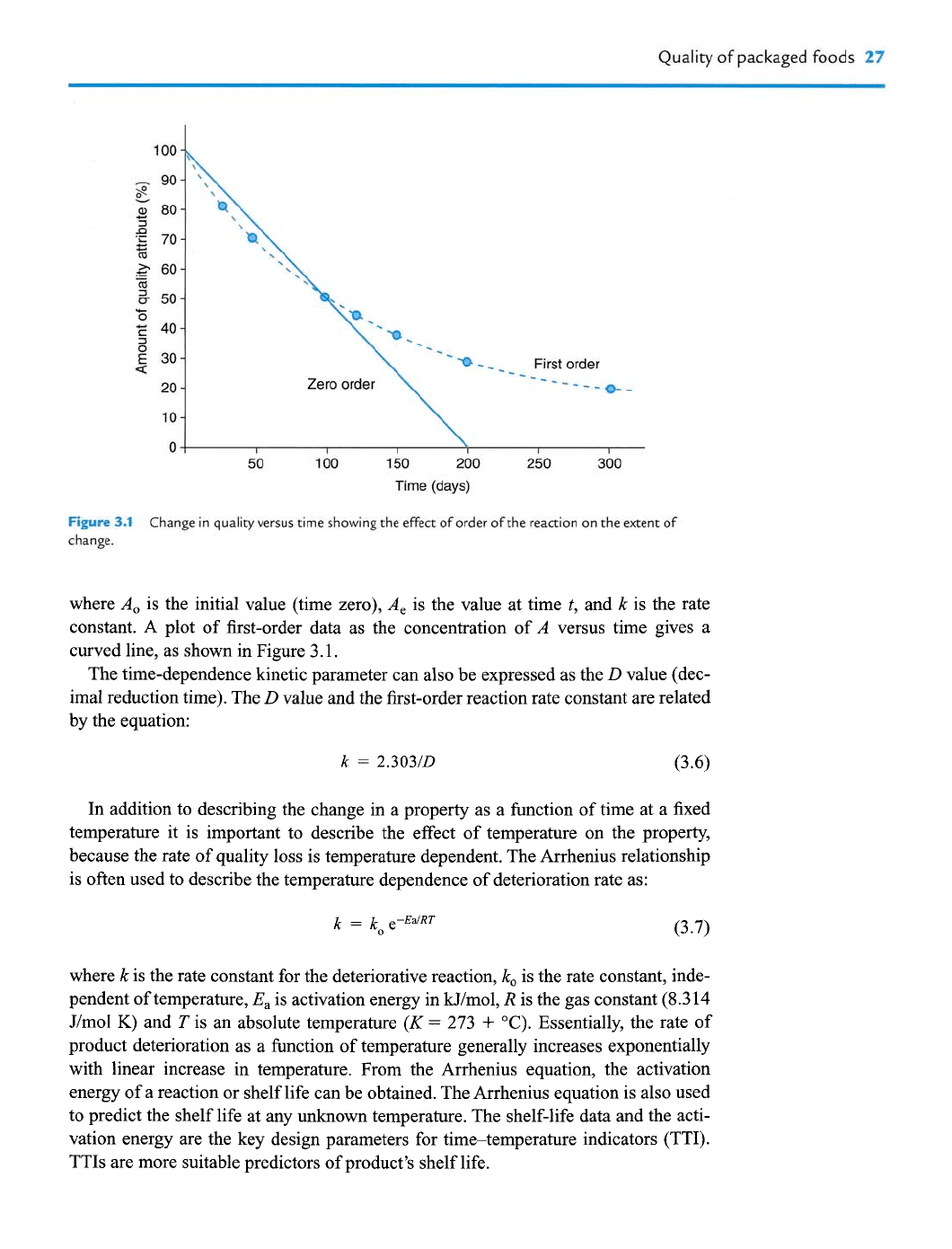
Figure 3.1 Change in quality versus time siiowing the effect of order of the reaction on the extent of
change.
where A^ is the initial value (time zero), A^ is the value at time t, and k is the rate
constant. A plot of first-order data as the concentration of A versus time gives a
curved line, as shown in Figure 3.1.
The time-dependence kinetic parameter can also be expressed as the D value (dec-
imal reduction
time).
The
D value and the
first-order
reaction rate constant are related
by the equation:
k = 2.303/Z)
(3.6)
In addition to describing the change in a property as a fiinction of time at a fixed
temperature it is important to describe the effect of temperature on the property,
because the rate of quality loss is temperature dependent. The Arrhenius relationship
is often used to describe the temperature dependence of deterioration rate as:
k = kQ
-EdJRT
(3.7)
where k
is
the rate constant for the deteriorative reaction,
k^
is the rate constant, inde-
pendent of temperature,
E^
is activation energy in kJ/mol, R is the gas constant (8.314
J/mol K) and T is an absolute temperature (K = 273 + °C). Essentially, the rate of
product deterioration as a function of temperature generally increases exponentially
with linear increase in temperature. From the Arrhenius equation, the activation
energy of a reaction or shelf life can be obtained. The Arrhenius equation is also used
to predict the shelf life at any unknown temperature. The shelf-life data and the acti-
vation energy are the key design parameters for time-temperature indicators (TTI).
TTIs are more suitable predictors of product's shelf
Hfe.

28 Innovations in Food Packaging
Shelf life
Shelf-life prediction
The shelf
Hfe
of novel foods that are not shelf stable may vary from batch to batch.
Accurate prediction of shelf life is, therefore, particularly important to ensure product
quality. Different approaches to shelf-life estimation have been
used.
Traditionally the
development of packaged foods has been largely an empirical procedure, involving
extensive laboratory evaluations of food formulations, packaging materials and vari-
ous package geometries. In an attempt to speed up the development process and opti-
mize food/package combinations, various mathematical models are able to predict the
shelf life of packed foods, and help in designing packaging systems to achieve the
desired results.
Mathematical modeling is the representation of real-life phenomena in terms of
a set of numerical expressions. The solutions of these mathematical systems are
supposed to stimulate the natural behavior of a food/package load. Whiting and
Buchanan (1994) proposed a three-level model classification scheme comprised of
primary, secondary, and tertiary
models.
Primary-level models describe the change in
microbial numbers over time, and secondary-level models indicate how the features
of primary models change with respect to one or more environmental factors - such
as pH, temperature and water activity. Tertiary-level models are personal computer
software packages that use the pertinent information from primary- and secondary-
level models to generate desired graphs, predictions and comparisons.
Predictive microbiology has been used to forecast the growth of spoilage micro-
organisms in order to determine the shelf life of a food
product.
Predictive models for
the growth of micro-organisms include temperature, pH and water activity as the main
growth-determining factors. However, other factors can significantly influence the
growth characteristics of the modeled micro-organisms, such as nitrite content,
organic acids and atmosphere (Devlieghere et
aL,
1999).
The effects of initial product
contamination, product characteristics, and storage conditions can be included in pre-
dictive models. However, specific spoilage organisms and spoilage reactions depend
upon intrinsic and extrinsic parameters. Recently, more attention has been paid to the
atmosphere as a fourth important growth-determining factor (Farber et aL, 1996;
Sutherland et
aL,
1996, 1997; Dalgaard et
aL,
1997; Fernandez et
aL,
1997). In these
studies the effect of storage temperature was described by the relative rate of spoilage
models, and the responses of numerous sensory, microbial, chemical, biochemical,
and physical measurements were correlated with remaining product shelf life.
Temperature models may predict the slope of the regression lines relating sensory or
microbial measurements and remaining shelf
life.
However, in general the regression
models are only applicable to products stored in given conditions (Olley and
Ratkowsky, 1973; Gibson, 1985; Bremner et aL, 1987). DevHeghere et al (1999)
compared two types of predictive models - an extended Ratkowsky model and a
response surface model - for the effect of temperature, concentration of dissolved
CO2,
and water activity
on the lag phase and the
maximum specific growth
rate
of
Lacto-
bacillus
sake for gas-packed cooked meat product. They reported that both developed

Quality of packaged foods 29
models proved to be useful in the prediction of the microbial shelf life of packed meat
products.
More recently, multivariate statistical methods have been used to relate the sensory
attributes of products (Girard and Nakai, 1994). Risbo (2003) observed that for a
packaged raisin-cereal mixture, shelf-life calculations based on the kinetic theory
show that the optimal product composition and product permeability for overall
product quality can be identified. In food packaging, measurements and predictions
of the shelf life of a product independent of the packaging structure are made first
and then integrated with the measurements and predictions made regarding the
effect of the package. Among the more relevant package properties are water
vapor transfer, gas transfer, odor transfer, flavor scalping, and other product/package
interactions.
To perform predictive modeling, the food model must be integrated with the pack-
age model. The package model must fit the food model - the package should be ana-
lyzed for its ability to function as a barrier against those variables that have been
identified as critical for the food's shelf life. Distribution parameters should be quan-
tified to determine their variability within the distribution environment. As a first
assumption, suppose that the main characteristics are the package barrier properties
(such as water vapor, oxygen or carbon-dioxide barrier) and that the package material
is unaffected by the packaging operations and distribution. In the predictive model,
assume that the internal package environment (water vapor, carbon dioxide, or oxy-
gen) changes as a permeant enters or leaves the package. Transmission of materials
will always occur from the high-concentration to the lower-concentration side of the
material. Standardize the transmission rate for the package area, gauge, time of
meas-
urement, and permeation properties for the package material/structure, the difference
of partial pressure of permeant across the package, and the environmental conditions -
in particular, temperature. Permeation is a function of dissolution into and diffusion
across the package material due to partial pressure P. The general models are based
on a standard differential equation which describes mass transfer across a permeable
membrane, with the effect on the product being the net gain or loss of the permeant by
the product (e.g. loss of carbon dioxide in a carbonated beverage, or loss of oxygen
attributable to reaction with a food component). The predictive model for the package
takes the general form:
dWIdt = (k/l)AdP (3.8)
where d^is the change in the weight of the critical food component, t is time, k is the
permeation coefficient of the package material, / is the package material thickness,
A is surface area of the package, and dP is the difference in partial pressures of the
permeant inside and outside of the package structure. Partial pressure requires knowl-
edge of the permeant: for oxygen external to the package it is 0.209; for carbon dioxide
outside the package it is 0.0003; for water vapor on the exterior it is six times the
relative of the distribution environment; for flavors and volatile components it is
essentially zero, etc. The elementary predictive model is for a monolayer material
structure of uniform thickness. When thickness changes, differential equations for the
thickness are incorporated into the model. When two or more materials are present, as

30 Innovations in Food Packaging
in a lamination, the net permeation is described by the sum of reciprocals of the indi-
vidual permeations:
IIP = l/Pj + I/P2 + ... + 1/^n (3-9)
plus the reciprocal of permeation across the boundary of different materials.
Shelf-life modeling and prediction have been limited by a serious paucity of key
variables, such as using only water vapor gain as a measure with no consumer accept-
ability input. The models do not replace the need for actual storage studies. Storage is
still necessary to verify product changes, find unusual effects, and, in some cases,
meet compliance criteria with government regulations. The use of these predictive
models in the food industry has been warranted by the need for rapid and cost-effective
provision of useful information for making decisions regarding product development,
prediction of safety and shelf life of
a
product, and identifying critical control points
in quality control
tests.
The use of models serves to reduce the time and effort neces-
sary to identify optimal packaging protection, and eliminate storage studies on mate-
rials with insufficient barrier properties to be reasonable candidates for the product.
The ability of the computer models to incorporate all of the known relevant variables
permits the food scientist, in concert with the packaging technologist, to predict accu-
rately and precisely the results of
a
package material and/or structural design change
on the shelf life of most product contents.
Storage conditions
The shelf life of a packaged food product is determined by numerous interactions
between parameters related to the product itself and/or associated with storage condi-
tions.
The quality changes in packaged products could be chemical, physical, enzy-
matic or microbiological, and are mainly due to mass transfer between foods and their
environment. Several studies have shown that barrier efficiency of packaging materi-
als depends not only on chemical composition of packaging material and penetrant,
but also on storage conditions (Giacin, 1995). Storage conditions (i.e. temperature,
humidity, atmospheric gas composition, and light) affect the appearance, aroma, flavor,
texture, and acceptability of packaged food
products.
Hence, appropriate packaging of
shelf-stable foods is essential, particularly during handling, transportation/distribution,
extended storage, and marketing.
Temperature
Storage temperature is probably the most important factor in maintaining the quality
and extending the shelf life of packaged foods. In most cases, an increase in storage
temperature degrades the quality and acceptability of packaged foods. To determine
the deleterious effects of temperature, it is often necessary to know how long a food
is exposed to that
temperature.
Biological reactions tend to increase by a factor of two
to three for each 10°C increase in temperature (Beaudry et al., 1992; Exama et al.,
1993).
Changes in environmental temperature create a specific problem in packaged
foods design because the respiration rate is more influenced by temperature changes

Quality of packaged foods 31
than by the O2 and CO2 permeabiHty of the permeable films used to obtain equilib-
rium modified atmosphere (EMAs). Owing to this fact, it is difficult to obtain an opti-
mum atmosphere inside a package when the surrounding temperature is not constant.
A film that produces a favorable atmosphere at the temperature for which the package
was designed may cause excessive accumulation of CO2 and/or depletion of O2 at
higher temperatures caused by an increased respiration rate. This anoxic situation
could lead to metabolic disorders such as fermentation, with production of off-flavors
(Kader et al., 1989; Silva et ai, 1999). In the case of a lower storage temperature,
decreased respiratory activity will lead to an accumulation of O2 above the optimal
3%,
resulting in a less effective EMA package. The influence of temperature on res-
piration rate was first quantified with the
QIQ
value, which is the respiration rate
increase for a 10°C rise in temperature:
Q,, = (Rr,/Rv^)mT2-n) ^^ ^^^
where
RY2
is the respiration rate at temperature T2, and
RYI
is the respiration rate at
temperature Ti. For various products,
QIQ
values may range from
1
to 4 depending on
the temperature range (Kader, 1987).
The Arrhenius equation is also used to quantify the effect of temperature on respi-
ration rate. The simultaneous use of this equation to describe the influence of temper-
ature on film permeability simplifies the mathematical modeling of MAP systems
(Exama et aL, 1993; Susana et al., 2002):
i?r = 8 X Qxp(-E/RT) (3.11)
The above equation may be rewritten with a reference temperature (Nelson, 1983;
Van Boekel, 1996):
Rv = 8^^f X Qxp[-E/R (l/T -
1/r^^f)]
(3.12)
Temperature variations during transportation and storage affect the produce respi-
ration rate (Exama et al, 1993). Kang et al. (2000) observed that temperature fluctua-
tions between 5°C and 15°C did not induce a harmfiil atmosphere inside the polyolefin
package, though high temperatures accelerated the weight loss, stipe elongation, and
darkening of the mushrooms. Temperature also has a significant effect on the sensory
quality of packaged food
products.
Enzymatic browning and shriveling, being the most
limiting sensory disorders of fresh cut produce, are inhibited by low-temperature storage
(Willocx, 1995; Garcia-Gimeno et al, 1998). The barrier efficiency of packages, too,
depends on temperature. Labuza and Contreas-Medellin (1981) observed that water
permeability decreases when temperature decreases, but may increase again at very
low temperatures. Morillon et al (2000) reported that corrected permeabilities of
cel-
lophane and polyethylene films decrease with temperature due to a preponderant
effect of temperature on water diffusion. Jacobsson et al. (2003) reported that change
of temperature during storage induced changes in smell, flavor, taste, and texture of
broccoli, depending on the packaging material used. However, the appearance of the
broccoli was found to be more dependent on the atmosphere than on the temperature
of storage.

32 Innovations in Food Packaging
Equilibrium relative humidity
Equilibrium relative humidity (ERH) is critical to the quality and safety of packaged
foods.
Moisture loss or gain from one region or food component
to
another region will
continuously occur in order to reach thermodynamic equilibrium with the surround-
ing food components and the environment.
The concept of water activity (a^) is an important property of food safety. Water
activity is widely used to predict the stability of food with respect to the potential for
growth of micro-organisms, and also some of the physical, chemical, and enzymic
changes that lead to deterioration. In packaged foods, the water-vapor permeability of
the packaging material is a decisive factor in controlling changes in moisture content
and thus the water activity (a^) of packaged foods. Water activity is defined as the
ratio of partial pressure of water vapor in the product {p) to that in presence of pure
water (/?o). A difference in the water activities of food components, food domains,
and the external environment outside the package introduces a driving force for water
transport (Labuza and
Hyman,
1998).
Water transport ceases when differences
in
water
activity have leveled out - i.e. water activities converge to a common equilibrium
value. For some systems, the equilibrium water activity is acceptable from the point of
view of textural (Katz,
1981;
Bourne, 1987), chemical (Leung, 1987), and microbio-
logical stability (Leneovich, 1987), and the shelf life is not limited by water transport.
For systems where this equilibrium water activity is undesirable to one or more of the
components, the product shelf life is determined by the dynamics of the water trans-
port process.
By measuring the water activity of foodstuffs, it is possible to predict which micro-
organisms will be potential sources of spoilage and infection. Non-enzymatic brown-
ing reactions and spontaneous autocatalytic lipid oxidation reactions are strongly
influenced by water activity. Troller and Christian (1978) observed that at very low
water activity levels, foods containing unsaturated fats and exposed to atmospheric
oxygen are highly susceptible to the development of oxidative rancidity. The high
oxidative rancidity occurs at a^ levels below the monolayer level, and as a^ increases,
both the rate and the extent of auto-oxidation increase until a^ in the range of
0.3-0.5
is reached. Above this point the rate of oxidation increases until a steady state is
reached, normally at a^ levels in excess of
0.75.
Water activity can also play a significant role in determining the activity of vitamins
in packaged foods. The rate of degradation of vitamins A, Bj, B2 and C increases as
a^ increases over the range 0.24-0.65. The rate of ascorbic acid and riboflavin degra-
dation increases with an increase in a^. Browning reactions generally increase with an
increasing a^ at low moisture content, reach a maximum at a^ of 0.4-0.8, and
decrease with a fiirther increase in water activity.
Gas atmosphere
The sensory properties of packaged foods depend on different physiological and bio-
chemical pathways that are induced by atmospheric conditions. Lipid oxidation and
non-enzymatic browning are the major chemical reactions that lead to deterioration in
the sensory quality of packaged foods. Responses to atmospheric modifications are
found to vary dramatically among packaged foods, and include both unwanted and
Quality of packaged foods 33
beneficial physiological
changes.
Desirable responses include
a
reduction
in
respiration,
oxidative tissue damage or discoloration, rate of chlorophyll degradation, and ethylene
sensitivity, with the concomitant reduction in the rate of ripening. Undesirable
responses include the induction of fermentation, development of disagreeable
flavors,
a
reduction in aroma biosynthesis, induction of tissue injury, and an alteration in the
makeup of microbial fauna.
Atmospheric oxygen generally has a detrimental effect on the nutritive quality of
foods,
and it is therefore desirable to maintain many types of foods at a low oxygen
concentration - or at least to prevent a continuous supply of oxygen into the package.
However, in the presence of sufficient oxygen, the sensory quality is less affected
(Watkins, 2000). Oxygen penetrating the packages affects the rate of carotenoid degra-
dation,
and thus
the color and ascorbic
acid
degradation
(Nagy,
1980;
Gvozdenovic et al,
2000).
The severity of off-flavor production will depend on the time of exposure to
conditions below the minimum required oxygen concentration and/or above the max-
imum tolerated carbon dioxide concentration (Beaudry, 1999, 2000; Watkins, 2000).
The diffusion of gases through
flexible
packaging material depends on the physico-
chemical structure of the barrier. The gas permeabilities of the common thermoplas-
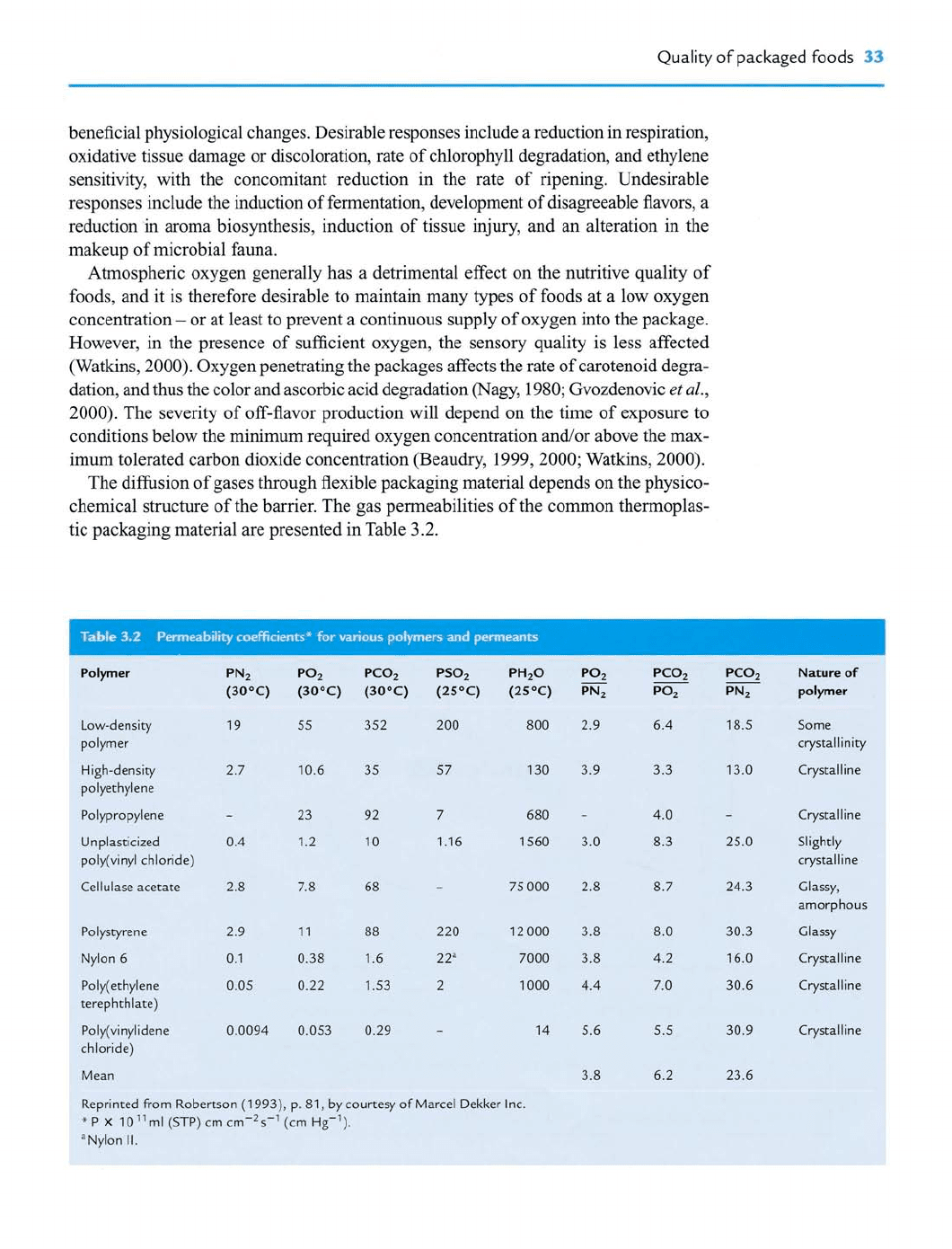
tic packaging material are presented in Table 3.2.
Table 3.2 Permeability coeffid^^^ibr various |^^
Polymer
Low-density
polymer
High-density
polyethylene
Polypropylene
Unplasticized
poly(vinyl chloride)
Cellulase acetate
Polystyrene
Nylon 6
Poly( ethylene
terephthlate)
Poly(vinylidene
chloride)
PN2
(30°C)
19
2.7
-
0.4
2.8
2.9
0.1
0.05
0.0094
PO2
(30<»C)
55
10.6
23
1.2
7.8
11
0.38
0.22
0.053
PCO2
(30°C)
352
35
92
10
68
88
1.6
1.53
0.29
PSO2
(25°C)
200
57
7
1.16
-
220
22a
2
-
PH2O
(ZS^'C)
800
130
680
1560
75 000
12
000
7000
1000
14
PO2
PN2
2.9
3.9
-
3.0
2.8
3.8
3.8
4.4
5.6
PCO2
PO2
6.4
3.3
4.0
8.3
8.7
8.0
4.2
7.0
5.5
PCO2
PN2
18.5
13.0
-
25.0
24.3
30.3
16.0
30.6
30.9
Nature of
polymer
Some
crystal linity
Crystalline
Crystalline
Slightly
crystalline
Glassy,
amorphous
Glassy
Crystalline
Crystalline
Crystalline
Mean
3.8
6.2
Reprinted from Robertson (1993), p. 81, by courtesy of Marcel Dekker Inc.
*P X 10^^ml(STP)cmcm-2s-'' (cm Hg""").
^Nylon II.
23.6
34 Innovations in Food Packaging
Light
Exposure of packaged foods to both ultraviolet radiation and visible light causes
oxidative deterioration of
lipids,
vitamins, proteins, and colorants in foods, leading to
the formation of off-flavors, nutrient losses, and discoloration. Factors influencing the
deteriorative effect of light include the intensity of light, duration of light exposure,
and light transmittance of the packaging material. The interplay between product,
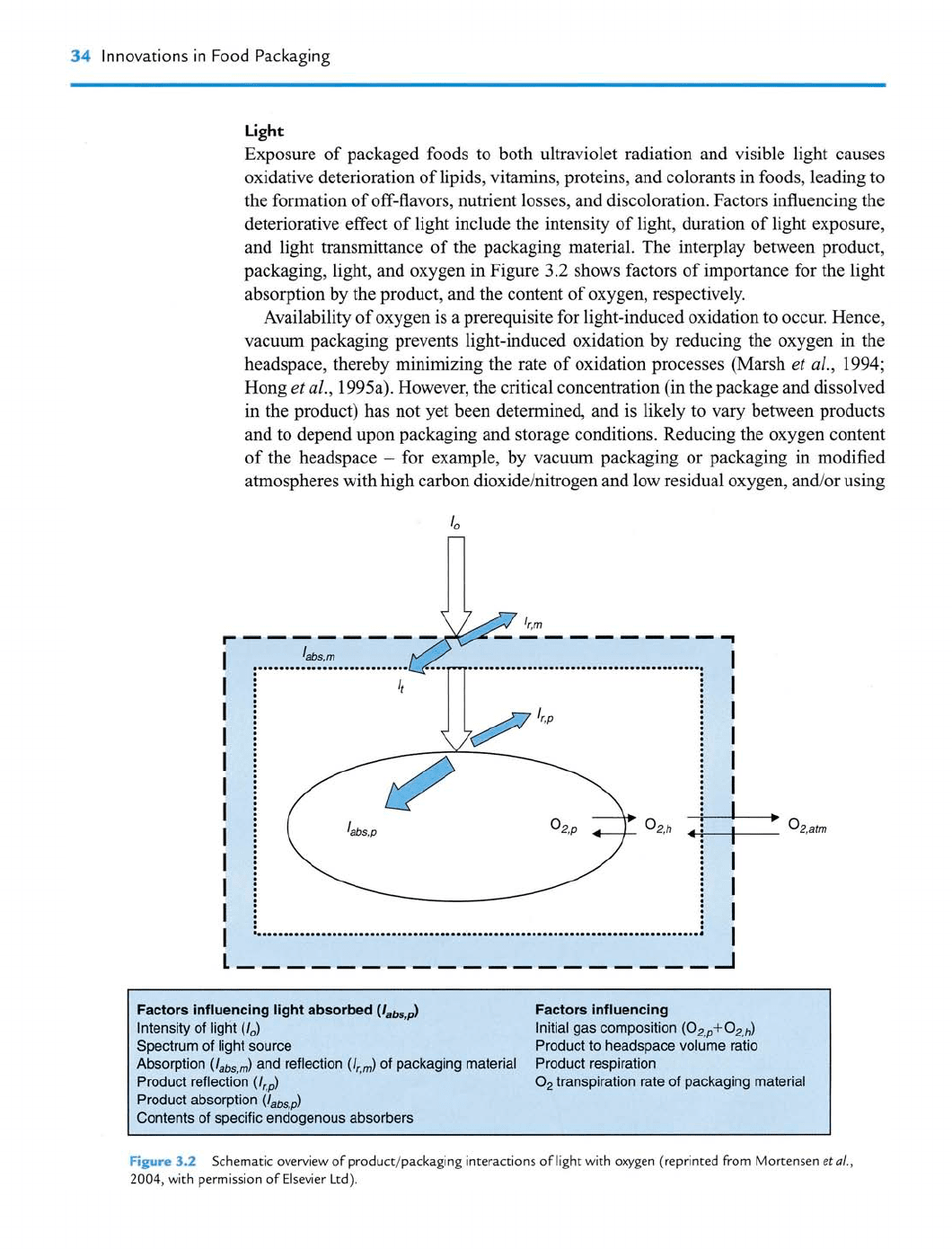
packaging, light, and oxygen in Figure 3.2 shows factors of importance for the light
absorption by the product, and the content of oxygen, respectively.
Availability of oxygen is
a
prerequisite for light-induced oxidation to occur. Hence,
vacuum packaging prevents light-induced oxidation by reducing the oxygen in the
headspace, thereby minimizing the rate of oxidation processes (Marsh et al., 1994;
Hong et
aL,
1995a). However, the critical concentration (in the package and dissolved
in the product) has not yet been determined, and is likely to vary between products
and to depend upon packaging and storage conditions. Reducing the oxygen content
of the headspace - for example, by vacuum packaging or packaging in modified
atmospheres with high carbon dioxide/nitrogen and low residual oxygen, and/or using
Factors influencing light absorbed
{Igbs^f)
Intensity of light
(/Q)
Spectrum of light source
Absorption
{labs,m)
^^^ reflection (/^^) of packaging material
Product reflection (/^p)
Product absorption
(labs,p)
Contents of specific endogenous absorbers
Factors influencing
Initial gas composition (O^^p+O^,/,)
Product to headspace volume ratio
Product respiration
O2 transpiration rate of packaging material
Figure 3.2 Schematic overview of product/packaging interactions of light with oxygen (reprinted from Mortensen
etal.,
2004,
with permission of Elsevier Ltd).

Quality of packaged foods 35
oxygen impermeable packaging - may actually reduce light-induced oxidation by
limiting the oxygen pool available for dissolution, and thereby the reaction in the
aqueous
phase.
Light protection offered
by
packaging materials depends on numerous
factors, including:
1.
The inherent absorption characteristics of the material
2.
The thickness of the material
3.
The material processing conditions
4.
The coloration of the material.
These may all
be
combined
to
optimize the photo-oxidative protection of specific foods.
Different packaging material categories offer varying degrees of protection against
light-induced changes due to differences in reflectance, transmittance, and oxygen
permeability determined by molecular composition of the material. Generally, metals
offer the best protection, followed by paper/paperboard, various plastics, and finally
glass,
through which up to approximately 90% of light is transmitted (Bosset et al,
1994;
Lennersten and Lingert, 1998). Unbleached paper provides
a
better light barrier
than bleached paper, especially at short wavelengths, due to the removal of and alter-
ations in the light-absorbing pigments (the lignins) during the bleaching process
(Mortensen et ai, 2003a). Hence, very different light transmission characteristics are
notable within different categories - a fact that should be utilized for optimization of
packaging with respect to photo-oxidation. For instance, a secondary packaging
consisting of cardboard may protect against photo-oxidation as well as providing
mechanical stability and ease of handling for the retailers.
Nelson and Cathcart (1984) examined
the
effect of the wall thickness of polyethylene
milk bottles on light transmission characteristics, and found that increasing the wall
thickness led to lower light transmission
rates.
Moreover, material processing (orienta-
tion of the polymer, crystallinity, and incorporation of additives) may have an impact
on the light transmission characteristics. Incorporation of titanium oxide into plastic
materials increases light scattering, thus reducing light transmittance - especially light
at wavelengths shorter
than
400 nm (Nelson and Cathcart,
1984;
Lennersten and Lingert,
1998).
Compounds such as carbon black, chalk and talc may also be applied to reduce
light transmittance. Cavitation, which may be used in, for example, the production of
polypropylene, is another approach to increase the light
barrier.
Lennersten and Lingert
(1998) concluded that cavitied films reflect more light than non-cavitied films, which
results in reduced light transmittance.
The catalytic effects of light
are
most pronounced for light in
lower
wavelengths of the
visible/ultraviolet spectrum (Bekbolet,
1990;
Lennersten and Lingert,
1998).
Increasing
light intensity, i.e. the photon flux, accelerates light-induced oxidation (Deger and
Ashoor, 1987; Hong et ah, 1995b; Alves et ai, 2002). Packaging materials absorb
most of the energy-rich ultraviolet light, which is generally not as harmful to the pack-
aged dairy product as is light in the blue-violet region (400-500
nm)
of the spectrum.
Hansen (1996) noted that monochromatic light at 405 and
448
nm was more detri-
mental to the examined dairy spread model than was monochromatic light at 460 nm.
Evidently, prolonged exposure time increases the light-induced damage (Kristensen
et ai,
2000;
Alves et al, 2002; Mortensen et ai, 2002a, 2002b, 2003a, 2003b, 2003c;

36 Innovations in Food Packaging
Wold et ai, 2002; Juric et
aL,
2003;
Kim et
aL,
2003).
These examples show that light
can play an important role in deterioration of nutrients. Depending on the light trans-
mission characteristics of the packaging materials, suitable packaging material can
offer protection of quality packaged foods by absorption or reflection of all or part of
the incident light. Lennersten and Lingnert (2000) observed that the effect on lipid
oxidation in low-fat mayonnaise was most pronounced with ultraviolet radiation,
although short-wavelength visible light also had a significant effect. Polymer materials
offered some protection against lipid oxidation by
filtering
out the ultraviolet radiation
to varying
degrees.
PEN and PET/PEN copolymer offered better protection than PET.
None of the materials offered sufficient protection against color changes.
The total amount of light absorbed by a packaged food can be calculated using the
following formula:
where
labs
= intensity of light absorbed by the food, 4 = intensity of incident light,
Ifp
= fractional transmission by the packaging material, 4^ =
firaction
reflected by the
packaging material, and
I^f
=
fi*action
reflected by the food.
The fraction of the incident light transmitted by any given material can be consid-
ered to follow the Beer-Lambert law:
/ = / e-^
(3.14)
where / = intensity of light transmitted by the packaging material, k =
a.
characteris-
tic constant (absorbance) for the packaging material, andx = thickness of
the
pack-
aging material.
The absorbance k
varies
not only with the nature of the packaging material but also
with the wavelength. Thus the amount of light transmitted through a given package
will be dependent on the incident light and the properties of packaging material.
Aseptic packaging
Aseptic packaging is an alternative to conventional canning in
the
production of
shelf-
stable packaged food
products.
The word "aseptic", derived from the Greek word
septi-
cos,
means the absence or exclusion of putrefactive micro-organisms. Aseptic packaging
technology is
fimdamentally
different
fi-om
that of traditional food-processing systems.
Canning processes commercially sterilize
filled
and sealed containers, while in aseptic
packaging the presterilized product is filled into sterilized containers that are hermet-
ically sealed in a commercially sterile environment. This form of heat treatment enables
the commercial sterilization of the product,
and results in
minimal loss of product qual-
ity and nutrition. A typical flow diagram is shown in Figure 3.3.
In aseptic packaging, raw or unprocessed product is heated, sterilized by holding at
a high temperature for a predetermined amount of
time,
cooled, and then delivered to
