Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

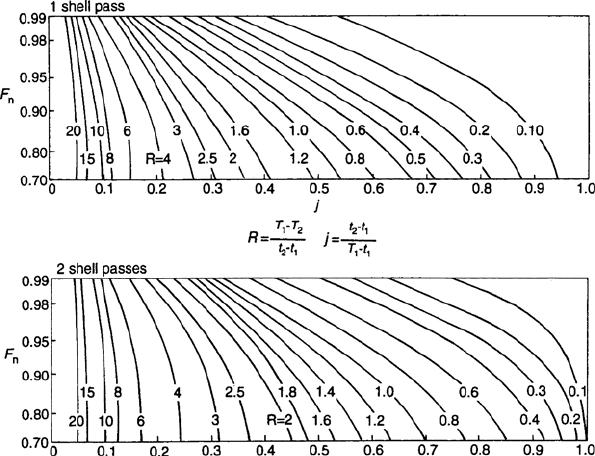
1066 CHAPTER 18
APPENDICES
Figure 18.A.1. LMTD correction factors.
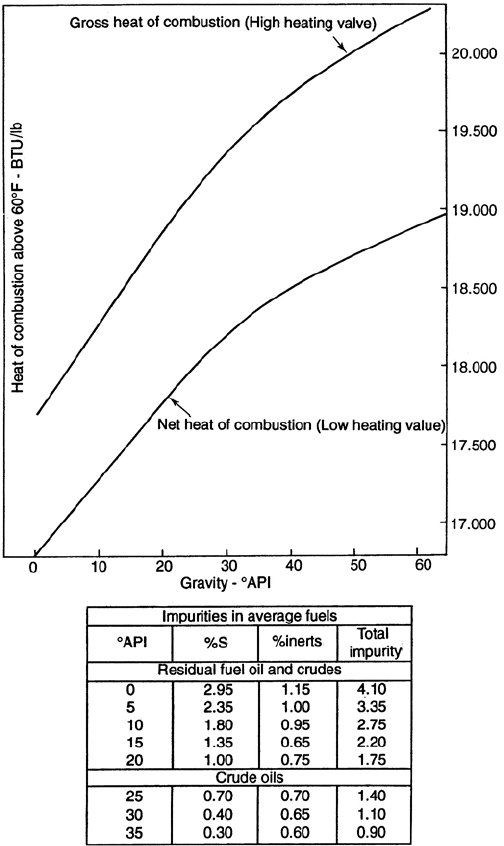
PROCESS EQUIPMENT IN PETROLEUM REFINING 1067
Figure 18.A.2. Heat of combustion of fuel oils.
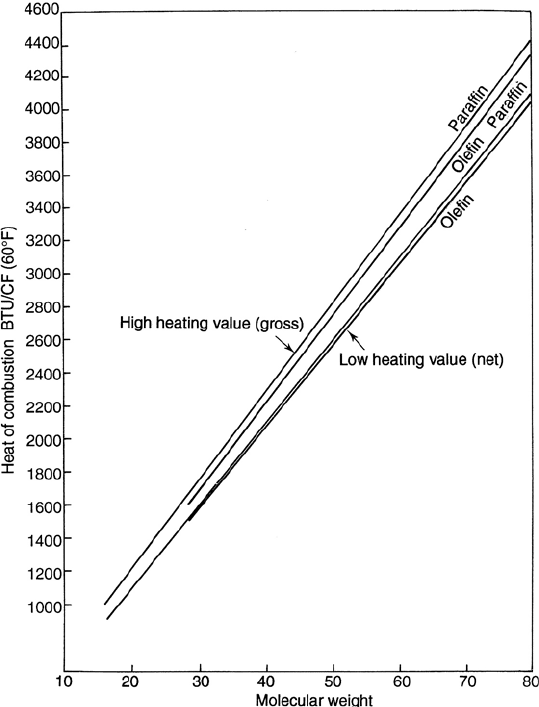
1068 CHAPTER 18
Figure 18.A.3. Heat of combustion of fuel gasses.

PROCESS EQUIPMENT IN PETROLEUM REFINING 1069
Table 18.A.1. Values for coefficient C.

1070 CHAPTER 18
Table 18.A.2. Some common heat transfer coefficients Uo.
Fluid being cooled Fluid being heated
U
0
(BTU/h.f
2
.
◦
F)
Exchangers
C
4
s and lighter Water 75–110
C
4
s and lighter LPG 75
Naphtha Naphtha 75
Naphtha Water 80–100
BPT 450 (kero) Hy oil (crude) 70–75
Gas oils Crude 40–50
Gas oils Water 40–70
Light fuel oil Crude 20–30
Waxy distillates Hy oils 30–40
Slurries Waxy distillate 40
MEA or DEA Water 140
MEA or DEA MEA or DEA 120–130
Water Water 180–200
Air Water 20–30
Lt HC vapour H
2
-rich stream 35–40
Lt HC vapour Naphtha 38
Condensers
Full-range naphtha Water 70–80
Amine stripper O/heads Water 100
C
4
s and lighter Water 90
Reformer effluent (Lt HC) Water 65
Table 18.A.3. Standard exchanger tube sheet data.
External
d
0
= o.d. of BWG l = thickness d
i
=i.d. of Internal area surface per foot
tubing (in.) gauge (ft) tubing (in.) (in.
2
) length (ft
2
)
3
4
10 0.0112 0.482 0.1822 0.1963
3
4
12 0.00908 0.532 0.223 0.1963
3
4
14 0.00691 0.584 0.268 0.1963
3
4
16 0.00542 0.620 0.302 0.1963
3
4
18 0.00408 0.652 0.334 0.1963
1 8 0.0137 0.670 0.355 0.2618
1 10 0.0112 0.732 0.421 0.2618
1 12 0.00908 0.782 0.479 0.2618
1 14 0.00691 0.834 0.546 0.2618
1 16 0.00542 0.870 0.594 0.2618
1 18 0.00408 0.902 0.639 0.2618
1
1
2
10 0.0112 1.232 1.192 0.3927
1
1
2
12 0.00908 1.282 1.291 0.3927
1
1
2
14 0.00691 1.334 1.397 0.3927
1
1
2
16 0.00542 1.37 1.474 0.3927
Chapter 19
A dictionary of terms and expressions
D.S.J. Jones and P.R. Pujad´o
A
Abel flash points
This is a test procedure for determining the flash point of light distillate such as
kerosene. The flash points determined by this method will be in the range of 85–120
◦
C.
The apparatus consists of a water bath with a heating source into which is suspended
a cup containing the material to be tested. The water bath is heated and retained at a
fixed temperature. The temperature of the sample being tested in the cup is measured
by a thermometer. The lid of the cup contains a shutter and a small gas burner from
which a flame of determined length exits. The rate of temperature rise of the test
material is noted and at predetermined temperatures the shutter is opened and the
burner flame exposed to the space above the test liquid in the cup. The temperature
at which a flame is observed crossing the surface of the oil sample when the burner
is dipped into the cup is the flash point. The ASTM name and number for this test is
D56-01 Standard Test Method for Flash Point by Tag Closed Tester.
Absorption units
Absorption units usually consist of a trayed or packed tower in which a gas stream is
contacted with a lean solvent in a counter current flow. Usually the gas stream enters
below the bottom tray or packed bed and rises up the tower meeting the solvent liquid
stream which enters the tower above the top tray or packed bed flowing down the tower.
Undesirable material in the gas stream is selectively absorbed into the liquid stream.
This liquid stream then enters a stripping column (usually a Steam Stripper), where
the absorbed material is stripped off and leaves as an overhead product. The stripped
solvent stream is then rerouted to the absorber tower to complete the cycle. These
processes are used in petroleum refining to remove heavy hydrocarbons from a light
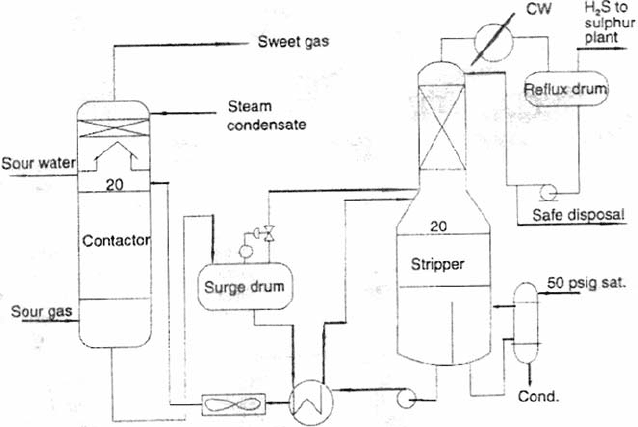
1072 CHAPTER 19
Figure 19.A.1. A schematic of a typical absorption unit.
gas stream. More commonly this type of unit is used in gas treating for the removal
of H
2
S from a gas stream (see Chapter 10 of this book). A schematic flowsheet of an
absorption (gas treating unit) is shown as Figure 19.A.1.
Anhydrous hydro fluoric acid
Anhydrous hydrofluoric acid (AHF) is a colorless, mobile liquid that boils at 67
◦
F
at atmospheric pressure, and therefore requires pressure containers. The acid is also
hygroscopic therefore its vapor combines with the moisture of air to form “fumes”.
This tendency to fume provides users with a built-in detector of leaks in AHF storage
and transfer equipment. On the other hand, care is needed to avoid accidental spillage
of water into tanks containing AHF. Dilution is accompanied by a high release of heat.
Hydrofluoric acid is very corrosive. It attacks glass, concrete, and some metals—
especially cast iron and alloys that contain silica (e.g., Bessemer steels). The acid also
attacks such organic materials such as leather, natural rubber, and wood, but does not
promote their combustion (see Chapter 15 for details).
Air condensers and coolers
Air cooling of process streams or condensing of process vapors is more widely used in
the process industry than cooling or condensing by exchange with cooling water. The
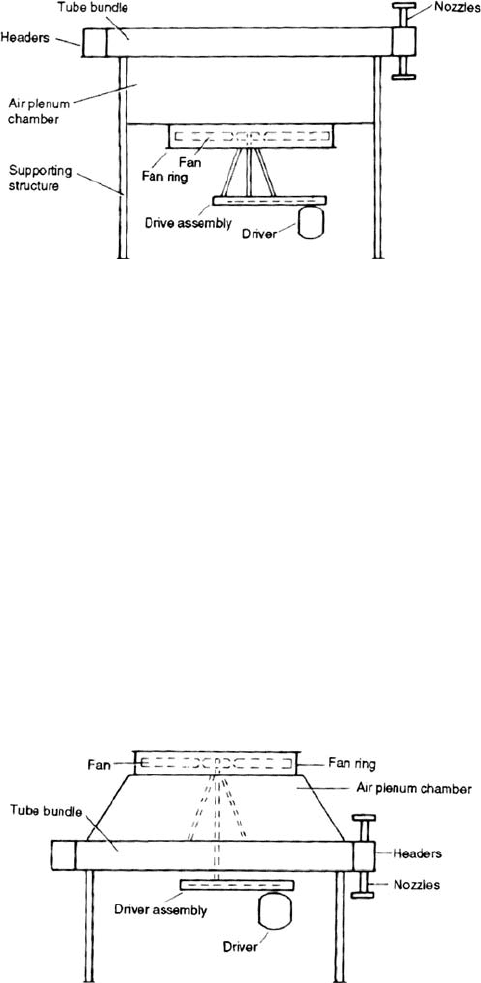
A DICTIONARY OF TERMS AND EXPRESSIONS 1073
Figure 19.A.2. A forced air flow arrangement.
use of individual air coolers for process streams using modern design techniques has
economized in plant area required. It has also made obsolete those large cooling towers
and ponds associated with product cooling. This item in Chapter 18 describes air
coolers in general and outlines a method to estimate surface area, motor horsepower,
and plant area required by the unit.
As in the case for shell and tube exchangers there are many excellent computer
programs that can be used for the design of air coolers. The method given in Chapter 18
for such calculation may be used in the absence of a computer program or for a good
estimate of a unit. The method also emphasizes the importance of the data supplied
to manufacturers for the correct specification of the units.
Figures 19.A.2 and 19.A.3 show the two types of air coolers used in the process
industry. Both units consist of a bank of tubes through which the fluid to be cooled or
condensed flows. Air is passed around the tubes either by a fan located below the tubes
Figure 19.A.3. An induced air flow arrangement.
1074 CHAPTER 19
(Figure 19.A.2) forcing air through the tube bank or a fan located above the tube bank
drawing air through the tube bank (Figure 19.A.3). The first arrangement is called
‘Forced draft’and the second ‘Induced draft’.
Air in both cases is motivated by a fan or fans driven by an electric motor or a
steam turbine or in some cases a gas turbine. The fan and prime driver are normally
connected by a ‘V’belt or by a shaft and gear box. Electric motor drives are by far
the most common prime drivers for air coolers.
The units may installed on a structure at grade or as is often the case on a structure
above an elevated pipe rack. Most air coolers in condensing service are elevate above
pipe racks to allow free flow of condensate into a receiving drum.
Air systems
All chemical and petroleum plants require a supply of compressed air to operate the
plant and for plant maintenance. There are usually two separate systems and these
are:
r
Plant air system
r
Instrument air system
Plant air is generally supplied by a simple compressor with an after cooler. Very
often when plant air is required only for maintenance this is furnished by a mobile
compressor connected to a distribution piping system. Air for catalyst regeneration and
the like is normally supplied by the regular gas compressor on the unit. Instrument air
should always be a separate supply system. Compressed air for instrument operation
must be free of oil and dry for the proper function of the instruments it supplies. This
is a requirement which is not necessary for most plant air usage. A reliable source
of clean dry instrument air is an essential requirement for plant operation. Failure of
this system means a complete shut down of the plant.
Figure 13.36 in Chapter 13 of this Handbook shows a typical instrument air supply
system. Atmospheric air is introduced into the suction of one of two compressors via
an air filter. The compressors are usually reciprocating or screw type non-lubricating.
Centrifugal type compressors have been used for this service when the demand for
instrument air is very high. The air compressors discharge the air at the required
pressure (usually above 45 psig) into an air cooler before the air enters one of two
dryers. One of the compressors is in operation while the other is on standby. The
operating compressor is usually motor driven with a discharge pressure operated
on/off start up switch. The standby compressor is turbine (or diesel engine) driven
with an automatic start up on low/low discharge pressure switch.
A DICTIONARY OF TERMS AND EXPRESSIONS 1075
The cooled compressed air leaves the cooler to enter the dryers. There are two dryer
vessels each containing a bed of desiccant material. This material is either silica
gel (the most common), alumina, or in special cases zeolite (molecular sieve). One
of the two dryers is in operation with the compressed air flowing through it to be
dried and to enter the instrument air receiver. The desiccant in the other dryer is
being simultaneously regenerated. Regeneration of the desiccant bed is effected by
passing through the bed a stream of heated air and venting the stream to atmosphere.
This heated stream removes the water from the desiccant to restore its hygroscopic
properties. At the end of this heating cycle cooled air is reintroduced to cool down
the bed to its operating temperature. When cool, the unit is then shut in ready to be
switched into operation for the first dryer to start its regeneration cycle. The various
operating and regeneration phases are automatically obtained by a series of solenoid
valves operated by a sequence timer switch control. These dryers (often including the
compressor and receiver items) are packaged units supplied, skid mounted, and ready
for operation.
The instrument air receiver vessel is a pressure vessel containing a crinkled wire
mesh screen (CWMS) before the outlet nozzle. It is high-pressure protected by a
pressure control valve venting to atmosphere, and of course is also protected by a
pressure safety valve. The air leaves the top of this vessel to enter the instrument air
distribution system servicing all the plants in the complex.
Alkylation
Alkylation as utilized in the petroleum industry was developed independently by UOP,
Shell, the Anglo Iranian Company, and Texaco in 1932–1936. Motor fuel alkylation
in the petroleum refining industry refers to the acid catalyzed conversion of C
3
–C
5
olefins with isobutane into highly branched C
5
–C
12
isoparaffins collectively called
alkylate, a valuable gasoline blending component. A major constituent of alkylate is
2,2,4-trimethyl pentane which is defined as 100 on the octane scale.
Alkylation reactions are catalyzed by liquid and solid acids, including H
2
SO
4
, AlCl
3
–
HCl, HF, HF–BF
3
,H
2
SO
4
–HSO
3
F (fluorosulfuric acid), trifluoromethane sulfonic
acid chlorided Pt alumina, BF
3
on alumina, zeolites, and ion exchange resins. How-
ever, the catalysts and associated processes commercialized during World War II
for aviation gasoline, were HF alkylation and sulfuric acid alkylation. These are the
process met with in most of today’s refineries (see Chapter 9 for a fuller historical
description).
The chemistry of the alkylation process is quite complex and is given in some detail
in Chapter 9 of this Handbook. Briefly it can be summarized by the following reaction
stages:
