Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

NON-PLAQUE INDUCED INFLAMMATORY GINGIVAL LESIONS • 273
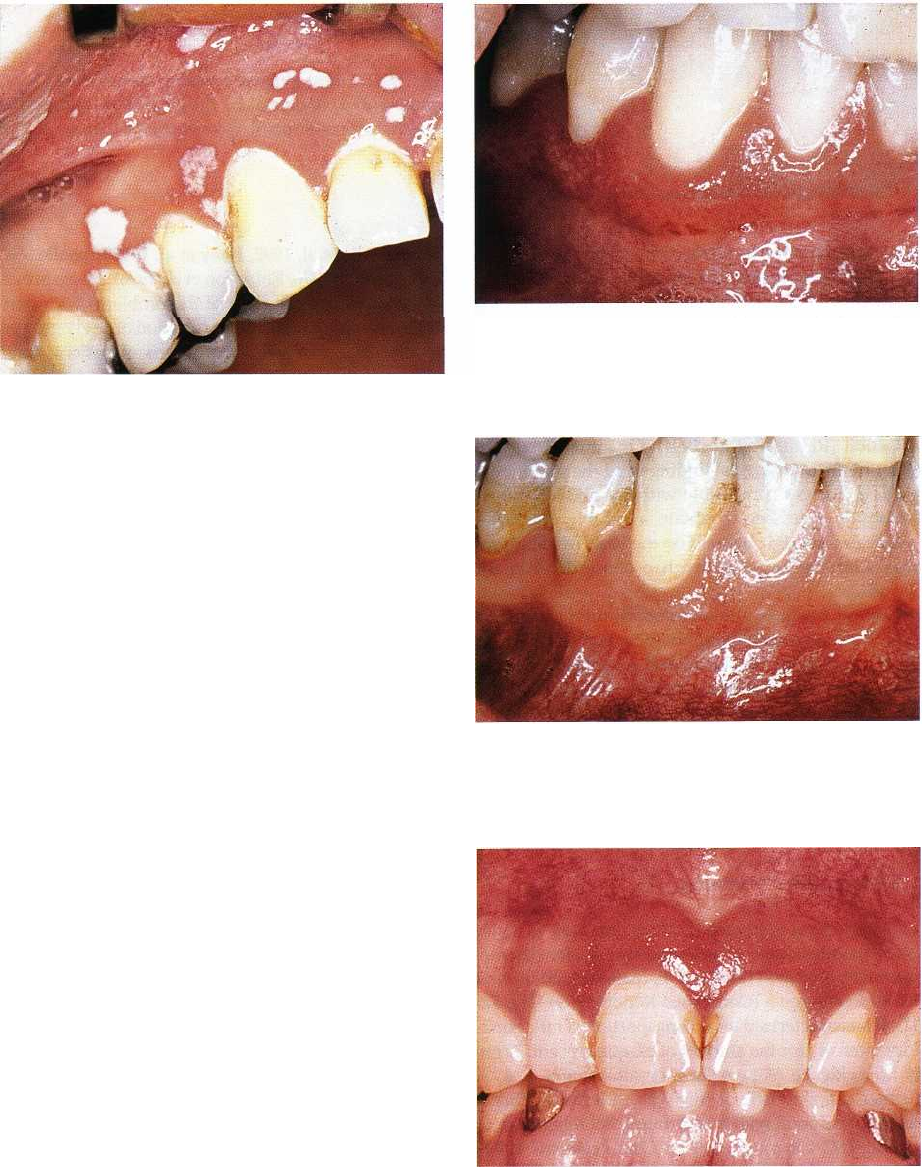
Fig. 12-8. Erythematous candidosis of attached
mandibular gingiva of HIV seropositive mucosa. The
mucogingival junction is invisible.
Fig. 12-7. Pseudomembranous candidosis of maxillary
gingiva and mucosa in HIV seropositive patient. The le
sions can be scraped off, leaving a slightly bleeding
surface.
host defense posture (Holmstrup & Johnson 1997),
including immunodeficiency (Holmstrup & Sama-
ranayake 1990) (Figs. 12-7 and 12-8), reduced saliva
secretion, smoking and treatment with corticoste-
roids, but may be due to a wide range of predisposing
factors. Disturbances in the oral microbial flora, such
as after therapy with broad-spectrum antibiotics, may
also lead to oral candidosis. The predisposing factors
are, however, often difficult to identify. Based on their
site, infections may be defined as superficial or sys-
temic. Candidal infection of the oral mucosa is usually
a superficial infection, but systemic infections are not
uncommon in debilitated patients.
In otherwise healthy individuals, oral candidosis
rarely manifests in the gingiva. This is surprising
when considering the fact that C.
albicans
is frequently
isolated from the subgingival flora of patients with
severe periodontitis (Slots et al. 1988). The most com-
mon clinical characteristic of gingival candidal infec-
tions is redness of the attached gingiva often associ-
ated with a granular surface (Fig. 12-10).
Various types of oral mucosal manifestations are
pseudomembranous candidosis (also known as thrush
in neonates), erythematous candidosis, plaque-type
candidosis, and nodular candidosis (Holmstrup &
Axell 1990). Pseudomembranous candidosis shows
whitish patches (Fig. 12-7), which can be wiped off
the mucosa with an instrument or gauze leaving a
slightly bleeding surface. The pseudomembranous
type usually has no major symptoms. Erythematous
lesions can be found anywhere in the oral mucosa (
Fig. 12-10). The intensely red lesions are usually
associated with pain, sometimes even severe. The
plaque-type of oral candidosis is a whitish plaque,
which cannot be removed. There are usually no symp-
toms and the lesion is clinically indistinguishable
from oral leukoplakia. Nodular candidal lesions are
infrequent in the gingiva. Slightly elevated nodules of
Fig. 12-9. Same patient as shown in Fig. 12-8 after topi-
cal antimycotic therapy. The mucogingival junction is
visible.
Fig. 12-10. Chronic erythematous candidosis of maxil
lary attached gingiva of the incisor region.
white or reddish color characterize them (Holmstrup
& Axell 1990).
A diagnosis of candidal infection can be accom-
plished on the basis of culture, smear and biopsy. A
culture on Nickersons medium at room temperature is
easily handled in the dental premises. Microscopic
examination of smears from suspected lesions is an-
other easy diagnostic procedure, either performed as
274 • CHAPTER 12
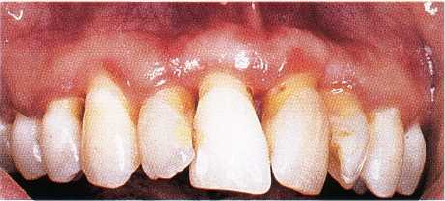
Fig. 12-11. Linear gingival erythema of maxillary
gingiva. Red banding along the gingival margin,
which does not respond to conventional therapy.
direct examination by phase contrast microscopy or as
light microscopic examination of periodic-acid-Schiff-
stained or Gram-stained smears. Mycelium forming
cells in the form of hyphae or pseudohyphae and
blastospores are seen in great numbers among masses
of desquamated cells. Since oral carriage of C.
albicans
is common among healthy individuals, positive cul-
ture and smear does not necessarily imply candidal
infection (Rindum et al. 1994). Quantitative assess-
ment of the mycological findings and the presence of
clinical changes compatible with the above types of
lesions is necessary to obtain a reliable diagnosis,
which can also be obtained on the basis of identifica-
tion of hyphae or pseudohyphae in biopsies from the
lesions.
Topical treatment involves application of antifun-
gals, such as nystatin, amphotericin B or miconazole.
Nystatin may be used as an oral suspension. Since it
is not resorbed it can be used in pregnant or lactating
women. Miconazole exists as an oral gel. It should not
be given during pregnancy and it can interact with
anticoagulants and phenytoin. The treatment in the
severe or generalized forms also involves systemic
antifungals such as fluconazole.
Linear gingival erythema
Linear gingival erythema (LGE) is regarded as a gin-
gival manifestation of immunosuppression charac-
terized by a distinct linear erythematous band limited
to the free gingiva (Consensus Report 1999) (Fig. 12-
11). It is characterized by a disproportion of inflamma
tory intensity for the amount of plaque present. There
is no evidence of pocketing or attachment loss. A
further characteristic of this type of lesion is that it
does not respond well to improved oral hygiene or to
scaling (EC Clearinghouse on Oral Problems 1993).
The extent of gingival banding measured by number
of affected sites has been shown to depend on tobacco
usage (Swango et al. 1991). While 15% of affected sites
were originally reported to bleed on probing and 11%
exhibited spontaneous bleeding (Winkler et al. 1988),
a key feature of linear gingival erythema is now con-
sidered to be lack of bleeding on probing (Robinson et
al. 1994).
Some studies of various groups of HIV-infected
patients have revealed prevalences of gingivitis with
band-shaped patterns in 0.5-49% (Klein et al. 1991,
Swango et al. 1991, Barr et al. 1992, Laskaris et al. 1992,
Masouredis et al. 1992, Riley et al. 1992, Ceballos-Sa-
lobrena et al. 1996, Robinson et al. 1996). These preva
lence values reflect some of the problems with non-
standardized diagnosis and selection of study groups.
A few studies of unbiased groups of patients have
indicated that gingivitis with band-shaped or punc-
tate marginal erythema may be relatively rare in HIV-
infected patients, and probably a clinical finding
which is no more frequent than in the general popu-
lation (Drinkard et al. 1991, Friedman et al. 1991).
It is interesting to note that, whereas there was no
HIV-related preponderance of red banding, diffuse
and punctate erythema was significantly more preva-
lent in HIV-infected than in non-HIV-infected indi-
viduals in a British study (Robinson et al. 1996). Red
gingival banding as a clinical feature alone was, there-
fore, not strongly associated with HIV infection.
There are indications that candidal infection is the
background of some cases of gingival inflammation
including LGE (Winkler et al. 1988, Robinson et al.
1994), but studies have revealed a microflora compris-
ing both C.
albicans,
and a number of periopathogenic
bacteria consistent with those seen in conventional
periodontitis, i.e.
Porphyromonas gingivalis, Prevotella
intermedia, Actinobacillus actinomycetemcomitans, Fuso-
bacterium nucleatum and
Campylobacter rectus
(Murray
et al. 1988, 1989, 1991). By DNA probe detection, the
percentage of positive sites in HIV associated gingivi-
tis as compared with matched gingivitis sites of HIV
seronegative patients for
A. actinomycetemcomitans
was
23% and 7% respectively, for P. gingivalis 52% and
17%, P. intermedia 63% and 29%, and for C. rectus 50%
and 14% (Murray et al. 1988, 1989, 1991). C.
albicans
has been isolated by culture in about 50% of HIV
associated gingivitis sites, in 26% of unaffected sites
of HIV seropositive patients and in 3% of healthy sites
of HIV seronegative patients. The frequent isolation
and the pathogenic role of C.
albicans
may be related
to the high levels of the yeasts in saliva and oral
mucosa of HIV-infected patients (Tylenda et al. 1989).
An interesting histopathologic study of biopsy
specimens from the banding zone has revealed no
inflammatory infiltrate but an increased number of
blood vessels, which explains the red color of the
lesions (Glick et al. 1990). The incomplete inflamma-
tory reaction of the host tissue may be the background
of the lacking response to conventional treatment.
A number of diseases present clinical features re-
sembling those of LGE and which, accordingly, do not
resolve after improved oral hygiene and debridement.
NON-PLAQUE INDUCED INFLAMMATORY GINGIVAL LESIONS •
2
75
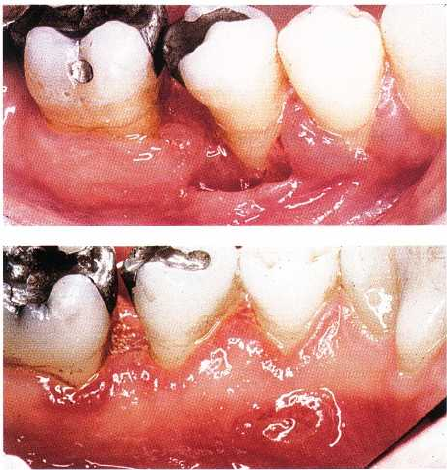
Fig. 12-12. Gingival histoplasmosis with loss of peri
odontal tissue around second premolar.
Fig. 12-13. Same patient as shown in Fig. 12-12. Lin-
gual aspect with ulceration in the deeper part of crater
-formed lesion.
Examples are oral lichen planus, which is frequently
associated with a similar inflammatory red band of
the attached gingiva (Holmstrup et al. 1990) and so is
sometimes mucous membrane pemphigoid (Pind-
borg 1992) or erythematous lesions associated with
renal insufficiency because of the salivary ammonia
production associated with the high levels of urea.
There is little information about treatment based on
controlled studies of this type of affection. Conven-
tional therapy plus rinsing with 0.12% chlorhexidine
gluconate twice daily has shown significant improve-
ment after 3 months (Grassi et al. 1989). It was men-
tioned above that LGE in some cases might be related
to the presence of
Candida
strains. In accordance with
this finding, clinical observations suggest that im-
provement is frequently dependent on successful
eradication of intraoral
Candida
strains, which results
in disappearance of the characteristic features (
Winkler et al. 1988). Consequently, attempts to iden-
tify the presence of fungal infection either by culture
or smear is recommendable followed by antimycotic
therapy in Candida-positive cases.
Histoplasmosis
Histoplasmosis is a granulomatous disease caused by
Histoplasma capsulatum,
a soil saprophyte found
mainly in feces from birds and cats. The infection
occurs in the north-eastern, south-eastern, mid Atlan-
tic and central states of the US. It is also found in
Central and South America, India, East Asia and Aus-
tralia. Histoplasmosis is the most frequent systemic
mycosis in the US. Airborne spores from the mycelial
form of the organism mediate it (Rajah & Essa 1993).
In the normal host, the course of the infection is sub-
clinical (Anaissie et al. 1986). The clinical manifesta-
tions include acute and chronic pulmonary histoplas-
mosis and a disseminated form, mainly occurring in
immunocompromised patients (Cobb et al. 1989). Oral
lesions have been seen in 30% of patients with pulmo-
nary histoplasmosis and in 66% of patients with the
disseminated form (Weed & Parkhill 1948, Loh et al.
1989). The oral lesions may affect any area of the oral
mucosa (Chinn et al. 1995) including the gingiva. They
initiate as nodular or papillary and later may become
ulcerative with loss of gingival tissue, and painful (
Figs. 12-12 and 12-13). They are sometimes granu-
lomatous and the clinical appearance may resemble a
malignant tumor (Boutros et al. 1995). The diagnosis
is based on clinical appearance and histopathology
and/or culture, and the treatment consists of systemic
antifungal therapy.
GINGIVAL LESIONS OF GENETIC
ORIGIN
Hereditary gingival fibromatosis
Gingival hyperplasia (synonymous with gingival
overgrowth, gingival fibromatosis) may occur as a
side effect to systemic medications including pheny-
toin, sodium valproate, cyclosporine and dihydro-
pyridines. These lesions are to some extent plaque-de-
pendent and they are reviewed in Chapter 7. Gingival
hyperplasia may also be of genetic origin. Such lesions
are known as hereditary gingival fibromatosis (HGF),
which is an uncommon condition characterized by
diffuse gingival enlargement, sometimes covering
major parts of, or the total, tooth surfaces. The lesions
develop irrespective of effective plaque removal.
HGF may be an isolated disease entity or part of a
syndrome (Gorlin et al. 1990), associated with other
clinical manifestations, such as hypertrichosis (Horn-
ing et al. 1985, Cuestas-Carneiro & Bornancini 1988),
276 • CHAPTER 12
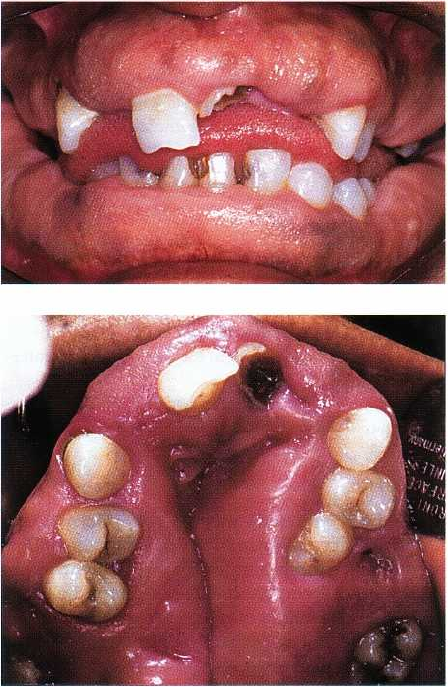
Fig. 12-14. Hereditary gingival fibromatosis. Facial as-
pect with partial coverage of teeth.
Fig. 12-15. Same patient as shown in Fig.12-15. The
maxillary gingival fibromatosis is severe and has re
sulted in total disfiguration of the dental arch.
mental retardation (Araiche & Brode 1959), epilepsy
(Ramon et al. 1967), hearing loss (Hartsfield et al.
1985), growth retardation (Bhowmick et al. 2001) and
abnormalities of extremities (Nevin et al. 1971, Skrin-
jaric & Basic 1989). Most cases are related to an autoso
mal dominant mode of inheritance, but cases have
been described with an autosomal recessive back-
ground (Emerson 1965, Jorgensen & Cocker 1974,
Singer et al. 1993). The most common syndrome of
HGF includes hypertrichosis, epilepsy and mental
retardation; the two latter features, however, are not
present in all cases (Gorlin et al. 1990).
Typically, HGF presents as large masses of firm,
dense, resilient, insensitive fibrous tissue that covers
the alveolar ridges and extends over the teeth result-
ing in extensive pseudopockets. The color may be
normal or erythematous if inflamed (Figs. 12-14 and
12-15). Depending on extension of the gingival en-
largement, patients complain of functional and es-
thetic problems. The enlargement may result in pro-
trusion of the lips, and they may chew on a consider-
able hyperplasia of tissue covering the teeth. HGF is
seldom present at birth but may be noted at an early
age. If the enlargement is present before tooth erup-
tion, the dense fibrous tissue may interfere with or
prevent the eruption (Shafer et al. 1983).
Studies have suggested that an important patho-
genic mechanism may be enhanced production of
transforming growth factor (TGF-beta 1) reducing the
proteolytic activities of HGF fibroblasts, which again
favor the accumulation of extracellular matrix (Colet-
ta et al. 1999). Recently a locus for autosomal domi-
nant HGF has been mapped to a region on chromo-
some 2 (Hart et al. 1998, Xiao et al. 2000), although at
least two genetically distinct loci seem to be responsi-
ble for this type of HGF (Hart et al. 2000).
The histological features of HGF include moderate
hyperplasia of a slightly hyperkeratotic epithelium
with extended rete pegs. The underlying stroma is
almost entirely made up of dense collagen bundles
with only few fibroblasts. Local accumulation of in-
flammatory cells may be present (Shafer et al. 1983).
Histological examination may facilitate the differen-
tial diagnosis from other genetically determined gin-
gival enlargements such as Fabry disease, charac-
terized by telangiectasia.
The treatment is surgical removal, often in a series
of gingivectomies, but relapses are not uncommon. If
the volume of the overgrowth is extensive, a reposi-
tioned flap to avoid exposure of connective tissue by
gingivectomy may better achieve elimination of
pseudopockets.
NON-PLAQUE INDUCED INFLAMMATORY GINGIVAL LESIONS • 277
GINGIVAL DISEASES OF
SYSTEMIC ORIGIN
Mucocutaneous disorders
A variety of mucocutaneous disorders present gingi-
val manifestations sometimes in the form of
desquamative lesions or ulceration of the gingiva. The
most important of these diseases are lichen planus,
pemphigoid, pemphigus vulgaris, erythema multi-
forme and lupus erythematosus.
Lichen planus
Lichen planus is the most common mucocutaneous
disease manifesting on the gingiva. The disease may
affect skin and oral as well as other mucosal mem-
branes in some patients while others may present
either skin or oral mucosal involvement alone. Oral
involvement alone is common and concomitant skin
lesions in patients with oral lesions have been found
in 5-44% of the cases (Andreasen 1968, Axel &
Rundquist 1987). The disease may be associated with
severe discomfort and since it has been shown to
possess a premalignant potential, although this is still a
controversial issue (Holmstrup 1992), it is important to
diagnose and treat the patients and to follow them at
the regular oral examinations (Holmstrup et al. 1988).
The prevalence of oral lichen planus (OLP) in
various populations has been found to be 0.1-4% (
Scully et al. 1998a). The disease may afflict patients at
any age although it is seldom observed in childhood (
Scully et al. 1994).
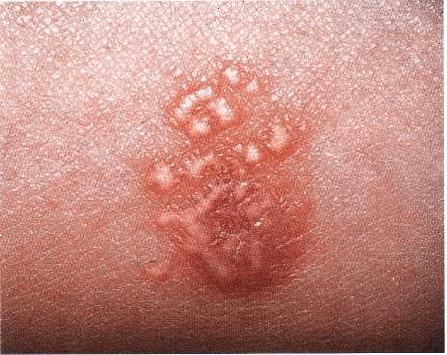
Skin lesions are characterized by red papules with
white striae (Wickham striae) (Fig. 12-16). Itching is a
common symptom, and the most frequent locations
are the flexor aspects of the arms, the thighs and the
neck. In the vast majority of cases the skin lesions
disappear spontaneously after a few months, which is
in sharp contrast with the oral lesions, which usually
remain for years (Thorn et al. 1988).
A variety of clinical appearances is characteristic of
OLP. These include:
• papular (Fig. 12-17)
• reticular (Figs. 12-18 and 12-19)
• plaque-like (Fig. 12-20)
• atrophic (Figs. 12-21 to 12-25)
• ulcerative (Figs. 12-22 and 12-27)
• bullous (Fig. 12-29)
Simultaneous presence of more than one type of lesion
is common (Thorn et al. 1988). The most characteristic
clinical manifestations of the disease and the basis of
the clinical diagnosis are white papules (Fig. 12-17)
and white striations (Figs. 12-18, 12-19, 12-26 and
12-27), which often form reticular patterns (Thorn et
al. 1988). Sometimes atrophic and ulcerative lesions
Fig. 12-16. Skin lesions of lichen planus. Red papules
with delicate white striations.
are referred to as erosive (Rees 1989). Papular, reticular
and plaque-type lesions usually do not give rise to
significant symptoms, whereas atrophic and ulcera-
tive lesions are associated with moderate to severe
pain, especially in relation to oral hygiene procedures
and eating. OLP frequently persists for many years (
Thorn et al. 1988). Any area of the oral mucosa may
be affected by OLP, but the lesions often change in
clinical type and extension over the years. Such
changes may imply the development of plaque-type
lesions, which are clinically indistinguishable from
oral leukoplakia. This may give rise to a diagnostic
problem if other lesions more characteristic of OLP
have disappeared (Thorn et al. 1988).
A characteristic histopathologic feature in OLP is a
subepithelial, band-like accumulation of lymphocytes
and macrophages characteristic of a type IV hypersen-
sitivity reaction (Eversole et al. 1994). The epithelium
shows hyperortho- or hyperparakeratinization and
basal cell disruption with transmigration of lympho-
cytes into the basal and parabasal cell layers (Eversole
1995). The infiltrating lymphocytes have been identi-
fied as CD4+ and CDS+ positive cells (Buechner 1984,
Walsh et al. 1990, Eversole et al. 1994). Other charac-
teristic features are Civatte bodies, which are dyskera-
totic basal cells. Common immunohistochemical find-
ings of OLP-lesions are fibrin in the basement mem-
brane zone, but deposits of IgM, C3, C4, and C5 may
also be found. None of these findings are specific of
OLP (Schie dt et al. 1981, Kilpi et al. 1988, Eversole et
al. 1994).
The subepithelial inflammatory reaction in OLP
lesions is presumably due to a so far unidentified
antigen in the junctional zone between epithelium and
connective tissue or to components of basal epithelial
cells (Holmstrup & Dabelsteen 1979, Walsh et al. 1990,
Sugerman et al. 1994). A lichen planus specific antigen
in the stratum spinosum of skin lesions has been
described (Camisa et al. 1986), but this antigen does
not appear to play a significant role in oral lesions
since it is rarely identified there. It is still an open
278 • CHAPTER 12
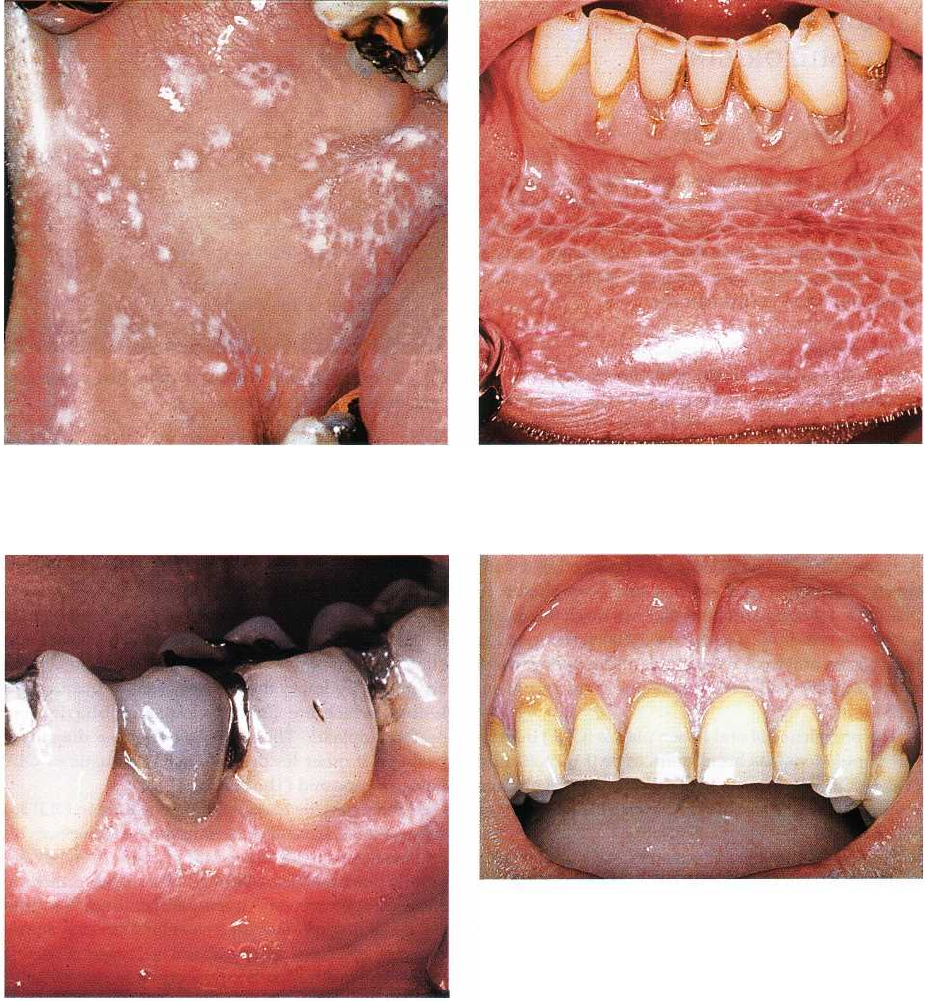
Fig. 12-17. Oral lichen planus. Papular lesion of right
buccal mucosa.
Fig. 12-18. Oral lichen planus. Reticular lesion of lower
lip mucosa. The white striations are denoted Wickham
striae.
Fig. 12-19. Oral lichen planus. Reticular lesions of
gingiva in lower left premolar and molar region.
question whether OLP is a multivariate group of eti-
ologically diverse diseases with common clinical and
histopathological features or a disease entity charac-
terized by a type IV hypersensitivity reaction to an
antigen in the basement membrane area. The clinical
diagnosis is based on the presence of papular or re-
ticular lesions. The diagnosis may be supported by
histopathological findings of hyperkeratosis, degen-
erative changes of basal cells and subepithelial inflam-
mation dominated by lymphocytes and macrophages (
Holmstrup 1999).
The uncertain background of OLP results in several
border zone cases of so-called oral lichenoid lesions (
OLL) for which a final diagnosis is difficult to establ-
ish. The most common OLLs are probably lesions in
Fig. 12-20. Oral lichen planus. Plaque-type lesion of
maxillary gingiva.
contact with dental restorations (Holmstrup 1991) (
see later in this chapter). Other types of OLL are
associated with various types of medications
including antimalarials, quinine, quinidine, non-
steroidal anti-inflammatory drugs, thiazides,
diuretics, gold salts, penicillamine, beta-blockers and
others (Scully et al. 1998a). Graft-versus-host
reactions are also characterized by a lichenoid
appearance (Fujii et a1.1988) and a group of OLL is
associated with systemic diseases of which liver
disease has been revealed lately (Fortune & Buchanan
1993, Bagan et al. 1994, Carrozzo et al. 1996). This
appears to be particularly evident in South-ern Europe
and Japan where hepatitis C has been found in 20-
60% of OLL cases (Bagan et al. 1994, Gandolfo et
al. 1994, Nagao et al. 1995).
Several follow-up studies have demonstrated that
OLP is associated with increased development of oral
cancer, the frequency of cancer development being in
the range of 0.5-2% (Holmstrup et al. 1988).
NON-PLAQUE INDUCED INFLAMMATORY GINGIVAL LESIONS • 279
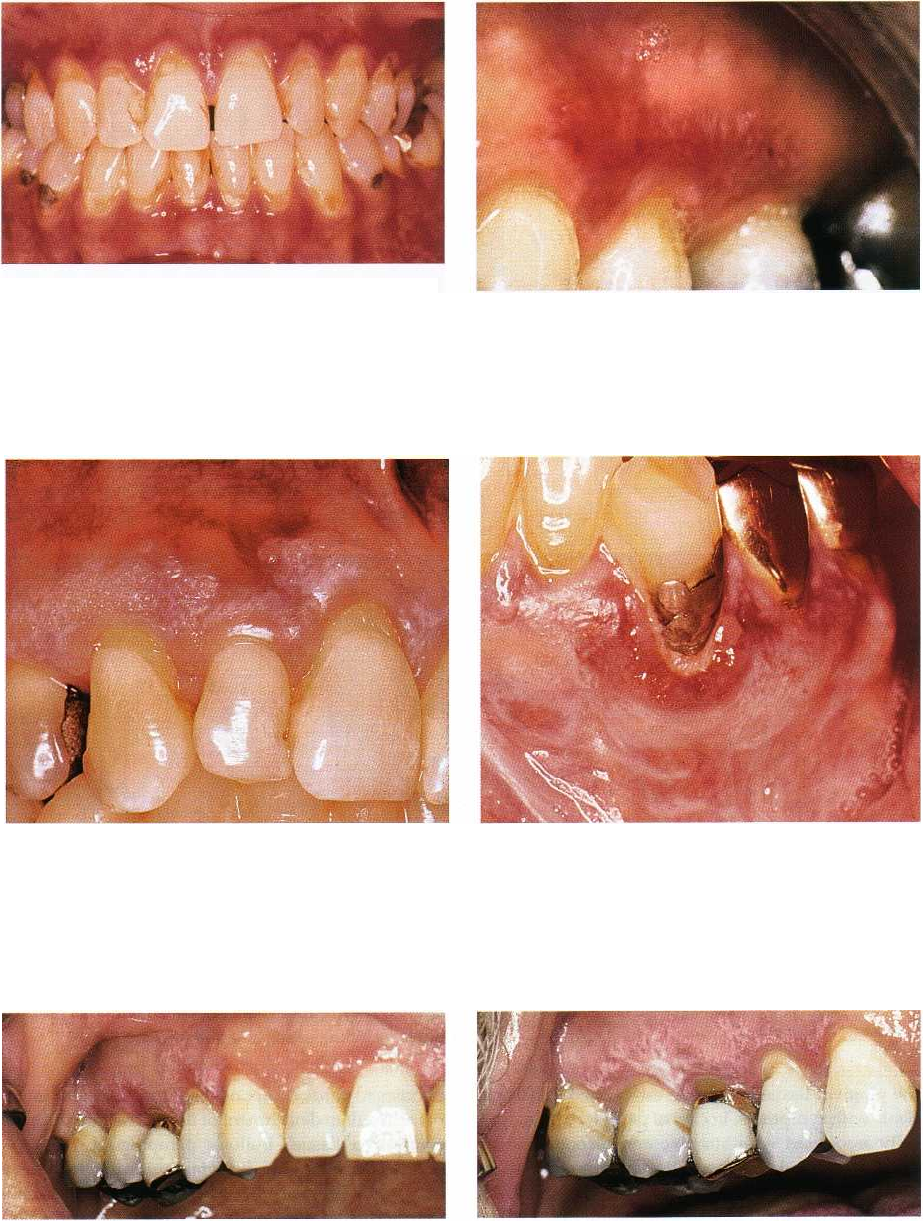
Fig. 12-21. Oral lichen planus. Atrophic lesions of facial
maxillary and mandibular gingiva. Such lesions were
previously often termed desquamative gingivitis.
Fig. 12-22. Oral lichen planus. Atrophic and ulcerative
lesion of maxillary gingiva. Note that the margin of the
gingiva has normal color in upper incisor region,
which distinguishes the lesions from plaque induced
gingivitis.
Fig. 12-23. Oral lichen planus. Atrophic and reticular le-
sion of maxillary gingiva. Several types of lesions are
often present simultaneously.
Fig. 12-24. Oral lichen planus. Atrophic and reticular le-
sion of lower left canine region. Plaque accumulation
results in exacerbation of oral lichen planus, and atro-
phic lesions compromises oral hygiene procedures. This
may lead to a vicious circle that the dentist can help in
breaking.
Fig. 12-25. Oral lichen planus. Atrophic and reticular le-
sion of right maxillary gingiva in a patient using an
electric toothbrush, which is traumatic to the marginal
gingiva. The physical trauma results in exacerbation of
the lesion with atrophic characteristics and pain.
The most important part of the therapeutic regimen is
an atraumatic meticulous plaque control, which results
in significant improvement in many patients
Fig. 12-26. Same patient as shown in Fig. 12-25 after
modified toothbrushing procedure with no traumatic
action on marginal gingiva.
(Holmstrup et al. 1990) (Figs. 12-25 to 12-28). Individ-
ual oral hygiene procedures with the purpose of effec-
tive plaque removal without traumatic influence on
280 • CHAPTER 12
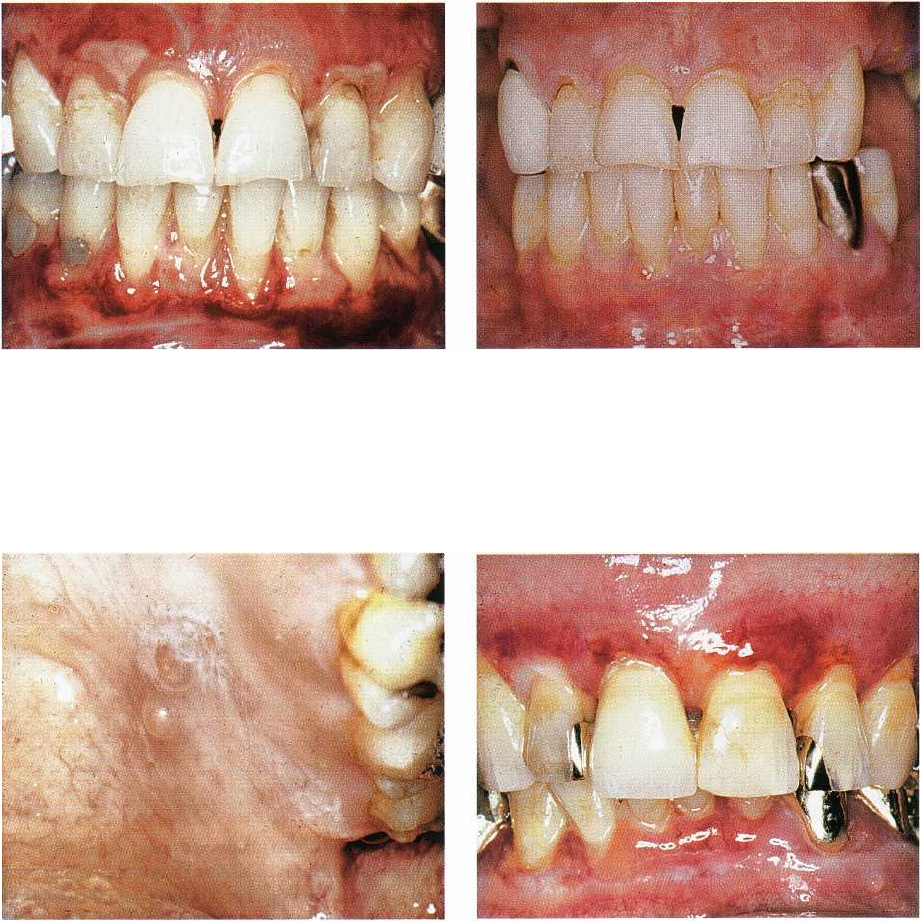
Fig. 12-27. Oral lichen planus. Atrophic and ulcera-
tive/reticular lesions of maxillary and mandibular inci-
sor region. The patient, who is a 48-year-old woman,
suffers from severe discomfort from food, beverages
and toothbrushing.
Fig. 12-28. Same patient as shown in Fig. 12-27 after
periodontal treatment and extraction of teeth with
deep pockets. An individual oral hygiene program,
which ensured gentle, meticulous plaque removal has
been used by the patient for three months. The atro-
phic/ulcerative lesions are now healed and no more
symptoms left.
Fig. 12-29. Oral lichen planus. Bullous/reticular lesion
of left palatal ucosa.
the gingival tissue should be established for all pa-
tients with symptoms. In case of persistent pain, typi-
cally associated with atrophic and ulcerative affec-
tions, antifungal treatment may be necessary if the
affections are hosting yeast, which occurs in 37% of
OLP-cases (Krogh et al. 1987). In painful cases, which
have not responded to the treatment above, topical
corticosteroids, preferably in a paste or an ointment,
should be used three times daily for a number of
weeks. However, in such cases relapses are very com-
mon, which is why intermittent periods of treatment
may be needed over an extended period of time.
Pemphigoid
Pemphigoid is a group of disorders in which autoan-
Fig. 12-30. Benign mucous membrane pemphigoid af-
fecting the attached gingiva of both jaws. The lesions
are erythematous and resemble atrophic lichen planus
lesions. They result in pain associated with oral proce
dures including eating and oral hygiene.
tibodies towards components of the basement mem-
brane result in detachment of the epithelium from the
connective tissue. Bullous pemphigoid predominantly
affects the skin, but oral mucosal involvement may
occur (Brooke 1973, Hodge et al. 1981). If only
mucous membranes are affected, the term benign mu-
cous membrane pemphigoid (BMMP) is often used.
The term cicatricial pemphigoid is also used to de-
scribe subepithelial bullous disease limited to the
mouth or eyes and infrequently other mucosal areas.
This term is problematic, because usually oral lesions
do not result in scarring, whereas this is an important
danger for ocular lesions (Scully et al. 1998b). It is now
evident that BMMP comprises a group of disease
entities characterized by an immune reaction involv-
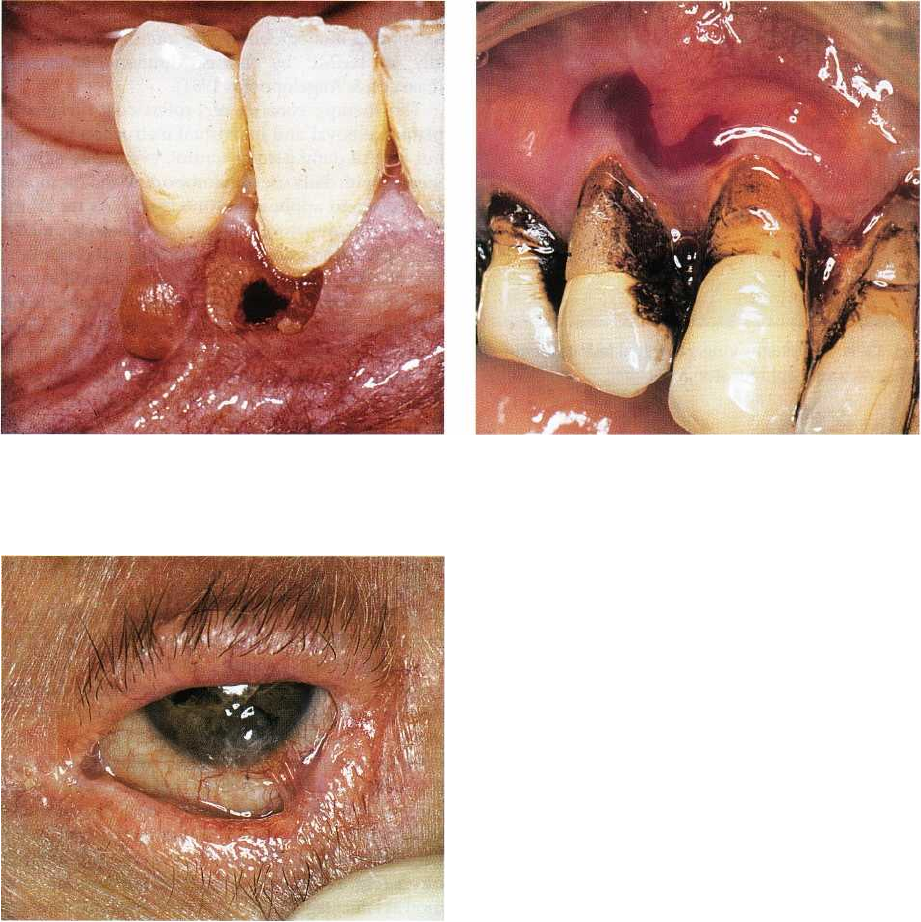
NON-PLAQUE INDUCED INFLAMMATORY GINGIVAL LESIONS • 281
Fig. 12-31. Benign mucous membrane pemphigoid
with intact and ruptured gingival bulla.
Fig. 12-32. Benign mucous membrane pemphigoid
with hemorrhagic gingival bulla. The patient uses
chlorhexidine for daily plaque reduction.
Fig. 12-33. Benign mucous membrane pemphigoid.
Eye lesion with scar formation due to coalescence of
palpebral and conjunctival mucosa.
ing autoantibodies directed against various basement
membrane zone antigens (Scully & Laskaris 1998).
These antigens have been identified as hemides-
mosome or lamina lucida components (Leonard et al.
1982, 1984, Manton & Scully 1988, Domloge-Hultsch
et al. 1992, 1994). In addition, complement-mediated
cell destructive processes may be involved in the
pathogenesis of the disease (Eversole 1994). The trig-
ger mechanisms behind these reactions, however,
have not yet been revealed.
The majority of affected patients are females with a
mean age at onset of 50 years or over (Shklar &
McCarthy 1971). Oral involvement in BMMP is almost
inevitable and usually the oral cavity is the first site of
disease activity (Silverman et al. 1986, Gallagher &
Shklar 1987). Any area of the oral mucosa may be
involved in BMMP, but the main manifestation is
desquamative lesions of the gingiva presenting in-
tensely erythematous attached gingival (Laskaris et
al. 1982, Silverman et al. 1986, Gallagher & Shklar
1987) (Fig. 12-30). The inflammatory changes, as al-
ways when not caused by plaque, may extend over
the entire gingival width and even over the muco-gin-
gival junction. Rubbing of the gingiva may precipitate
bulla formation (Dahl & Cook 1979). This is denoted a
positive Nicholsky sign and is caused by the de-
stroyed adhesion of the epithelium to the connective
tissue. The intact bullae are often clear to yellowish or
they may be hemorrhagic (Figs. 12-31 and 12-32). This,
again, is due to the separation of epithelium from
connective tissue at the junction resulting in exposed
vessels inside the bullae. Usually, the bullae rupture
rapidly leaving fibrin coated ulcers. Sometimes, tags
of loose epithelium can be found due to rupture of
bullae. Other mucosal surfaces may be involved in
some patients. Ocular lesions are particularly impor-
tant because scar formation can result in blindness (
Williams et al. 1984) (Fig. 12-33).
The separation of epithelium from connective tis-
sue at the basement membrane area is the main diag-
nostic feature of BMMP. An unspecific inflammatory
reaction is a secondary histologic finding. In addition,
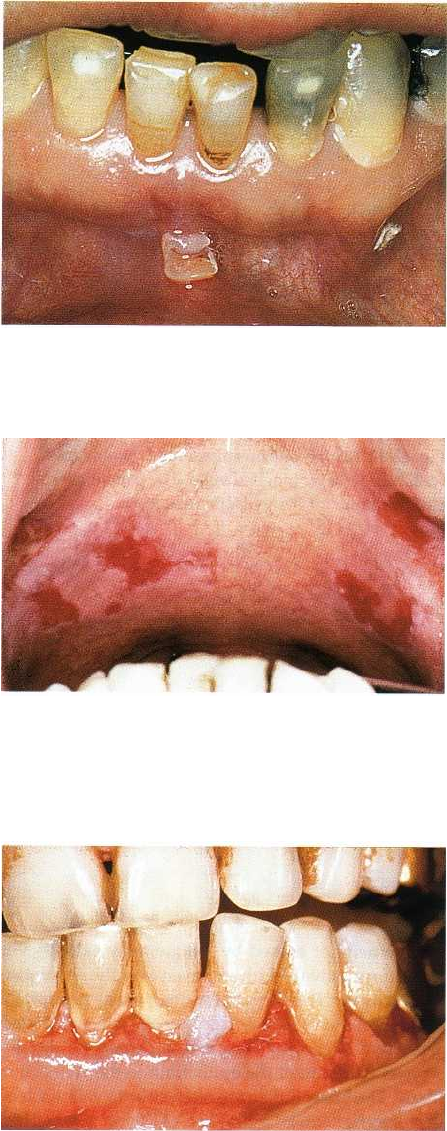
282 • CHAPTER 12
Fig. 12-34. Pemphigus vulgaris. Initial lesion resem-
bling recurrent aphtous stomatitis.
Fig. 12-35. Pemphigus vulgaris. Erosions of soft palatal
mucosa. The erosive lesions are due to loss of the super
ficial part of the epithelium, leaving the connective tis-
sue covered only by the basal cell layers.
Fig. 12-36. Pemphigus vulgaris. Erosive lesions of the
attached gingiva.
immunohistochemical examination can help distin-
guish BMMP from other vesiculobullous diseases, in
particular pemphigus which is life threatening. De-
posits of C3, IgG, and sometimes other immunoglobu-
lins as well as fibrin are found at the basement mem-
brane zone in the vast majority of cases (Laskaris &
Nicolis 1980, Daniels & Quadra-White 1981, Manton &
Scully 1988). It is important to involve peri-lesional
tissue in the biopsy because the characteristic features
may be lost within lesional tissue (Ullman 1988). Cir-
culating immunoglobulins are found only occasion-
ally in BMMP by indirect immunofluorescence (
Laskaris Sr Angelopoulos 1981).
The therapy consists of professional atraumatic
plaque removal and individual instruction in gentle
but careful daily plaque control, eventually supple-
mented with daily use of chlorhexidine and / or topical
corticosteroid application if necessary. As for all the
chronic inflammatory oral mucosal diseases, oral hy-
giene procedures are very important and controlling
the infection from plaque bacteria may result in con-
siderable reduction of disease activity and symptoms.
However, the disease is chronic in nature and forma-
tion of new bullae is inevitable in most patients. Topi-
cal corticosteroids, preferably applied as a paste at
night, temper the inflammatory reaction.
Pemphigus vulgaris
Pemphigus is a group of autoimmune diseases char-
acterized by formation of intraepithelial bullae in skin
and mucous membranes. The group comprises sev-
eral variants, pemphigus vulgaris (PV) being the most
common and most serious form (Barth & Yenning
1987).
Individuals of Jewish or Mediterranean back-
ground are more often affected by PV than others. This
is an indication of a strong genetic background of the
disease (Pisanti et al. 1974). The disease may occur at
any age, but is typically seen in the middle-aged or
elderly. It presents with widespread bulla formation
often including large areas of skin, and if left untreated
the disease is life threatening. Intraoral onset of the
disease with bulla formation is very common and
lesions of the oral mucosa including the gingiva are
frequently seen. Early lesions may resemble aphtous
ulcers (Fig. 12-34), but widespread erosions are com-
mon at later stages (Fig. 12-35). Gingival involvement
may present as painful desquamative lesions or as
erosions or ulcerations, which are remains of ruptured
bullae (Fig. 12-36). Such lesions may be indistinguish-
able from BMMP (Zegarelli & Zegarelli 1977, Sciubba
1996). Since the bulla formation is located in the spi-
nous cell layer, the chance of seeing an intact bulla is
even more reduced than in BMMP. Involvement of
other mucous membranes is common (Laskaris et al.
1982). The ulcers heal slowly, usually without scar
formation, and the disease runs a chronic course with
recurring bulla formation (Zegarelli & Zegarelli 1977).
The diagnosis is based on the characteristic histo-
logical feature of PV that is intraepithelial bulla for-
mation due to destruction of desmosomes resulting in
acantholysis. The bullae contain non-adhering free
epithelial cells, denoted Tzank cells, which have lost
their intercellular bridges (Coscia-Porrazzi et al. 1985,
Nishikawa et al. 1996). Mononuclear cells and neutro-
phils dominate the associated inflammatory reaction.
Immunohistochemistry reveals pericellular epithelial
deposits of IgG and C3. Circulating autoantibodies
against interepithelial adhesion molecules are detect-
