Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

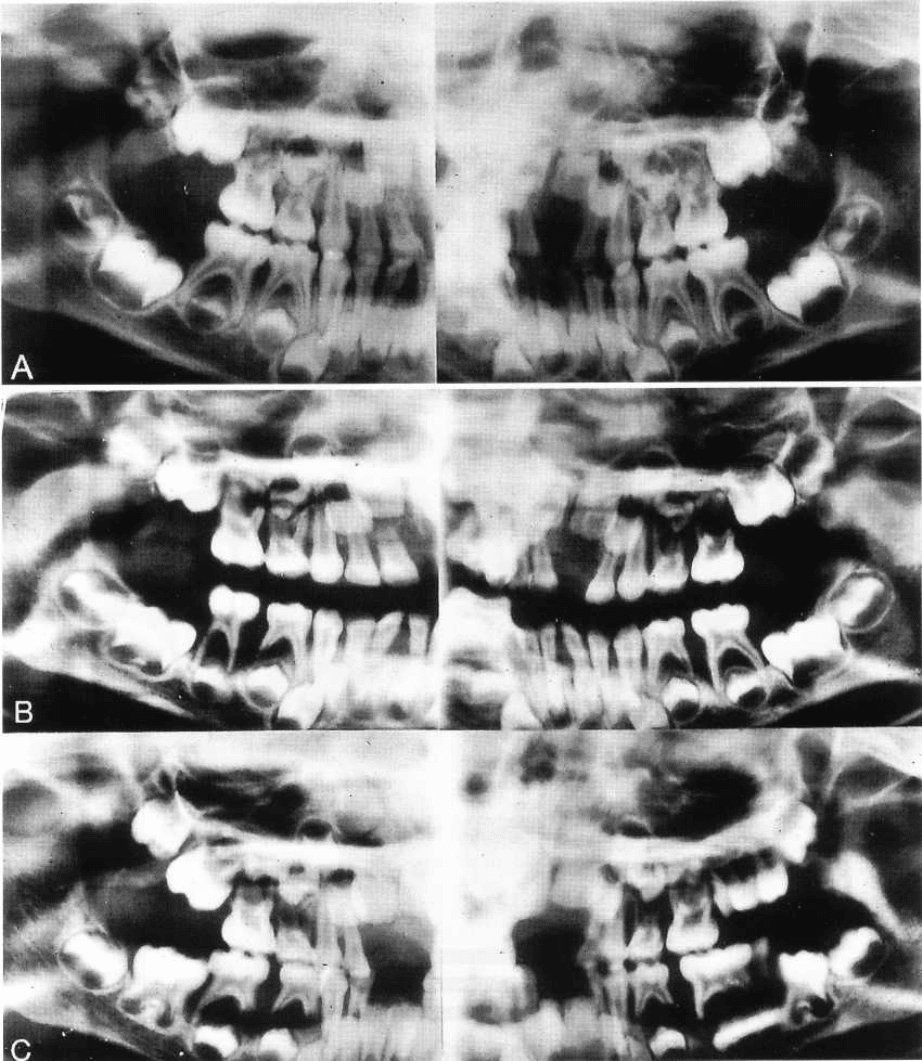
AGGRESSIVE PERIODONTITIS • 223
Fig. 9-4. Radiographs obtained from a Caucasian female with generalized pre-pubertal periodontitis. Radiographic
situation in (A) April 1978 when she was 4-5 years old, (B) December 1978; and (C) August 1979. The
radiographs illustrate the extent of alveolar bone loss that occurred over the 15-month period. Note the
widespread bone loss. During infancy, this patient had severe, recurrent skin and ear infections sustained by
Staphylococcus aureus and
Pseudomonas aeruginosa,
respectively. Delayed healing was also observed following
minor injuries. White cell counts revealed a persistent leukocytosis, with absolute neutrophil counts always above
8000/mm
3
. Gingival biopsy indicated that the inflammatory infiltrate consisted almost completely of plasma cells
and lymphocytes. No neutrophils were present, in spite of the abundance of these cells in the circulation. This
history and clinical manifestation appears to be consistent with the diagnosis of periodontal manifestations of
systemic disease in a subject with leukocyte adhesion deficiency (LAD). From Page et al. 1983.
dontitis. A distance of 2 mm between the cemento- and 9-7) (Sjodin & Mattson 1992). This tentative diagenamel
junction and the alveolar crest, in the absence nosis will have to be confirmed by a complete peri-
of the above-mentioned local factors, argues therefore odontal examination. In utilizing bite-wing radio-
for a suspected diagnosis of periodontitis (Figs. 9-6 graphs for the screening of patients, clinicians should
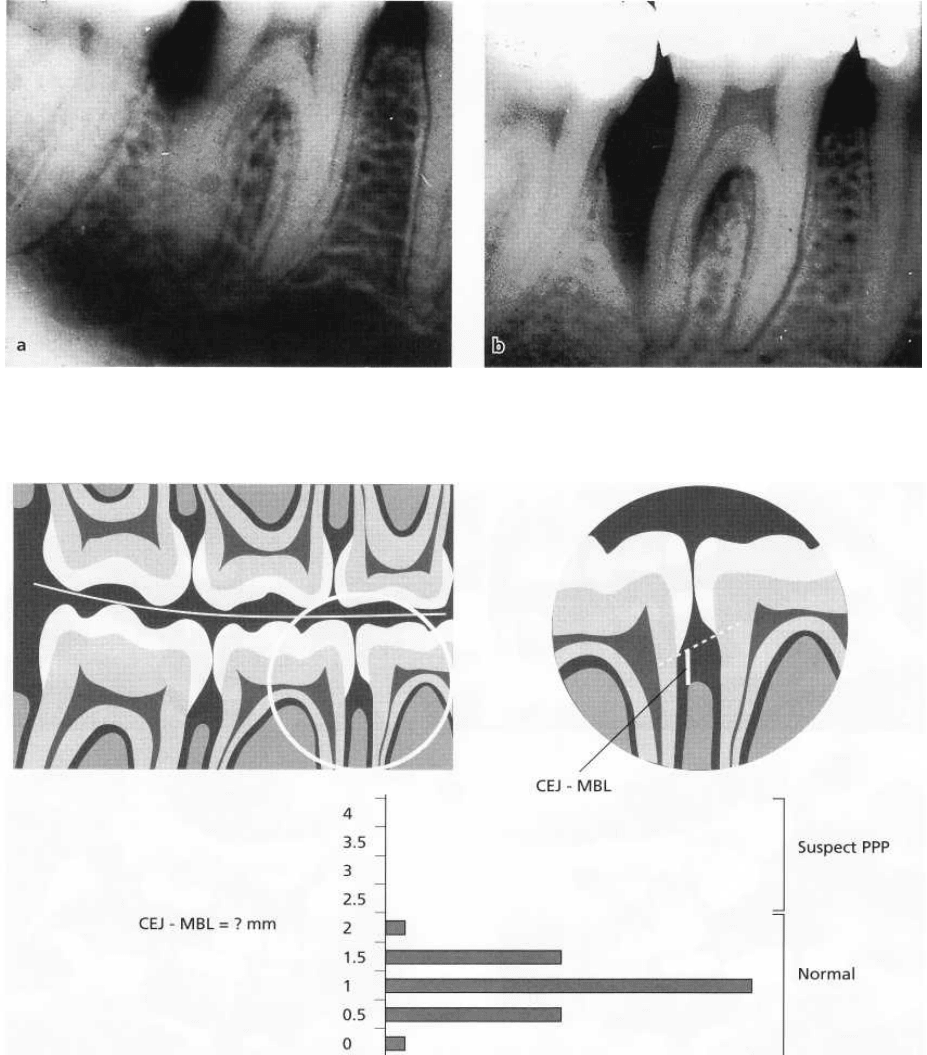
224 • CHAPTER 9
Fig. 9-5. Radiographs illustrating bone loss at the distal aspect of the mandibular first molar in a 15-year-old girl (a)
and progression of disease 1 year later (b).
Fig. 9-6. Diagrammatic representation of the use of bite-wing radiographs to screen for prepubertal periodontitis in
mixed dentition. The distance from the cemento-enamel junction (CEJ) and the marginal bone level (MBL) is meas-
ured from a line connecting the CEJ of the two adjacent teeth. Measurements are taken for each mesial and distal
surface. Normal CEJ-MBL distances for 7-9 year olds are less than 2.0 mm. If the measurement exceeds this
value, prepubertal periodontitis should be suspected, and a comprehensive periodontal examination should be
per-
formed.
be aware that radiographic marginal bone loss (in the
presence of probing attachment loss) is a highly spe-
cific diagnostic sign of periodontitis. Its sensitivity,
however, is lower than that of periodontal probing
because initial intrabony lesions may not appear on
radiographs as a result of the masking effects of intact
cortical plates (Suomi et al. 1968, Lang & Hill 1977).
Some initial cases of periodontitis may therefore re-
main undetected.
In older adolescents and adults, periodontal probing
is a more appropriate screening examination than the
use of radiographs. It is in this respect important to
differentiate between clinical use of periodontal
probing to perform a complete periodontal examina-
tion, and its use as a screening tool. Using probing to
detect attachment loss during a screening examination
requires circumferential probing to evaluate all sites
around the tooth. In a screening examination,
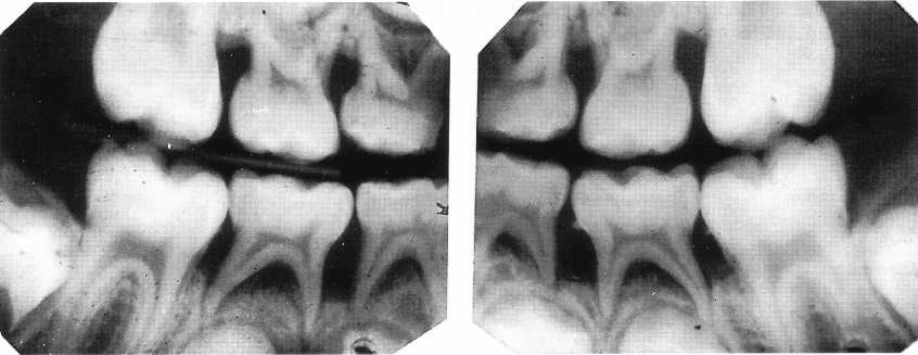
AGGRESSIVE PERIODONTITIS • 225
Fig. 9-7. Bite-wing radiographs illustrating advanced bone loss at primary molars, and initial involvement of
the mesial aspect of the first molar in a child with early onset periodontitis. Note the marginal pattern of bone
loss, which is significantly different from the pattern expected in association with the normal exfoliation of
deciduous teeth. Subgingival calculus can also be observed.
however, attachment loss values for all sites are usu-
ally not recorded. Furthermore, the screening exami-
nation can be stopped once evidence of attachment
loss has been detected, and therefore the need for a
comprehensive examination has been established.
The American Academy of Periodontology has re-
cently endorsed a simplified screening examination
for this purpose. This examination is based on a modi-
fication of the Community Periodontal Index of Treat-
ment Needs (CPITN) (Ainamo et al. 1982, American
Academy of Periodontology & American Dental As-
sociation 1992).
Once a case has been detected by a screening exami
nation, a comprehensive periodontal examination will
be necessary to establish a proper diagnosis. At this
stage, once a case of periodontitis has been con-
firmed, a differential diagnosis between aggressive (
type 1) periodontitis and chronic (type 2) periodonti-
tis needs to be made in accordance with the criteria
mentioned above and keeping in mind that cases
which do not fit the AgP criteria should be diagnosed
as chronic periodontitis.
Conclusion
Screening periodontal examinations should be per-
formed as part of every dental visit. Marginal bone
loss assessed on bite-wing radiographs, though less
sensitive than periodontal probing, may be used as a
screening tool in subjects with primary and mixed
dentitions. Attachment loss evaluated by periodontal
probing is the most sensitive screening approach cur-
rently available; it should be used in older adolescents
and adults. Differential diagnosis between AgP and
chronic periodontitis is made based on exclusion of
AgP.
ETIOLOGY AND PATHOGENESIS
As a group, aggressive forms of periodontitis are char-
acterized by severe destruction of the periodontal at-
tachment apparatus at an early age. This short time of
manifestation of clinically detectable lesions is gener-
ally interpreted as being the expression of highly viru-
lent causative agents or high levels of susceptibility of
the individual patient, or a combination of the two.
Bacterial etiology
The evidence implicating bacterial etiology in perio-
dontitis has been described in Chapter 4. The most
abundant evidence regarding the bacterial etiology of
AgP comes from studies of LAP. Evidence relating to
other forms of AgP (GAP) will be discussed only when
specifically different from LAP.
Acceptance of bacterial etiology of aggressive
forms of periodontitis has been particularly difficult
since clinical presentation of cases frequently shows
little visible plaque accumulation. Of great impor-
tance, in this respect, were microscopic studies dem-
onstrating the presence of a layer of bacterial deposits
on the root surface of advanced AgP lesions (List-
garten 1976, Westergaard et al. 1978). Early studies
attempting the identification of the involved bacteria
using culture techniques were performed by Newman
et al. and by Slots (Newman et al. 1976, Slots 1976,
Newman & Socransky 1977). In these studies, Gram-
negative organisms comprised approximately two-
thirds of the isolates from deep periodontal pockets.
In contrast, these organisms averaged only about one-
third of the isolates in control sites with normal
gingiva. The dominant microorganisms in LIP in-
cluded Actinobacillus actinomycetemcomitans, Capnocy-
tophaga
sp.,
Eikenella corrodens, Prevotella intermedia
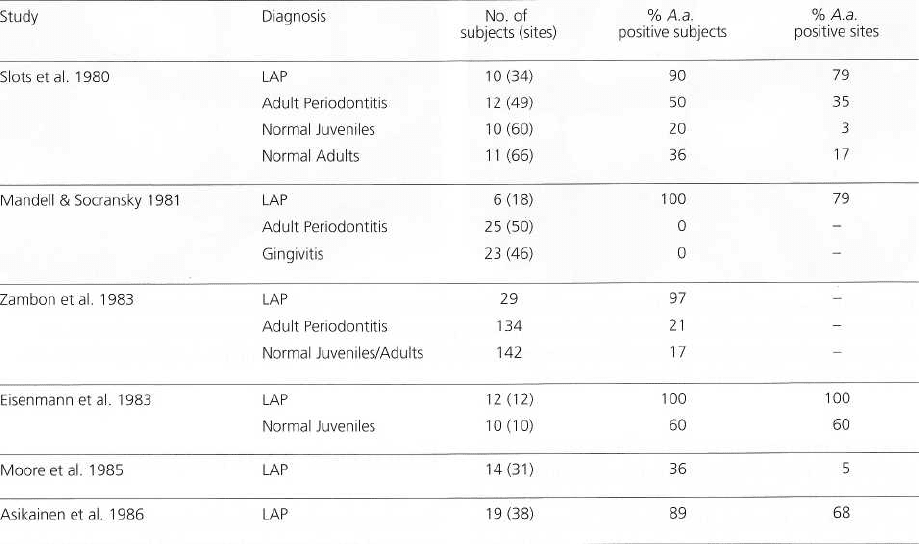
226 • CHAPTER 9
Table 9-1. Classical studies on the distribution of A. a. in LAP, gingivitis, adult periodontitis and in normal
non-diseased subjects
See text for a selection of more recent investigations.
and motile anaerobic rods, such as
Campylobacter rec-
tus. Gram-positive isolates were mostly streptococci,
actinomycetes and peptostreptococci.
One of these organisms, A. actinomycetemcomitans
(A.a.),
has received particular attention in recent years
and is regarded as being a key microorganism in LAP.
This view is based on four lines of evidence (Socran-
sky & Haffajee 1992):
1. Association studies, linking the organism to the dis-
ease: A.a. is generally isolated in periodontal le-
sions from more than 90% of LAP patients and is
much less frequent in periodontally healthy indi-
viduals (Table 9-1) (for more recent investigations,
see also Ashley et al. 1988, Van der Velden et al.
1989, Albandar et al. 1990, Gunsolley et al. 1990,
Slots et al. 1990, Asikainen et al. 1991, Aass et al.
1992, Ebersole et al. 1994, Listgarten et al. 1995). In
some studies it was possible to demonstrate ele-
vated levels of A.a. in sites showing evidence of
recent or ongoing periodontal tissue destruction (
Haffajee et al. 1984, Mandell 1984, Mandell et al.
1987).
2. Demonstration of virulence factors: A.a. produces
several potentially pathogenic substances, includ-
ing a leukotoxin, and is capable of inducing disease
in experimental animals and non-oral sites (for
review see Zambon et al. 1988, Slots & Schonfeld
1991). Furthermore, it can translocate across epi-
thelial membranes.
3. Findings of immune responses towards this bacte-
rium: investigators have repeatedly reported sig-
nificantly elevated levels of serum antibodies to A.
a. in LAP patients (Listgarten et al. 1981, Tsai et al.
1981, Altman et al. 1982, Ebersole et al. 1982,
1983, Genco et al. 1985, Vincent et al. 1985, Mandell
et al. 1987, Sandholm et al. 1987). Such patients also
locally produce antibodies against this organism at
diseased sites (Schonfeld & Kagan 1982, Ebersole
et al. 1985b, Tew et al. 1985).
4. Clinical studies showing a correlation between
treatment outcomes and levels of
A.a.
after therapy:
unsuccessful treatment outcomes have been linked
to a failure in reducing the subgingival load of A.a. (
Slots & Rosling 1983, Haffajee et al. 1984, Chris-
tersson et al. 1985, Kornman & Robertson 1985,
Mandell et al. 1986, 1987, Preus 1988).
A.a. is one of the few oral microorganisms regarded as
true infectious agents in periodontal disease. The ac-
ceptance of this concept has far-reaching conse-
quences with regards to strategies for prevention and
therapy of LAP or AgP. If A.a. is a real exogenous
pathogen, (1) avoidance of exposure to the organism
becomes a relevant issue in prevention, and (2) elimi-
nation of A.a. becomes a valid treatment goal. Thus,
the mere presence of A.a. would have to be regarded
as an indication for intervention. Consequently,
highly sensitive tests to detect the bacterium would be
useful diagnostic tools. Several studies have, in fact,
provided evidence for transmission of A.a. between
humans, e.g. from parent to child or between spouses
AGGRESSIVE PERIODONTITIS • 227
Table 9-2. Determinants of virulence and pathogenic potential of
A. actinomycetemcomitans
Factor
Significance
Leukotoxin
Destroys human polymorphonuclear leukocytes and macrophages
Endotoxin
Activates host cells to secrete nflammatory mediators (prostaglandins, interleukin-1 [3, tumor necrosis factor-a)
Bacteriocin
May inhibit growth of beneficial species
Immunosuppressive factors
May inhibit IgG and IgM production
Collagenases
Cause degradation of collagen
Chemotactic inhibition factors May inhibit neutrophil chemotaxis
(DiRienzo et al. 1990, 1994, Preus et al. 1992, Petit et al.
1993a,b, Poulsen et al. 1994, Von Troil-Linden et al.
1995). Other studies have indicated that
A.a.
can be
eliminated with appropriate mechanical treatment
and adjunctive antibiotic therapy (Rams et al. 1992,
Pavicic et al. 1994).
The view of LAP as an
A.a.
infection is, however,
not undisputed. It has been opposed on the basis of
cross-sectional studies, showing a high prevalence of
this organism in certain populations, particularly
from developing countries (Eisenmann et al. 1983,
Dahlen et al. 1989, McNabb et al. 1992, Al-Yahfoufi et
al. 1994, Gmur & Guggenheim 1994), and for the fact
that there are patients with LJP who apparently nei-
ther show presence of
A.a.
in the oral flora nor have
elevated antibody titers to the organism (Loesche et
al. 1985, Moore 1987).
A.a. is
a short, facultatively anaerobic, non-motile,
Gram-negative rod. Using monoclonal antibody tech-
nology five serotypes (a, b, c, d, e) of
A.a.
can be
distinguished. Serotype-dependent variance in viru-
lence has been suggested. Differences in serotype dis-
tribution have been noted between patients with peri-
odontal disease and apparently non-affected carriers
of
A.a.
Serotype b has been found particularly often in
patients with LAP (Asikainen et al. 1991, Zambon et
al. 1996).
Several properties of
A.a.
are regarded as important
determinants of virulence and pathogenic potential (
Table 9-2). Among them, leukotoxin production is
considered highly significant since it may play an
important role in A.a.'s evasion of local host defences.
The leukotoxin produced by A.a. exhibits cytotoxic
specificity and destroys human polymorphonuclear
leukocytes and macrophages, but neither epithelial
and endothelial cells nor fibroblasts. It belongs to the
family of the RTX (Repeats in ToXin) toxins, which are
pore-forming lytic toxins (for details the reader is
referred to Lally et al. 1996).
A.a.
strains exhibit a wide
range of variability in leukotoxin production. High
leukotoxin-producing strains have been linked to the
etiology of AgP. A substantially higher prevalence of
highly leukotoxic strains has been reported in patients
with LAP than in chronic periodontitis patients or
healthy subjects (Zambon et al. 1996).
All Gram-negative bacteria are enveloped by two
membranes, of which the outer is rich in endotoxin.
This identifying feature of Gram-negative bacteria
consists of a lipid and a polysaccharide part and is
therefore frequently termed lipopolysaccharide (LPS).
LPS is set free when bacterial cells die or multiply. The
LPS of
A.a.
can activate host cells, and macrophages
in particular, to secrete inflammatory mediators such
as prostaglandins, interleukin-113 and tumor necrosis
factor-a. It is also highly immunogenic, since high
titers of antibodies against its antigenic determinant
are frequently detected in infected individuals.
A bacteriocin of A.a., capable of inhibiting the
growth of some streptococci and some actinomyces,
has furthermore been detected (Hammond et al. 1987).
Additional virulence factors interfering with fi-
broblast proliferation have been described for certain
strains of
A.a.
Furthermore, immunosuppressive
properties of A.a., as well as collagenolytic activity and
inhibition of neutrophil chemotaxis, have been dem-
onstrated (for review see Fives-Taylor et al. 1996).
Secretion of membrane vesicles by
A.a.
has been
observed. These vesicles may be important virulence
factors since they may contain leukotoxin, endotoxin
and other factors and may serve as a transport vehicle
to spread pathogenic substances produced by the bac-
terium.
A.a.,
Capnocytophaga sputigena
and
Prevotella inter-
media have also been shown to be the most prominent
members of the subgingival microbiota of periodonti-
tis lesions in the primary dentition. The microbial
patterns observed in periodontal lesions of the pri-
mary dentition, however, seem to be more complex
than the ones detected in LAP patients.
Generalized aggressive periodontitis (GAP), for-
merly named generalized early onset periodontitis (
G-EOP) and rapidly progressive periodontitis (RPP),
have been frequently associated with the detection of
Porphyromonas gingivalis, Bacteroides forsythus and
A.a.
In contrast to A.a., which is facultatively anaerobic, P.
gingivalis and
B. forsythus
are fastidious strict anaer-
obes. P. gingivalis produces several potent enzymes, in
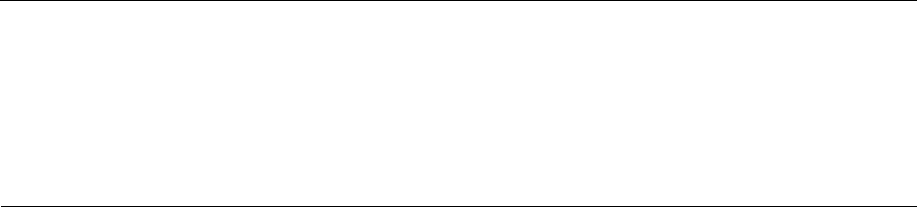
228 • CHAPTER 9
Table 9-3. Host defense mechanisms in the gingival sulcus
Intact epithelial barrier and epithelial attachment
Salivary flushing action, agglutinins, antibodies
Sulcular fluid flushing action, opsonins, antibodies, complement and other plasma components
Local
antibody production
High levels of tissue turnover
Presence of normal flora or beneficial species
Emigrating PMNs and other leukocytes
Modified from Page (1990).
particular collagenases and proteases, endotoxin,
fatty acids and other possibly toxic agents (Shah 1993).
A relationship between the clinical outcome of ther-
apy and bacterial counts has also been documented
for P.
girigivalis,
and non-responding lesions often con-
tain this organism in elevated proportions. High local
and systemic immune responses against this bacte-
rium have been demonstrated in patients with GAP (
Tolo & Schenck 1985, Vincent et al. 1985, Ebersole et
al. 1986, Murray et al. 1989).
Bacterial damage to the periodontium
Disease-associated bacteria are thought to cause de-
struction of the marginal periodontium via two re-
lated mechanisms: (1) the direct action of the microor-
ganisms or their products on the host tissues, and/or (
2) as a result of their eliciting tissue-damaging inflam-
matory responses (see Chapter 5 and Tonetti 1993).
The relative importance of these two mechanisms in
AgP remains speculative. Human investigations have
indicated that Actinobacillus actinonnfcetemcomitans is
able to translocate across the junctional epithelium
and invade the underlying connective tissue (Saglie et
al. 1988). These data support the hypothesis that direct
bacterial invasion may be responsible for some of the
observed tissue breakdown. Data from chronic perio-
dontitis, however, seem to indicate that two-thirds of
attachment loss and alveolar bone resorption is pre-
ventable through the action of non-steroidal anti-in-
flammatory drugs, and therefore tissue destruction
seems to be driven by the inflammatory process (Wil-
liams et al. 1985, 1989). Apical spread of bacteria
loosely adhering to the hard, non-shedding surface of
the tooth is thought to be controlled through a first line
of defense consisting of mechanisms such as the high
turnover of junctional epithelium keratinocytes, the
outward flow of crevicular fluid and the directed mi-
gration of polymorphonuclear leukocytes through the
junctional epithelium; the efficiency of these innate
immune mechanisms is highly enhanced by the pres-
ence in the gingival fluid of specific antibodies and
complement fragments (Page 1990) (Table 9-3).
Host response to bacterial pathogens
Both local and systemic host responses to AgP-associ-
ated microflora have been described. Local inflamma-
tory responses have been characterized by an intense
recruitment of polymorphonuclear leukocytes both
within the tissues and into the periodontal pocket.
Such a preponderance of PMNs underlines the impor-
tance of these cells in the local defense against bacte-
rial aggression and their potential role in host-medi-
ated tissue destruction. B cells and antibody-produc-
ing plasma cells represent a significant component of
the mononuclear cell-dominated connective tissue le-
sion (Liljenberg & Lindhe 1980). Plasma cells have
been shown to be predominantly IgG-producing cells,
with a lower proportion of IgA-producing cells (
Mackler et al. 1977, 1978, Waldrop et al. 1981, Ogawa
et al. 1989). Local IgG
4
-producing cells, in particular,
seem to be elevated. Another important component of
the local inflammatory infiltrate are T cells. Subset
analysis of local T cells has indicated a depressed T-
helper to T-suppressor ratio as compared to both
healthy gingiva and peripheral blood. These findings
have been interpreted to suggest the possibility of
altered local immune regulation (Taubman et al. 1988,
1991). Peripheral blood mononuclear cells from AgP
patients have been reported to exhibit a reduced
autologous mixed lymphocyte reaction, as well as a
higher than normal response to B cell mitogens (for
review see Engel 1996). Local inflammatory responses
are characterized by high levels of prostaglandin E
2
,
interleukin-1c and interleukin-113 in both crevicular
fluid and tissue (Masada et al. 1990, Offenbacher et al.
1993). Prostaglandin E
2
production, in particular, has
been shown to be highly elevated in AgP subjects
when compared to periodontally healthy individuals
and chronic periodontitis patients.
Specific antibodies against AgP-associated micro-
organisms (Lally et al. 1980, Steubing et al. 1982, Eber
sole et al. 1984, 1985a,b) and cleaved complement
fragments (Schenkein & Genco 1977, Patters et al.
1989) have also been detected in crevicular fluid from
AgP lesions. Of interest is the evidence indicating that
crevicular fluid titers of antibodies against AgP-asso-
ciated microorganisms are frequently higher than in
the serum of the same patient (Ebersole et al. 1984,
AGGRESSIVE PERIODONTITIS • 229
(Fig. a)
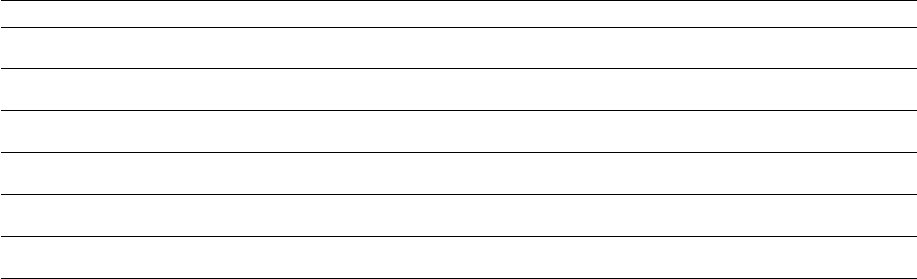
Localized aggressive periodontitis in siblings of 22 families
Fig. 9-8. (a) Patients suffering
from LAP in 22 families are repre
sented by solid black figures. In
each family the proband is on the
left. (h) Diagrammatic repre-
sentation of sibships involved in
study group. Numbers are the
same as in (a). Solid black figures
represent patients exhibiting de-
pressed neutrophil chemotaxis. In
this group, after correcting for
sampling bias, 40% of subjects pre
-sent with abnormal chemotaxis.
Subjects in sibship 8 are identical
twins. From Van Dyke et al. (1985).
Prevalence of LAP
67% of siblings (> 12 yrs), uncorrected
34% of siblings (> 12 yrs)corrected for proband bias
1985a,b). This observation, together with substantial
in vitro and
ex vivo
data, strongly suggests that sub-
stantial fractions of these antibodies are locally pro-
duced in the inflammatory infiltrate (Steubing et al.
7982, Hall et al. 1990, 1991, 1994). Substantial titers of
antibodies against A.a. and
P. gingivalis
have also been
detected in the serum of AgP patients. Furthermore,
in some patients, titers of antibodies reactive with A.a.
have been shown to be as high as the ones against
Treponema pallidum present in tertiary syphilis (0.1-1
g/ml); this clearly indicates the extent of host response
that can be mounted against these periodontal patho-
gens (for a review see Ebersole 1990, 1996).
Recent investigations have identified the immuno-
dominant A. actinomycetcmcomitans antigen to be the
serotype specific carbohydrate; furthermore, it has
been shown that the vast majority of antibodies reac-
tive with this carbohydrate in AgP patients consist of
IgG
2
(Califano et al. 1992). High titers and high avidity
of
A.a.
specific IgG
2
have been demonstrated in LAP
patients, where high antibody titers are thought to be
associated with the host's ability to localize attach-
ment loss to few teeth; conversely, GAP patients are
frequently seronegative for A.a. or display low titers
and avidity. Anti A.a. serotype polysaccharide IgG
2
,
therefore, are considered to be protective against
widespread AgP (Tew et al. 1996).
Of importance are findings reporting antibody re-
sponse to
P. gingivalis
in GAP forms. Patients suffering
from these forms of disease frequently show both low
levels of serum antibodies against P. gingivalis and low
levels of antibody avidity, indicating a specific inabil-
ity of some GAP patients to effectively cope with these
bacteria. Importantly, however, both titers and avidity
of antibodies reacting with
P. gingivalis
can be im-
proved as a result of therapy.
Another important aspect of host response towards
AgP microorganisms has been the recognition that
polymorphonuclear leukocytes (PMN) of some LAP
and GAP patients present decreased migration and
antibacterial functions (Genco et al. 1980, 1986, Van
Dyke et al. 1982, 1986, 1988). These abnormalities are
frequently minor in the sense that they are usually not
associated with infections other than periodontitis. A
(Fig. b)
Neutrophil chemotaxis in LAP families
230 • CHAPTER 9
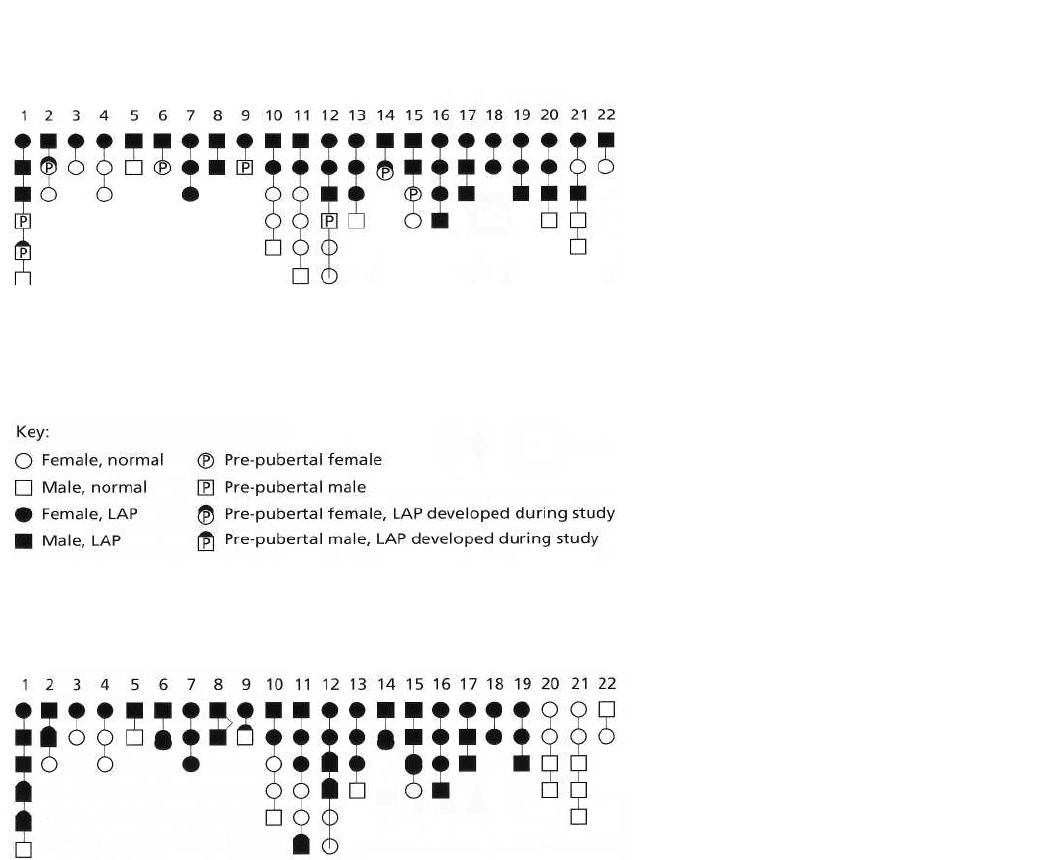
Major gene locus: AgP susceptibility gene
(Fig. a)
H = high IgG
2
titer, I = intermediate IgG
2
titer, L = low IgG
2
titer
--------------------------------------------------------
Clinical disease expression
(Fig. c)
Fig. 9-9. (a) Genetic predisposition
to AgP is determined by a single
gene of major effect, inherited as
an autosomal dominant trait. (b)
Modifying genes may control im-
mune responses that determine the
clinical extent and severity of
periodontal destruction in AgP.
Here an allele controlling IgG
2
lev-
els is inherited as a codominant
trait. (c) Independent inheritance of
major locus and modifying locus
illustrating how LAP and GAP may
segregate within the same family.
The propensity to develop AgP is
dependent upon inheritance of a
major susceptibility gene. The
clinical phenotype is de-pendent
upon host ability to pro-duce IgG
2
in response to periodontopathic
bacteria. High IgG
2
titers limit
disease extension. Intermediate
and low IgG
2
titers are less ef-
fective in limiting intermediate
disease progression. From
Schenkein (1994), as modified by
Hart (1996).
key report has indicated that PMN abnormalities in LAP
patients seem to cluster in families much in the same
way as AgP does (Van Dyke et al. 1985) (Fig. 9-8). This
evidence has been interpreted as a suggestion that the
LAP-associated PMN defect may be inherited. Other
recent reports have indicated that PMN abnor-
malities in LAP patients may be, at least in part, the
result of a hyper-inflammatory state resulting in the
presence of pro-inflammatory cytokines in the serum of
some AgP patients (Shapira et al. 1994, Agarwal et al.
1996).
AGGRESSIVE PERIODONTITIS • 231
Genetic aspects of host susceptibility
Several family studies have indicated that the preva-
lence of AgP is disproportionately high among certain
families, where the percentage of affected siblings
may reach 40-50% (Saxen & Nevanlinna 1984, Beaty
et al. 1987, Long et al. 1987, Boughman et al. 1992,
Marazita et al. 1994). Such a dramatic familial aggre-
gation of cases indicates that genetic factors may be
important in susceptibility to AgP. Genetic studies in
these families suggest that the pattern of disease trans-
mission is consistent with mendelian inheritance of a
gene of major effect (Saxen & Nevanlinna 1984, Beaty
et al. 1987, Boughman et al. 1992, Hart et al. 1992,
Marazita et al. 1994). This means that the observed
familial pattern can be partly accounted for by one or
more genes that could predispose individuals to de-
velop AgP. Segregation analyses have indicated that
the likely mode of inheritance is autosomal dominant (
Saxen & Nevanlinna 1984, Beaty et al. 1987, Hart et
al. 1992, Marazita et al. 1994; Fig. 9-9a). Most of these
investigations, however, were carried out in African-
American populations; it is therefore possible that
other modes of inheritance may exist in different
populations. Segregation analysis can provide infor-
mation about the mode of inheritance of a genetic trait
but does not provide information about the specific
gene(s) involved. The chromosomal location of a gene
of major effect for a trait such as AgP susceptibility can
be determined by linkage analysis. An investigation
utilizing this methodology reported linkage of LAP to
the vitamin D binding locus on region q of chromo-
some 4 in a large family of the Brandywine population
(Boughman et al. 1986). These results, however, were
Table 9-4. Genes known to affect human PMN function or host response to LPS load and/or thought to be
among the candidate genes of major effect in EOP susceptibility
Condition
OMIM*
Trans-
mission
Chromo-
some
location
Comments
Bactericidal
permeability
increasing
protein (BPIP)
109195
AD
20q11-12
BPIP is associated with PMN granules and is bactericidal to Gram
organisms. It binds to LPS with high affinity. BPIP is 45% homologous
to LPS binding protein.
Lipopolysaccharide
binding protein (LBP)
151990
AD
20q11-12 Produced during acute phase of infection: binds to LPS and functions
as a carrier for LPS; functions in monocyte response.
Monocyte
differentiation antigen
(CD 14)
158126
AD
5q31
Receptor for LBP-LPS complex.
Prostaglandin synthase
2 (PTGS2)
600262
AR
1q25.2-3
Major role in regulation of prostaglandin synthesis. Dramatic induction
of PTGS2 mRNA occurs in normal peripheral blood leukocytes in
response to LPS.
PMN actin
dysfunction (NAD)
257150
AR
?
Carriers (heterozygotes) have a 50% decrease in actin filament
assembly; affected individuals (homozygotes) have recurrent bacterial
infections. PMN severely defective in migration and particle ingestion;
basic defect due to failure of PMN actin polymerization.
Myeloperoxidase
deficiency (MPO)
254600
AR
17q12-21
Absence of MPO. MPO is a dimeric protein that catalyzes the
production of oxidating agents with microbicidal activity against a
wide range of microbes. Several variants have been described.
IgE elevation
with PMN
chemotaxis defect
147060
AD
?
Impaired lymphocyte response to Candida antigen; recurrent bacterial
infections.
Fc receptor
gamma IIA
polymorphism
(FCGR2A)
146790
AD
1 q21-q23
Allelic variants of the Fc-gamma receptor 2A confer distinct phagocytic
capacities providing a possible mechanism for hereditary susceptibility
to infection. The H131 allele is the only FGR2A that recognizes IgG
Z
efficiently, and optimal IgG
Z
handling occurs only in the homozygous
state for H131. The allelic variant R131 has low binding of IgG
Z
.
Immunoglobulin G2m
allotypes
N/A N/A
Specific allotypes associated with IgG
Z
response to specific bacterial
antigens. Subjects lacking specific allotypes may be selectively unable
to mount efficient antibody response against specific antigens.
* Online Mendelian Inheritance in Man (OMIM). Modified from Hart (1996).

232 • CHAPTER 9
Table 9-5. Effect of smoking on extent and severity of GAP
Smoking status
Mean % of sites with PAL 5
mm *
Mean PAL (mm) *
GAP
Smokers
49.0 ± 3.9 2.78 ± 0.2
Non-smokers
36.8 ± 3.8 2.14 ± 0.2
* Values adjusted for age and mean plaque index, subject as unit of analysis. Smokers showed significantly greater extent and Rverity of
periodontal disease than non-smokers after correcting for age and oral hygiene level.
Modified from Schenkein et al. (1995).
not confirmed in a subsequent study utilizing a differ-
ent population (Hart et al. 1993). Such data are cur-
rently considered to support the existence of genetic
heterogeneity in LAP forms, and of distinct forms of
AgP. Therefore, it is currently maintained that al-
though formal genetic studies of AgP support the
existence of a gene of major effect, it is unlikely that all
forms of AgP are due to the same genetic defect (Hart
1996). This notion is consistent with the fact that nu-
merous diseases and syndromes with similar clinical
appearance are known to result from different genetic
polymorphisms. Based on current knowledge that
AgP subjects present high prevalence of PMN func-
tion defects, and that they have been shown to pro-
duce high levels of inflammatory mediators in re-
sponse to LPS stimulation, several loci have been
proposed as genes conferring increased susceptibility
to AgP. These genes are associated with neutrophil
function and with the host ability to effectively deal
with LPS exposure, and are listed in Table 9-4 (for
review see Hart 1996).
Besides genes of major effect that may determine
susceptibility to AgP, other genes may act as modify-
ing genes and influence clinical expression of the dis-
ease. In this respect, particular interest has been fo-
cused on the impact of genetic control on antibody
responses against specific AgP associated bacteria and
against A.a. in particular. These studies have indicated
that the ability to mount high titers of specific antibod
ies is race-dependent and probably protective (Gun-
solley et al. 1987, 1988). This has been shown to be
under genetic control as a co-dominant trait, inde-
pendent of the risk for AgP. In individuals susceptible
to AgP, therefore, the ability to mount high titers of
antibodies (IgG
2
in particular) may be protective and
prevent extension of disease to a generalized form (
Schenkein 1994, Fig. 9-9b,c). Allelic variations in the
Fc receptor for IgG
2
immunoglobulins have also been
suggested to play a role in suboptimal handling of A.a.
infections. PMN expressing the R131 allotype of
FcyRIIa (i.e. an Fc receptor containing an arginine
instead of a histidine at aminoacid 131) show de-
creased phagocytosis of
A.a.
(Wilson & Kalmar 1996).
Environmental aspects of host susceptibility
Recent evidence has indicated that, besides genetic
influences, environmental factors may affect the clini-
cal expression of AgP. In a large study, cigarette smok
ing was shown to be a risk factor for patients with
generalized forms of AgP (Schenkein et al. 1995).
Smokers with GAP had more affected teeth and
greater mean levels of attachment loss than patients
with GAP who did not smoke (Table 9-5). Environ-
mental exposure to cigarette smoking, therefore,
seems to add significant risk of more severe and preva
lent disease to this group of already highly
susceptible subjects. The mechanism(s) for this
observation are not completely understood, but
findings from the same group indicate that IgG
2
serum
levels as well as antibody levels against A.a. are
significantly de-pressed in subjects with GAP who
smoked. Since these antibodies are considered to
represent a protective response against A.a., it is
possible that depression of IgG
2
in smokers may be
associated with the observed increase in disease
extent and severity in these subjects.
Current concepts
Aggressive forms of periodontitis are currently con-
sidered to be multifactorial diseases developing as a
result of complex interactions between specific host
genes and the environment. Inheritance of AgP sus-
ceptibility is probably insufficient for the develop-
ment of disease: environmental exposure to potential
pathogens endowed with specific virulence factors is
also a necessary step. Host inability to effectively deal
with the bacterial aggression and to avoid inflamma-
tory tissue damage results in the initiation of the dis-
ease process. Interactions between the disease process
and environmental (e.g. cigarette smoking) and ge-
netically controlled (e.g. IgG
2
response to A.a.) modi-
fying factors are thought to contribute to determining
the specific clinical manifestation of disease (Figs. 9-
9a-c and 9-10).
