Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

730 18 Titanium Silicalite-1
produced glycolic acid. Further oxidation yielded the α - dicarbonyl derivatives and,
at prolonged contact time, C
–
C bond oxidative cleavage.
The oxidation of tetrahydrofuran, tetrahydropyran and dihydropyran produced
γ - butyrolactone and δ - valerolactone in fairly good yields. Linear ethers, conversely,
produced exclusively the corresponding acids, presumably through the in situ
hydrolysis of intermediate esters [116] .
The Baeyer – Villiger rearrangement of cyclohexanone and acetophenone with
TS - 1/H
2
O
2
proved to be poorly selective [117] . Notably, Ti - β and Sn - β have different
chemoselectivities in the oxidation of unsaturated ketones, leading selectively to
corresponding epoxides and lactones, respectively [118] . The different oxidation
pathways were attributed to the preferential adsorption of hydrogen peroxide on
Ti - sites and of the carbonyl group on Sn - sites.
18.6
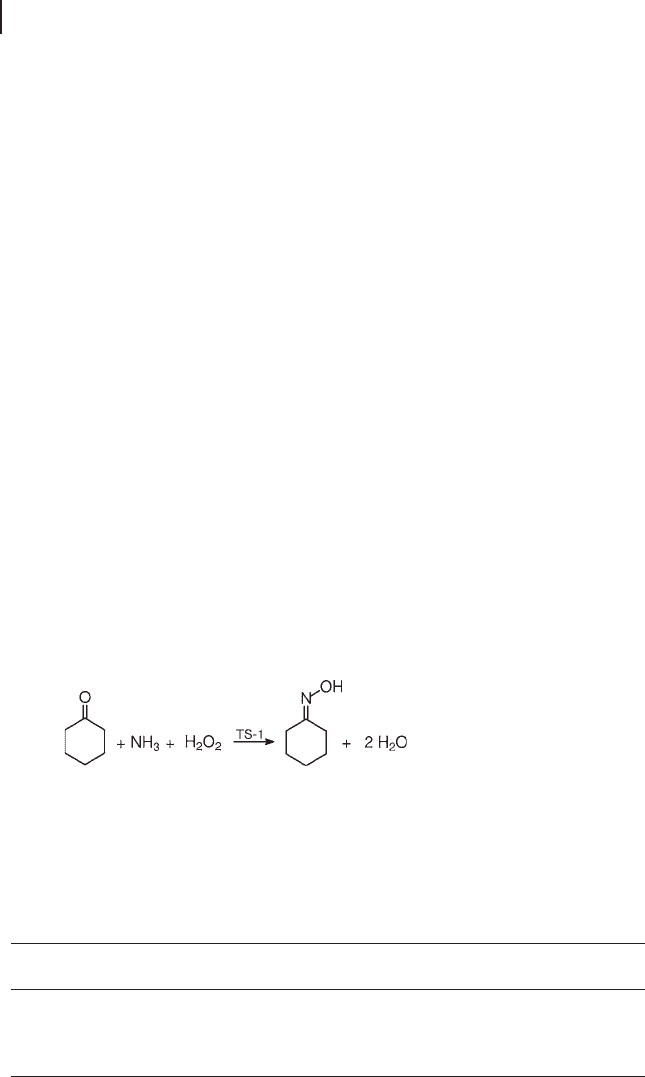
Ammoximation of Carbonyl Compounds
The word ammoximation defi nes the reaction of a carbonyl compound with
ammonia, under oxidative conditions, to yield the corresponding oxime (Equation
18.12 ). Early homogeneous and heterogeneous catalysts, for example heteropoly
compounds and amorphous Ti/SiO
2
, had too poor selectivity to envisage any
application. The prospects changed when Roffi a and coworkers discovered that
TS - 1 was an excellent ammoximation catalyst with hydrogen peroxide as oxidant
[119] . As Table 18.12 shows, TS - 1 and amorphous Ti/SiO
2
, albeit with similar Ti
contents, lead to completely different results: to quantitative yields for the former
and to a mixture of products, with only a minor amount of oxime, for the latter.
(18.12)
In contrast to the stability shown in other oxidations, TS - 1 undergoes a slow but
irreversible deactivation. The siliceous matrix of the catalyst, in fact, slowly dis-
solves in the basic medium, while Ti, released from the framework, aggregates to
Table 18.12 Ammoximation of cyclohexanone on different catalysts.
a)
Cat. Ti (wt%) Conv. C
6
H
10
O (%) Sel. on C
6
H
10
O (%) Yield on H
2
O
2
(%)
TS - 1 1.5 99.9 98.2 93.2
Ti/SiO
2
1.5 49.3 9.3 4.4
S - 1 – 59.4 0.5 0.3
a Solvent, t - butanol/water; T , 80 ° C; molar ratios, cyclohexanone:
NH
3
: H
2
O
2
1.0 : 2.0 : 1.1; t.o.s. 1.5 h.
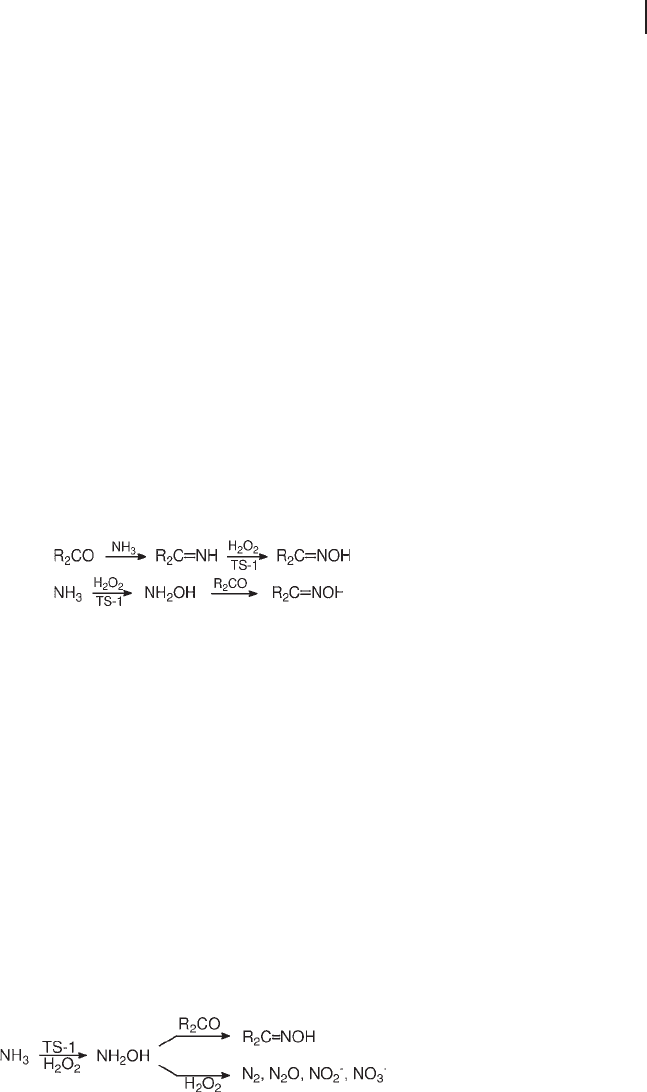
form TiO
2
particles. Accordingly, the activity decreases with time on - stream,
approaching that of conventional catalysts at prolonged times [120] . Major side
reactions consist of the oxidation of ammonia to N
2
, N
2
O, nitrites and nitrates and
in the decomposition of H
2
O
2
to molecular O
2
. Condensation reactions of cyclo-
hexanone in the basic medium and consecutive oxidation of the oxime produce
minor amounts of organic by - products. However, the losses of the oxidant are
generally moderate, for example < 7% under the conditions of Table 18.12 , and the
decay of the catalyst is not so fast as to preclude the development of an industrially
viable process.
The reaction of ammoximation is generally applicable and provides an effi cient
route to many other oximes in addition to the industrially relevant cyclohexanone
oxime. Acetone, butanone, acetophenone, C
5
–
C
8
cyclic ketones and methyl - sub-
stituted cyclohexanones produced the corresponding oximes with high conversion
and selectivity. Even the ammoximation of cyclododecanone and 4 - butylcyclohexa-
none, unable to diffuse in TS - 1, occurred with high yields [121 – 123] . Other ammo-
ximation catalysts are carbon - supported TS - 1, TS - 2, Ti - MOR and Ti - MWW, with
conversions and selectivities close to those of TS - 1 [124 – 128] .
Two ammoximation mechanisms were initially proposed, one envisaging the
formation of cyclohexanone imine (Equation 18.13 ) and the other that of hydrox-
ylamine (Equation 18.14 ) as key intermediates.
(18.13)
(18.14)
The high yields obtained from ketones unable to diffuse inside the pores of TS - 1,
namely cyclododecanone and 4 - butylcyclohexanone, strongly supports the second
mechanism [121, 123] . The imine intermediate, if present, could only play a sec-
ondary role in the overall balance of the ammoximation process. In this regard,
Mantegazza and others showed that TS - 1, under the reaction conditions, catalyzes
the oxidation of ammonia to hydroxylamine with yields greater than 60% [129] .
The subsequent condensation with the carbonyl group is a fast reaction and does
not require catalysis by TS - 1. It occurs in the external medium with bulky ketones,
while with smaller ones the intra - porous volume also could be involved. In the
absence of a carbonyl group acceptor, hydroxylamine undergoes consecutive oxida-
tion to N
2
, N
2
O, nitrites and nitrates. On these grounds, the overall ammoximation
process could be better defi ned as an oximation reaction with in situ production
of hydroxylamine. The relative rates of condensation and consecutive oxidation
determine the selectivity of the overall process (Scheme 18.13 ).
Scheme 18.13 Competing reactions of hydroxylamine with cyclohexanone and H
2
O
2
.
18.6 Ammoximation of Carbonyl Compounds 731
732 18 Titanium Silicalite-1
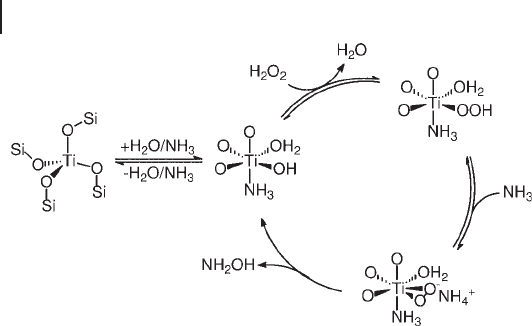
The nature of active species and the mode of generation of hydroxylamine are
the subject of several studies by various spectroscopic techniques [20, 130] . Accord-
ing to Zecchina and coworkers, the chemisorption of hydrogen peroxide produces
the familiar Ti
–
OOH species, which, however, in the basic medium evolves
towards a side - on - bonded anionic peroxide Ti( η
2
–
O
2
)
−
(Scheme 18.14 ). The adsorp-
tion of water and ammonia completes the coordination sphere of Ti, leading to
various species in which either adsorbate can be present with one or two molecules
at each time. In Scheme 18.14 , the suggestion is made, somewhat arbitrarily, of
the mixed adsorption species. A second issue that spectroscopic data do not help
solve is whether hydroxylamine is formed by intramolecular reaction between
chemisorbed species (in a Languimir – Hinshelwood - type mechanism) or by exter-
nal attack of physisorbed ammonia on peroxy oxygen (in an Eley – Rideal - type
mechanism). A recent kinetic study seems to favor the former pathway [131] . A
third issue concerns a possible role of Ti
–
OOH species in the formation of hydrox-
ylamine. The basic medium, indeed, shifts the acid – base equilibrium towards the
formation of the anionic peroxy species, but the presence of Ti
–
OOH in small
amounts cannot be ruled out completely. The greater electrophilicity of Ti
–
OOH
could compensate for its lower concentration.
18.7
Oxidation of N - Compounds
Primary amines, with at least one α - C
–
H bond, undergo consecutive oxidation to
yield unstable alkylnitroso intermediates. These can isomerize to corresponding
oximes, dimerize to nitroso dimers and produce nitro derivatives by further oxida-
tion (Scheme 18.15 ). The selectivity depends on the oxidant system and the reac-
tion conditions. The size restrictions of TS - 1 allow a greater selectivity to the
Scheme 18.14 Ammoximation mechanism on TS - 1.
(Adapted from Ref. [130] , with permission from Elsevier).
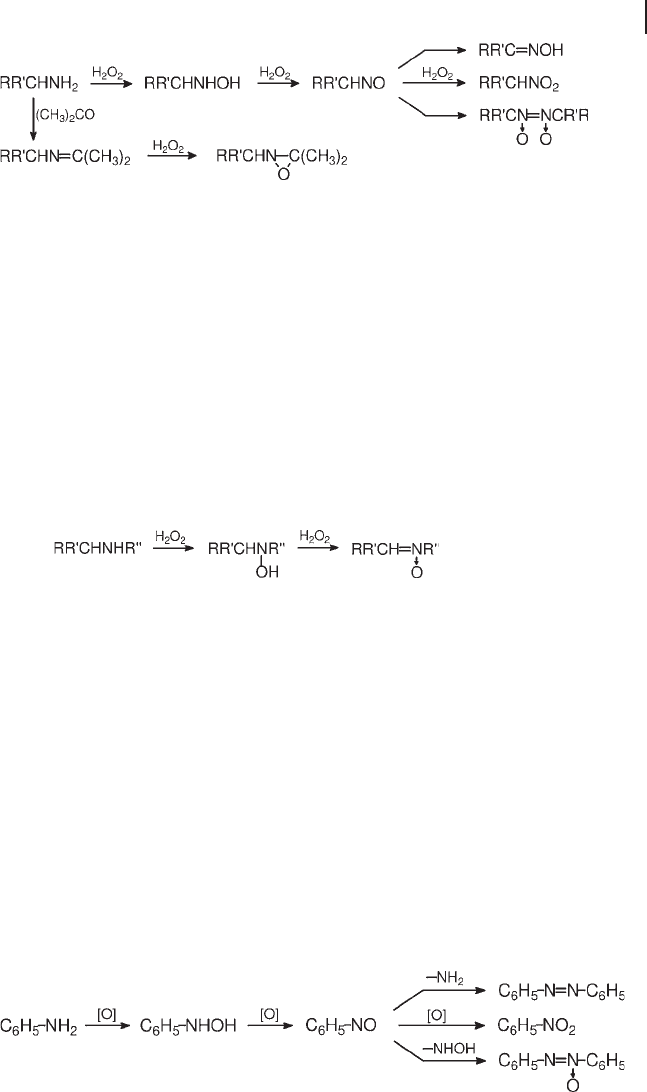
desirable oxime than conventional catalysts, at the expense of the bulkier dimer.
Methanol and t - butanol are suitable solvents, while acetone undergoes side reac-
tions with the amine and the oxidant [132] . The oxidation was broadened to include
several aliphatic and benzylic amines, owing to the versatility of alkyl oximes in
organic synthesis [133] .
The oxidation of secondary amines can produce the corresponding hydroxyl-
amines and nitrones (Equation 18.15 ) [134, 135] . TS - 1 and TS - 2 catalyzed the pro-
duction of the former, at H
2
O
2
/amine molar ratios below unity. The addition of
alkali metal additives improved the selectivity up to 90% [136] . High yields of
nitrones could be obtained at greater H
2
O
2
/amine ratios.
(18.15)
The oxidation of aniline and other arylamines yields a wide spectrum of prod-
ucts, among which azoxybenzenes are the most desirable (Scheme 18.16 ). TS - 1,
Ti - ZSM - 5, TS - 2, Ti,Al - β and Ti - MCM - 41, were studied as catalysts, with hydrogen
peroxide and t - butyl hydroperoxide as the oxidants [137 – 140] . The best yields, up
to 95%, were reported for Ti,Al - β and mesoporous Ti - silicates, with high aniline/
H
2
O
2
molar ratios to minimize over - oxidation to nitrobenzene. Anilines, with
either electron - donating or - withdrawing groups, and 1 - naphthylamine behaved
similarly. Owing to diffusional restrictions, TS - 1 was less effective than large - pore
and mesoporous catalysts.
The oxidation of aromatic and aliphatic oximes and of tosylhydrazones on TS - 1
with excess H
2
O
2
was reported to regenerate corresponding aldehydes [141, 142] .
These were obtained also by the one - pot oxidation of primary amines under analo-
gous conditions [143] .
Scheme 18.15 Oxidation of primary amines.
Scheme 18.16 Oxidation of aniline.
18.7 Oxidation of N-Compounds 733

734 18 Titanium Silicalite-1
18.8
Oxidation of S - Compounds
The oxidation of thioethers on TS - 1, Ti - β and Ti,Al - β produced corresponding
sulfoxides and, more slowly, sulfones by consecutive oxidation [144 – 149] . Allyl
methyl thioether was oxidized selectively on sulfur, with no reaction of the double
bond [148] . The kinetic law was obtained for the oxidation of dibutylsulfoxide on
Ti,Al - β [147] .
The oxidation of thiophene sulfi des combined with a selective separation
process of oxidized products, for example by adsorption on inert materials, might
offer an alternative to deep hydrotreating for desulfurization of oil fractions. On
these grounds, the oxidation of disulfi des has gained considerable interest [149] .
Owing to the nature of sulfur impurities, large - pore and even more mesoporous
Ti - silicates would be appropriate catalysts.
18.9
Industrial Processes Catalyzed by TS - 1
Three oxidation processes are already commercial. Other processes could be devel-
oped in the future, for example for the production of epichlorohydrin and phenol,
especially in the event that new production strategies reduced the cost of hydrogen
peroxide (see below). It is unlikely, however, that Ti - zeolites other than TS - 1 could
fi nd application in the foreseeable future.
18.9.1
Hydroxylation of Phenol to Catechol and Hydroquinone
In 1986 the EniChem process replaced the Brichima process, with signifi cant
materials and energy savings (Table 18.5 , entries 1 and 2). It operates with excess
phenol, fed with H
2
O
2
into a slurry of TS - 1 in aqueous acetone, at ca 100 ° C [5] .
While the conversion of the oxidant is driven to completeness, excess phenol is
recovered by distillation and recycled. The catalyst is structurally stable under
operating conditions, being deactivated only by pore plugging and active site
fouling. The regeneration is conveniently carried out thermally under fl owing air.
With an overall capacity of 10 000 t a
− 1
, it is a modest process when compared to
recent applications of TS - 1 in ammoximation and propene epoxidation. The intro-
duction of digital photography, which no longer needs hydroquinone for the devel-
opment of silver emulsions, risks the continuity of the process, unless the selectivity
to catechol is greatly improved or new uses are developed for hydroquinone.
18.9.2
Salt - Free Production of Cyclohexanone Oxime
A demonstration plant, built by EniChem at the industrial site of Porto Marghera,
near Venice, began operation in 1994 (12 000 t a
− 1
). The process was operated con-

tinuously in the liquid phase in a stirred reactor. Cyclohexanone, ammonia and
hydrogen peroxide were fed, at 80 – 90 ° C and slight over - pressure, into a slurry of
TS - 1 in aqueous t - butanol. The effl uent was separated from the catalyst through
a fi lter, and sent to a distillation column. Excess ammonia and the solvent, recov-
ered overhead, were recycled in the reactor, while the aqueous solution of oxime,
taken from the bottom, underwent further purifi cation. Periodic purging and
make - up operations of the catalyst were necessary to compensate for irreversible
decay.
The fi rst commercial application, made possible by an agreement between
EniChem and Sumitomo, went on - stream in 2003 in Japan, within the context of
an integrated process for the production of ε - caprolactam by a new salt - free tech-
nology (ca 60 000 t a
− 1
). Actually, besides the ammoximation step, no major by -
product is produced even in the gas - phase rearrangement carried out on silicalite - 1
as the catalyst. On the whole, the ammonium sulfate is no longer a burden and
the gaseous emissions too are drastically reduced.
The truly innovative nature of the EniChem process over earlier ones is appar-
ent. The preparation of hydroxylamine in the latter case necessitates multi - step
operation, often ending with major co - production of inorganic salts, as in the
Raschig process. The ammoximation of cyclohexanone, with its in situ generation
of the intermediate, reduces signifi cantly the investment and operation costs while
improving the environmental compatibility. The new process represents a good
example of how to combine profi tability and environmental concern.
18.9.3
Propene Oxide Synthesis ( HPPO )
The industrial process for propene oxide manufacture is commonly referred to as
the HPPO ( hydrogen peroxide propene oxide ) process. EniChem set up a prototype
plant in 2002 [150] . BASF/Dow Chemicals and Degussa, in turn, have the con-
struction of commercial plants already in progress or at the planning stage [151] .
In the EniChem process (6 t day
− 1
), a dilute solution of hydrogen peroxide and a
gaseous stream of propene were fed into a stirred reactor containing TS - 1 and
aqueous methanol [150] . The process was operated continuously, at moderate
pressure and temperature. The selectivity could be as high as 98%. Propene, the
epoxide and methanol were recovered by distillation of the effl uent, while glycol
by - products accumulated in the aqueous solution at the bottom of the column.
The latter, depending on the selectivity of epoxidation, was either processed to
recover the glycols or sent directly to the biological plant, for fi nal treatment before
disposal. The stability of the catalyst to deactivation, achieved by the appropriate
control of operating conditions, was very high and did not require frequent regen-
eration. According to Romano, the new process is characterized by lower environ-
mental impact, simpler process design and relatively lower investment and
operating costs than conventional ones [150] .
Less is known of the processes developed by other companies. BASF/Dow
Chemical, in a joint venture, started the construction of a 300 000 t a
− 1
plant at the
BASF site in Antwerp (Belgium) at the end of 2006 [151] . Hydrogen peroxide will
18.9 Industrial Processes Catalyzed by TS-1 735

736 18 Titanium Silicalite-1
be supplied by a 230 000 t a
− 1
plant based on Solvay ’ s anthraquinone technology and
located at the same site in Antwerp. The operative start - up is scheduled for early
2008. Both plants will be the world ’ s largest single train ones in operation. HPPO
technology is also under evaluation for other facilities in the USA and Asia.
Degussa, in turn, has recently announced the commercialization of a HPPO
process, jointly developed with the engineering company Uhde. Parallel to this,
Degussa with Headwaters is also working on the direct synthesis of H
2
O
2
, for
which a demonstration plant was completed in 2006 [151] . According to the news
release, hydrogen peroxide will be obtained in the new process as a dilute metha-
nol solution to be used directly in the epoxidation of propene.
18.10
Problems in the Use of H
2
O
2
and Possible Solutions
Hydrogen peroxide is currently produced by the anthraquinone autoxidation ( AO )
process. It is a relatively expensive chemical, used for bleaching in the pulp/paper
(ca 57%) and textile industries (6%), for waste water and effl uent treatment (5%)
and for laundry products (ca 12%). Only a minor amount (ca 10%) is currently
consumed for the production of fi ne chemicals (the fi gures refer to the early
2000s). The cost and the production technology, while compatible with its present
uses, fi t less well with petrochemical applications. For instance, the cost of com-
mercial hydrogen peroxide (0.69 $ /lb, 100% H
2
O
2
) is comparable, on a weight
basis, to that of propene oxide (0.70 $ /lb) [152] . The annual capacity of the plants,
mainly comprising the ranges of 10 000 – 20 000 and 40 000 – 70 000 tons, with a
very few slightly over 100 000 t a
− 1
, is undersized for average petrochemical pro-
duction. The shipment of large amounts of hydrogen peroxide could represent a
problem from the point of view of logistics and safety. In addition, the anthra-
quinone route involves complex technology, not easy to access (Figure 18.2 ).
Thus, the applications of TS - 1 in the petrochemical industry were, from the
beginning, strong incentives for the investigation of potential alternatives for
commercial hydrogen peroxide production. Direct synthesis from the elements,
Figure 18.2 Simplifi ed block diagram of anthrahydroquinone autoxidation (AO).
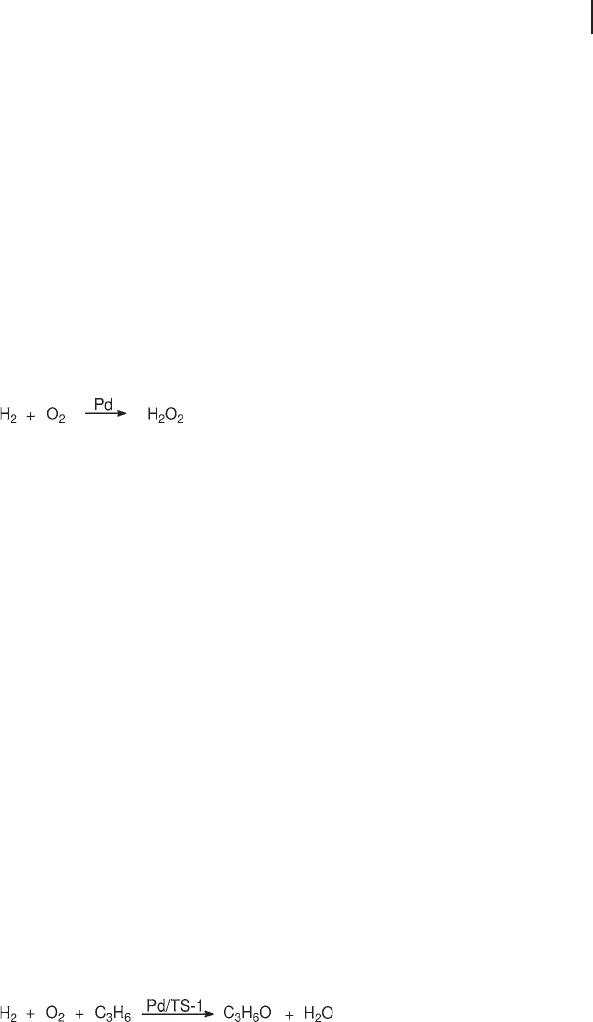
in situ production and process integration have been the most studied solutions.
Unless otherwise specifi ed, HPPO is the process of reference in the following
discussion.
18.10.1
Direct Synthesis of Hydrogen Peroxide
The catalyzed reaction of the elements produces hydrogen peroxide directly, in
competition with water (Equation 18.16 ). Most selective catalysts are based on
supported Pd, often modifi ed by other metals. Methanol is increasingly studied as
a reaction medium, from the perspective of the direct use of the solution obtained
in the HPPO process. For high selectivities, the reaction normally necessitates
relatively low temperatures, often room temperature or lower, and the presence
of mineral acids and other additives [153] .
(18.16)
The direct synthesis is in principle a one - step process, which is simpler and
easier to scale - up for large capacity plants than the AO route. However, it also
suffers from major drawbacks, for example continuing low yields and the hazards
of mixing fl ammable gases. Various technical solutions are envisaged in the patent
literature to minimize the risks of explosion and fi re, such as the dilution of
hydrogen and oxygen with an inert fl uid.
18.10.2
In Situ Production of Hydrogen Peroxide
In situ generation removes dependence on external supplies. The availability of
hydrogen at the chemical site is the only requisite for its feasibility. The approach
generally envisaged in the literature is of two types [154 – 158] . The fi rst requires
that TS - 1 is modifi ed by a metal species, generally Pd, able to catalyze the direct
synthesis. H
2
, O
2
and propene are fed to the reactor to produce propene oxide and
water directly (Equation 18.17 ). On a microscopic scale, hydrogen peroxide is
formed on metal particles, then diffuses in TS - 1 to react with propene on Ti sites.
The high activity of TS - 1 in very dilute solutions of hydrogen peroxide prevents
the accumulation of the oxidant in contact with metal particles that would other-
wise catalyze its decomposition. A major drawback of the method relates to the
excellent hydrogenation properties of Pd, leading to signifi cant losses of propene
as propane.
(18.17)
In early studies, carried out batch - wise on Pd - Pt/TS - 1, the maximum yield was
11.7%, with 46% selectivity based on propene [156] . By operating in a continuous
fi xed - bed reactor, Jenzer and others showed that the selectivity could initially be
18.10 Problems in the Use of H
2
O
2
and Possible Solutions 737

738 18 Titanium Silicalite-1
as high as 99% at 3.5% conversion. The catalyst, however, underwent rapid deac-
tivation with a parallel decrease of the selectivity. Methyl formate from the oxida-
tion of methanol, and acrolein, acetone and other compounds from that of propene
became the main products after few hours on - stream [157] . The use of supercritical
carbon dioxide, without the addition of methanol/water, allowed the reduction of
side reactions [158] . Other studies envisage in situ generation by an electrochemi-
cal cell [159] and gas - phase epoxidation on Ag/TS - 1 at 150 ° C [160] .
A second approach consists of the autoxidation of an organic carrier, in the
presence of propene and TS - 1 (Equations 18.18 and 18.19 ) [154, 155, 161, 162] .
The solvent is a crucial aspect in the use of alkylanthrahydroquinone compounds
[154] . It should be able to solubilize both the oxidized and the hydrogenated forms
of the carrier and possess a molecular size larger than the pores of TS - 1. On this
basis, the various components of the working solution cannot interfere in the
epoxidation mechanism or undergo oxidative degradation at the Ti sites. Normally,
the conditions are met by the use of a mixture of two or more exotic solvents, to
which methanol is also added to increase the rate of epoxidation. Specifi c tests
proved the feasibility of the method, albeit with somewhat lower yields than when
carried out with ex situ hydrogen peroxide [154] . A subsequent study, while con-
fi rming early results, provided useful information on the reactor and operative
conditions [161] . The use of a gas - lift loop reactor, allowed the process to be carried
out satisfactorily even in the presence of two liquid phases, one richer in metha-
nol/water and the other in the working solution. The best yields were 82%, with
85% selectivity based on alkylanthrahydroquinone.
(18.18)
(18.19)
Major shortcomings also affect this in situ route to propene oxide. The most
obvious one is the even greater complexity than for the AO process, owing to the
modifi cation required to accommodate the epoxidation of propene. This explains
why patents have been fi led on anthraquinone compounds and other organic car-
riers, soluble in aqueous methanol. The aim was a radical simplifi cation of the
working solution, trying to eventually use the simple methanol/water medium for
the whole series of reactions. Examples of soluble carriers are anthraquinone
compounds carrying hydrophilic alkyl ammonium or sulfonic substituent groups
and secondary alcohols.
18.10.3
Process Integration
The integration of propene oxide and hydrogen peroxide processes appears, from
a short - term perspective, a more realistic approach than in situ generation. It is
also favored by the location of both facilities at the same chemical site. Basically,
process integration envisages a simplifi cation of H
2
O
2
recovery that can even
result, at best, in the complete elimination of the purifi cation, concentration and
stabilization steps [154] . In this hypothesis, the aqueous H
2
O
2
extract obtained is
fed, without further processing, into the epoxidation reactor. Impurities from the
working solution, which may have been extracted with the H
2
O
2
, are hindered
from diffusing to Ti sites by their relatively large molecular size. Drawbacks could
ensue, for example for the activity of the catalyst, from inorganic additives that
may be required by the AO process [161] . Beside anthraquinone compounds, old
and new carriers such as isopropanol, once used industrially for the production
of hydrogen peroxide and 1 - phenylethanol, have also been studied from the per-
spective of process integration [29, 163] .
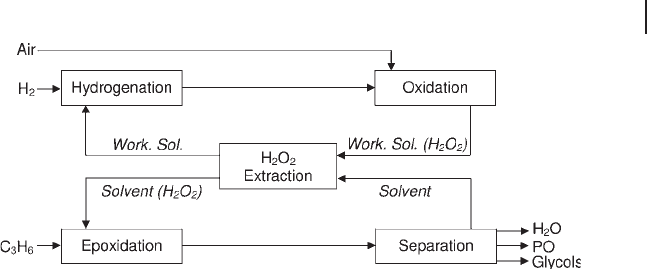
A variant envisages the use of aqueous methanol for both the extraction of
hydrogen peroxide and the epoxidation of propene as shown, in a simplifi ed form,
by Figure 18.3 . Thus, no extra water is added to the epoxidation reactor, which
decreases separation and waste - treatment costs. On the laboratory scale, the quan-
tity of methanol dissolving into the working solution is small and apparently does
not interfere with the AO cycle of reactions [154, 161] .
In principle, process integration applies even more to direct synthesis of hydro-
gen peroxide, further improving the advantages over the anthraquinone route.
Methanol can be used to replace water as the solvent and the dilute methanol
solution obtained fed into the epoxidation reactor. Minimal purifi cation may be
required, for example for the removal of hydrogen bromide and other additives
that may have been needed to increase the selectivity.
18.10.4
Miscellanea
The relatively small capacity of the phenol hydroxylation process does not justify
the development of alternatives to the use of commercial hydrogen peroxide. The
direct synthesis of phenol, for which process integration might be crucial, would
be a different matter.
The ammoximation process is not very compatible with in situ generation. From
one side, the possible reaction of ammonia with the working solution and the
complex recovery of cyclohexanone oxime rule out the use of organic carriers, and,
Figure 18.3 Integrated process scheme for the production of propene oxide.
18.10 Problems in the Use of H
2
O
2
and Possible Solutions 739
