Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


720 18 Titanium Silicalite-1
the rate of epoxidation of cyclohexene, found to be slower than that of 1 - hexene
by almost two orders of magnitude at near room temperature [62] . The second is
apparent in the epoxidation of freely diffusing olefi ns, for example of the methyl -
substituted butenes mentioned above, in which the reactivity order is the expected
one, but the relative rates are greatly different from those of a purely electrophilic
oxidant (Table 18.8 ) [65] . Most striking is 2 - methyl - 2 - butene compared to
1 - pentene, with a rate somewhat slower on TS - 1/H
2
O
2
and 240 times faster on
peracetic acid. Transition - state shape selectivity in these and other epoxidations is
likely to result from steric repulsions exerted by the surface and by the species
chemisorbed on Ti on alkyl substituents of the double bond.
The evidence provided so far, that is the quantitative yields (including glycols),
the absence of allylic oxidation, the substituent effects and the role of shape selec-
tivity, are consistent with a heterolytic mechanism, involving an electrophilic
oxidant species subject to major steric constraints. The retention of confi guration
in the epoxidation of sterically pure cis - and trans - butenes is further and convincing
support for this proposition. By analogy with many known transition metal per-
oxides, the active species may be identifi ed among Ti( η
1
–
OOH), Ti( η
2
–
OOH) and
Ti( η
2
–
O
2
) peroxy species (Figure 18.1 a – d). The detection of Ti superoxide radicals
(Figure 18.1 e), on addition of hydrogen peroxide to TS - 1, and the homolytic nature
of C
–
H abstraction do not weaken the idea of a heterolytic mechanism. Based on
the author ’ s and others ’ experience [66] (and in contrast to some other reports [9] ),
the superoxide species involve only a negligible fraction of Ti - sites.
The competition of one - electron pathways is sometimes detectable in the epoxi-
dations catalyzed by transition metal catalysts [67] . However, in the epoxidation of
unhindered olefi ns on TS - 1, the typical radical products are below the detection
limits. Their presence could no longer be neglected when the rate of epoxidation
is so slow as to become comparable to that of homolytic side reactions, for example
with bulky olefi ns (see also Section 18.11 ). It is possible that, within these limits
only, the epoxide is produced in part through the addition of a radical peroxy
intermediate to the double bond [68, 69] . Even so, a homolytic pathway has again
been proposed as a generally valid epoxidation mechanism [7] .
In early proposals based upon the mechanisms of Mo and V peroxides,
Ti( η
2
–
O
2
) was thought to be the active species [70, 71] . The epoxidation of the
olefi n occurred either by a coordinative mechanism, with the olefi n adsorbing on
Ti prior to the insertion into one Ti - peroxide bond, or by the attack of one peroxy
oxygen directly on the double bond. However, the chemical inertness of known
Ti( η
2
–
O
2
) peroxides, the unlikelihood of olefi n adsorption on oxophilic Ti in com-
petition with the protic medium and other evidence led subsequently to the loss
Figure 18.1 Active species proposed for epoxidations on TS - 1.
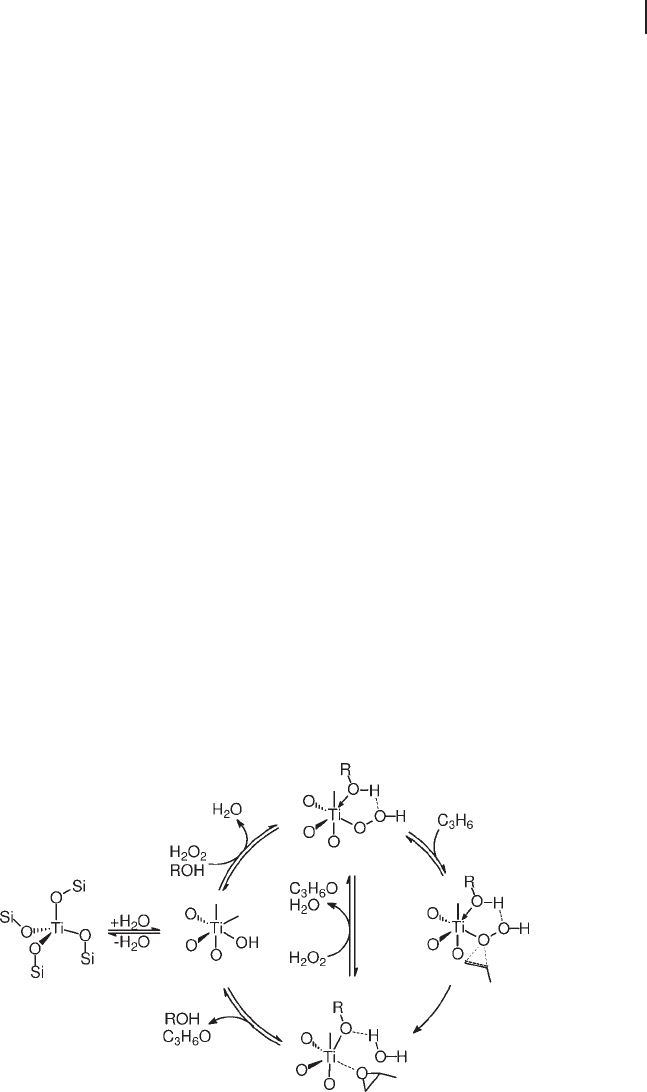
of credibility for this proposal. Currently, a general consensus involving a central
role of a Ti
–
OOH species seems to exist. There is less agreement on the details
of its structure, particularly on the presence and the function of a co - adsorbed
protic molecule (Figure 18.1 a and c) and on the mono - (Figure 18.1 a) or di - hapto
(Figure 18.1 b and c) coordination of the hydroperoxide group. In an early proposal,
the latter was monodentate and was stabilized in a fi ve - membered ring by a hydro-
gen - bonded protic molecule, typically methanol or water (Figure 18.1 a) [24, 62] .
This proposal was based on adsorption properties of Ti sites, on the reactivity
behaviour of organic and inorganic peroxides and on the smooth formation of
cyclic structures in hydroperoxides and other compounds. In the alternative
species, based on quantum chemical studies, the hydroperoxide group was η
2
coordinated and did not require stabilization (Figure 18.1 b) [72] . In another pro-
posal, also based on DFT calculations, η
2
coordination and hydrogen bonding with
a protic molecule coexist in the same structure (Figure 18.1 c) [73, 74] .
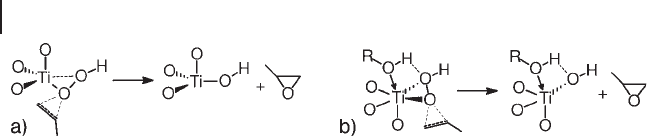
Scheme 18.7 illustrates a proposed early mechanism based on the intermediate
of Figure 18.1 a [62, 75] . The fi rst steps in the catalytic cycle are reversible and
envisage the adsorption of one molecule of H
2
O
2
, the formation of a Ti
–
OOH
species and its hydrogen bonding with one alcohol or water molecule. In the irre-
versible epoxidation step, the attack on the double bond by the peroxy oxygen
vicinal to Ti, leads to the formation of the epoxide, a Ti - alkoxide and a water mol-
ecule. Finally, the desorption of the epoxide and the reaction of Ti - alkoxide with
water, to form again the initial Ti - site, complete the catalytic cycle. As an alterna-
tive to it, H
2
O
2
could chemisorb directly on Ti - alkoxide to regenerate the Ti
–
OOH
species. The desorption of the product is an important step for the activity, since
the epoxide behaves as an inhibitor.
Scheme 18.8 illustrates the oxygen - transfer steps of species b and c in Figure
18.1 [72 – 74] . Both mechanisms are based on DFT studies and are similar to the
epoxidations with organic hydroperoxides on Group IV – VI metal catalysts [1] . The
Scheme 18.7 Epoxidation mechanism of species ( a ) from Figure 18.1 .
18.4 Oxidation of Olefi nic Compounds 721
722 18 Titanium Silicalite-1
coordination of distal peroxy oxygen on Ti facilitates its removal as a titanol. A
weakness of Scheme 18.8 a is the absence of adsorbates other than hydrogen per-
oxide on Ti. The hypothesis could be correct for epoxidations with organic hydro-
peroxides carried out under anhydrous conditions, but not for the use of aqueous
hydrogen peroxide. It is fully consistent, however, with epoxidations conducted
under reduced pressure, in the conditions studied by Lin and Frei [76] . The same
arguments for Scheme 18.7 could apply also to Scheme 18.8 b.
18.4.1.2 Other Ti - Zeolites
The number of metal zeolites and their application to the epoxidation of olefi ns
rose in parallel from the late 1980s. TS - 2, Ti,Al - β , Ti - β , Ti - MWW and, rarely,
Ti - MOR are catalysts that have been studied in some detail [7 – 9, 35, 77 – 84] . TS - 2
behaves, according to the few studies published, similarly to TS - 1. The greater
spaciousness of pores in Ti - Beta zeolites and of external cups in Ti - MWW allows
the epoxidation, under mild conditions, of olefi ns unable to diffuse in TS - 1 and
TS - 2, such as methylcyclohexenes, cyclododecene, norbornene, camphene and
methyl oleate [80 – 83] . Steric constraints still prevail over electronic factors,
however, as in medium pore Ti - zeolites, even in the epoxidation of linear olefi ns
(Table 18.9 ). It is generally believed that active sites and epoxidation mechanisms
are not signifi cantly different from those of TS - 1.
There are major differences that distinguish Ti,Al - β , Ti - β and Ti - MWW. The
fi rst concerns the activity and selectivity, which, under optimum conditions for
each catalyst, are considerably lower than for TS - 1. The activity in methanol
decreases in the order, TS - 1 > Ti - β > Ti,Al - β ≥ Ti - MWW, in a parallel trend with
the decrease of the hydrophobicity. Actually, the density of surface Si
–
OH species
increases in the reverse order: TS - 1 < Ti - β < Ti,Al - β ≤ Ti - MWW. The selectivity, in
turn, drops owing to a greater incidence of solvolysis and hydrogen peroxide
decomposition. Even in the absence of framework Al sites, as in Ti - β , the solvolysis
can signifi cantly reduce epoxide yields. Ion exchange with basic compounds or
just their addition in the reaction medium is an effective tool to limit the losses
of product [84] .
A second difference compared to TS - 1 concerns the solvent and its effects on
kinetics and selectivity. The choice is again restricted to alcohols, ketones and
acetonitrile, but the latter is now preferable to methanol for higher rates and lower
solvolysis [77, 78] . As a general rule, methanol is the best solvent for oxidations
catalyzed by TS - 1, whereas acetonitrile is preferable for Ti - β , Ti,Al - β and Ti - MWW
Scheme 18.8 (a) Epoxidation mechanism of species ( b ) from
Figure 18.1 ; (b) Epoxidation mechanism of species ( c ) from
Figure 18.1 .
[81, 85, 86] . The enhanced activity is likely the result of the effects of different
factors, while the greater selectivity has to be related to the reduction of acidity by
the mildly basic medium. Interestingly enough, in various instances Ti - β , Ti,Al - β
and Ti - MWW turn out to be more active catalysts than TS - 1, when acetonitrile is
a common solvent. In one study, however, trifl uorethanol emerged as the best
solvent for Ti,Al - β [84] .
A third difference concerns Ti - MWW only. The siting of Ti in different porous
environments, that is in external pockets, in internal supercages and in sinusoidal
10 - MR channels, leads to active species associated with different diffusional and
steric constraints [79] . Thus, the epoxidation of bulky olefi ns can occur exclusively
in external pockets, whereas the linear ones are not subject to site limitations.
Ti - MWW is also an unusual catalyst in the epoxidation of stereoisomers. At odds
with TS - 1 and Ti - Beta zeolites, trans - olefi ns are epoxidized faster than their cis
analogues [85] . Though the mechanism is still unclear, a better fi tting of the trans
confi guration to the tortuous nature of 10 - MR channels could be an explanation.
Ti - Beta zeolites and, even more, mesoporous Ti - silicates can be somewhat
unstable to aqueous hydrogen peroxide and to strongly chelating agents. A partial
collapse of the lattice and the release of Ti, in the form of TiO
2
particles or soluble
Ti peroxides, was sometimes observed under these conditions (see also Section
18.4.2 ). The structural instability grows in parallel with the hydrophilicity of the
surface and the defectiveness of the silica matrix: Ti - β < Ti,Al - β << Ti - MCM - 41
[87 – 89] . For the same reason, the stability of the catalyst is indirectly related to the
method of synthesis, as far as this is able to produce materials with a different
content of connectivity defects.
Table 18.9 Epoxidation of olefi ns over Ti - β , Ti,Al - β , TS - 1 and CH
3
CO
3
H.
Relative rates.
Olefi n
Ti,Al - β
a)
Ti - β
b)
TS - 1
a)
C H
3
CO
3
H
c)
1.0 1.0 1.0 1.0
0.70 0.77 0.63 ca 1
– 1.55 – 20 – 24
1.1 – 2.2 20 – 24
0.83 – – 20 – 24
1.6 1.2 – 27
1.6 – 0.63 ca 240
1.2 0.72 0
( ≥ 240)
a Data from Ref. [83] .
b Data from Ref. [81] .
c Data from Ref. [65] .
18.4 Oxidation of Olefi nic Compounds 723
724 18 Titanium Silicalite-1
Since water is detrimental to catalytic performance, and its presence is unavoid-
able with hydrogen peroxide as the oxidant, an alternative consists of the use of
t - butyl hydroperoxide ( TBHP ), compatible with the pores of Ti - Beta zeolites and
with external pockets of Ti - MWW. In the case of the former catalysts, rates are
lower than with hydrogen peroxide while with Ti - MWW they are comparable [79,
83] . It should be considered, however, that in this use both large - pore Ti - zeolites
and mesoporous Ti - silicates are in competition with the cheaper and easier to
prepare Ti/SiO
2
.
In summary, the poorer activity, the stronger acidity and sometimes the struc-
tural instability are major drawbacks that minimize the chances of application of
large - pore Ti - zeolites. The reduced adsorption of alkanes, alkenes and other apolar
reagents in favor of that of water, and the defectiveness of the surface, are the
principal reasons for this (for a further treatment, the reader is referred to Section
18.11 ). In relation to these issues, synthetic methods for the production of materi-
als with fewer defective sites and better connectivity, have been developed for both
Ti - Beta zeolites and mesoporous Ti - silicates [7 – 9] .
18.4.2
Epoxidation of Unsaturated Alcohols
In principle, the oxidation of allyl alcohols may concern the double bond or the
alcohol group. In the absence of steric constraints, TS - 1, TS - 2, Ti,Al - β , Ti - β and
Ti - MWW catalyze the epoxidation with generally high chemoselectivity [81, 88,
90 – 97] . Carbonyl compounds may form by competitive oxidation, to an extent that
depends on steric constraints (Table 18.10 ). Normally, TS - 1 is the most effective
Table 18.10 Oxidation of unsaturated alcohols over TS - 1 and Ti - β .
Olefi n TS - 1
a)
Ti - β
b)
Epoxide (%) Aldehyde (%) Epoxide (%) Aldehyde (%)
100 –
> 90
–
100 –
> 90
–
90 10 85 11
– – 8 9 1 1
7 6 2 4 – –
82 18 96 –
6 4 3 6 – –
a Data from Ref. [90] .
b Data from Ref. [81] .
catalyst for unhindered substrates. However, Ti - MWW showed the best activity
and selectivity for the synthesis of glycidol in acetonitrile and water: Ti - MWW >
TS - 1 > Ti - β [96] . Selective poisoning tests showed that the epoxidation occurred in
the external pockets of crystallites.
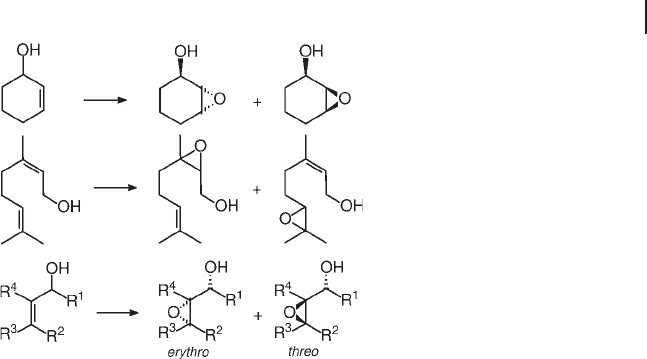
Stereo - , diastereo - and regioselectivity can be very high (Scheme 18.9 ). This
implies OH - assistance in the oxygen - transfer step, through the hydrogen bonding
of the alcohol group with the oxidant species, as for vanadium catalysts and per-
acids. Thus, with TS - 1, 3 - cyclohexenol and 3 - cyclopentenol yielded the cis epoxides
with 90% selectivity [91] . Chiral allyl alcohols produced, with TS - 1 and Ti,Al - β , a
mixture of threo and erythro epoxy alcohols, in a ratio that was apparently indepen-
dent of constraints produced by the pores [97] . The epoxidation of geraniol and
nerol on TS - 1 occurred on the vicinal double bond, while the remote and more
nucleophilic one was reported to be inert [91] . However, Schofi eld and others
observed epoxidation at either double bond, on TS - 1 and Ti,Al - β , in the ratio of ca
2 : 1 in favor of the distant one [93] . Interestingly enough, the use of methanol
completely inhibited epoxidation at the vicinal position, favoring the electron - rich
distant one. This suggests that the competition of methanol for adsorption on Ti
sites suppresses the coordinative interaction of the allyl alcohol with the active
species.
The use of aqueous methanol, through the opening of oxirane rings, can have
a detrimental effect on the stability of the catalyst. By - product triols coordinate
strongly on Ti, facilitating its removal from the silica matrix, to the point that after
an induction period the epoxidation of allyl alcohols on Ti,Al - β and Ti - β became
an essentially homogeneous process [88] . In the epoxidation of crotyl alcohol, the
trend of Ti leaching was: Ti,Al - β > Ti - β >> TS - 1, the process being negligible on
the latter. The use of acetonitrile and the adoption of anhydrous conditions pro-
tected the catalyst from structural degradation, by the reduction of solvolysis.
Specifi c tests revealed that the simultaneous presence of triol and hydrogen per-
oxide was necessary for leaching to occur, while taken alone they did not produce
any signifi cant structural damage.
Scheme 18.9 Oxidation of allyl alcohols.
18.4 Oxidation of Olefi nic Compounds 725

726 18 Titanium Silicalite-1
It is likely that the commercial value of glycidol is the unmentioned background
of most studies. However, process parameters were specifi cally studied in only
one case, in which TS - 2 was the catalyst. The conversion of the olefi n, under
optimum conditions, was ca 88% with 100% selectivity [94] . Methallyl alcohol was
the subject of a similar study [95] .
18.4.3
Epoxidation of Allyl Chloride and other Substituted Olefi ns
Several patents and two papers deal with the epoxidation of allyl chloride [98, 99] .
Actually, a process based on TS - 1 would represent an environmentally cleaner
alternative to current production of epichlorohydrin. In this regard, one study has
addressed the cost of commercial hydrogen peroxide with the in situ production
of the oxidant, by the use of molecular oxygen and anthrahydroquinone com-
pounds [99] . In a mechanistic study, the kinetic data were interpreted on the basis
of Eley – Rideal isotherms, with the rate of reaction being fi rst order on TS - 1 and
between 0 and 1 on H
2
O
2
and C
3
H
5
Cl (Equation 18.8 ) [98] .
(18.8)
Diallyl ether and diallyl carbonate produced monoglycidyl and diglycidyl ethers
in a ratio that depended on the conversion. Allyl methacrylate yielded the corre-
sponding glycidyl methacrylate [5] . Ti - MWW, in acetonitrile and acetone solvents,
was again reported to be more active and selective than TS - 1 [100] .
The epoxidation of α , β - unsaturated ketones produced corresponding epoxides
and minor amounts of glycols [101] . Methyl substitution on the double bond
reduced, or even suppressed, the oxidation. α , β - Unsaturated aldehydes yielded the
corresponding carboxylic acids and minor amounts of epoxides. α , β - Unsaturated
acids were inert under analogous reaction conditions. In this regard, Ti - zeolites
behave differently from homogeneous tungsten catalysts, in which epoxidation
does occur through a coordinative mechanism [102] . Consistently, acrylic acid
esters were inert to both kinds of catalyst.
18.4.4
Epoxidation with Solvolysis/Rearrangement of Intermediate Epoxide
The presence of acidity leads to a variety of end - products, depending on the cata-
lyst, the olefi nic substrate and the reaction conditions (e.g. the sort of solvent, the
temperature and the use of acidity moderators). The acid properties of Ti,Al - β in
this regard, and the use of basic additives to reduce their effects, have been illus-
trated [61, 84] . Ti - MOR is also acidic owing to residual Al in the framework [59] .
TS - 1, with Al, Ga, B or Fe inserted in the framework, behaves similarly [103] . Al -
free Ti - β and Ti - MWW are less acidic catalysts than the former ones, but signifi -
cantly more acidic than TS - 1. Most likely, the acid sites in the latter are the
Ti
–
OOH species a of Figure 18.1 (see also Section 18.11.3 ).
In some instances, combined redox/acid catalysis might be desirable, as in the
one - pot syntheses of β - phenylacetaldehyde by the oxidation of styrene. The primary
product is generally believed to be 1,2 - styrene oxide, which, in a second stage,
undergoes acid - catalyzed rearrangement to the aldehyde. Acidic zeolites are known
to behave as good catalysts in the latter reaction [104] . According to one study,
however, 1,2 - styrene oxide and β - phenyl acetaldehyde could be formed indepen-
dently by two competing mechanisms [105] .
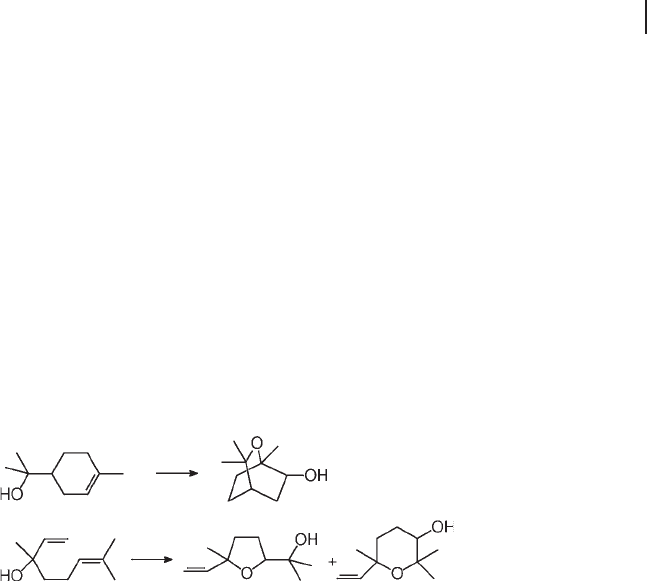
Derouane and coworkers exploited the dual catalytic function and the spacious-
ness of pores of Ti,Al - β to prepare trans - 2 - alkoxycycloalkanols, of interest as phar-
maceutical intermediates, by the oxidation of cyclohexene and cyclopentene [106] .
The one - pot epoxidation/cyclization of unsaturated terpene alcohols, for example
linalool and α - terpineol, to mono - and bicyclic derivatives also required the cataly-
sis of large - pore Ti,Al - β (Scheme 18.10 ) [93, 107] .
Scheme 18.10 Oxidation/cyclization of unsaturated alcohols on Ti,Al - β .
18.5 Oxidation of Alcohol and Other Oxygenated Compounds 727
On TS - 1, Ti - β , Ti,Al - β and Ti - MWW in water and, to a lesser extent, in acetoni-
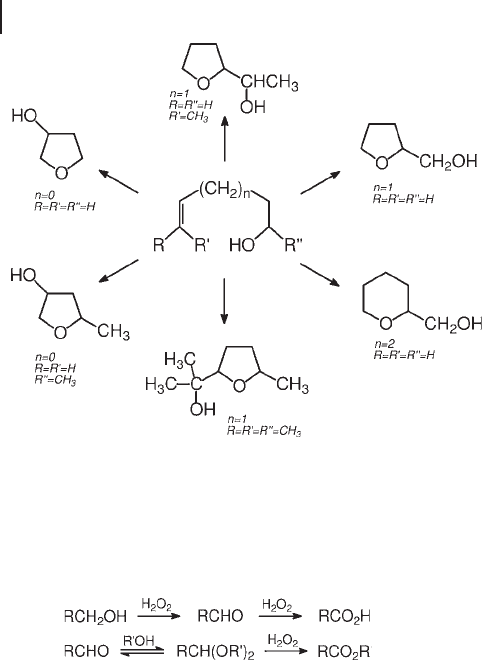
trile, tetralkyl - substituted olefi ns yielded corresponding pinacols (tetralkyl - substi-
tuted glycols) [108] . In the oxidation of 3,4 - and 4,5 - unsaturated alcohols on TS - 1,
the intramolecular cyclization, by internal attack of the hydroxy group on the
oxirane ring, competed successfully with hydrolysis by external attack of water
(Scheme 18.11 ) [107] . Cyclization occurred regioselectively on the less remote
carbon, with yields often higher than 85%. Oxidation of 5 - hexen - 1 - ol, in which the
fi ve - membered ring cannot form, produced the tetrahydropyran derivative. Triol
by - products increased with temperature and water content. Conversely, nearly
anhydrous conditions and the control of acidity with basic additives turned the
oxidation into epoxy alcohol production.
18.5
Oxidation of Alcohol and Other Oxygenated Compounds
The oxidation of primary and secondary alcohols using TS - 1 and Ti,Al - β produces
corresponding aldehydes and ketones [5, 77, 109 – 112] . While the latter are suffi -
ciently stable under the reaction conditions, the former can undergo further oxida-
tion to carboxylic acids. Acetals and esters are also produced when operating in
728 18 Titanium Silicalite-1
neat alcohol or in methanol solvent (Equations 18.9 and 18.10 ). Tertiary alcohols
yield corresponding hydroperoxides.
(18.9)
(18.10)
Primary alcohols react at a much slower rate than secondary ones, suggesting
an electrophilic oxidation mechanism (Table 18.11 ). The relative inertness of
methanol justifi es its use as an oxidation solvent for TS - 1. Within a homologous
series, the rate increases with the chain length of the alcohol up to a maximum
for octanol, then decreasing again [109] . It is likely that this trend involves the
combined effects of electronic factors, deactivation of the catalyst and adsorption
phenomena. A true kinetic ordering, only obtainable from initial rates, is substan-
tially lacking for this as for other oxidations. Shape selectivity is apparent in the
decrease of the rate of alcohols with the hydroxy group at more internal positions
or with vicinal methyl groups, the decrease being larger with the increase of the
number of methyl substituents [110, 111] .
Two papers deal with the kinetics of the oxidation on TS - 1 and Ti,Al - β [110, 111] .
Equation 18.11 illustrates the general rate law valid for both catalysts, according
to a Langmuir – Hinshelwood model. It is consistent with the competition of
alcohol/solvent for Ti sites and the adsorption of the substrate and the oxidant on
the same site. The inhibitory effect of water, implicit in the competitive adsorption
of the solvent, was proved by oxidation tests performed in acetonitrile containing
variable quantities of water [77, 111] .
Scheme 18.11 Oxidation of 3,4 - , 4,5 - and 5,6 - unsaturated alcohols on acidic Ti - zeolites.

(18.11)
The oxidation mechanism, based on an early one proposed for a tungsten cata-
lyst, entails a concerted step in which a C
–
H bond undergoes electrophilic attack
by the distal peroxy oxygen of Ti
–
OOH (Scheme 18.12 ) [110, 111, 113] .
The one - step synthesis of isoamyl butyrate from isoamyl alcohol and n - butyral-
dehyde, possibly through the formation and subsequent oxidation of an acetal
intermediate, could represent a special case in TS - 1 catalysis, since the oxidant
was molecular oxygen [114] . The authors did not advance any mechanistic hypo-
thesis. n - Butyl hydroperoxide, however, produced in situ by the autoxidation of
n - butyraldehyde, could have been the true oxidant, by virtue of its dimensional
compatibility with the narrow pores of TS - 1.
The oxidation of glycols occurred preferentially at the secondary alcohol group.
Thus, propene glycol and 2 - phenyl - 1,2 - ethanediol afforded hydroxyacetone and
β - hydroxyacetophenone, respectively, as the major product [115] . Ethene glycol
Table 18.11 Oxidation of alcohols catalyzed by TS - 1.
a)
Alcohol t
1/2
(h)
b)
H
2
O
2
decomposition (%)
c)
methanol 8.5 13
ethanol 0.7 2.5
1 - propanol 1.0 5
1 - butanol 1.3 6
1 - octanol 3.0 –
2 - methyl - 1 - propanol 3.4
d)
–
2 - propanol 0.01
< 0.5
2 - butanol 0.05
< 0.5
2 - pentanol 0.06
< 0.5
3 - pentanol 0.9 5
cyclohexanol 35 50
a Reprinted from Ref. [110] . Copyright 1994, with permission of Elsevier.
Solvent, pure alcohol; T , 45 ° C; TS - 1, 5 wt%; H
2
O
2
, 0.5 mol l
− 1
.
b Time required for 50% conversion.
c Fraction of H
2
O
2
decomposed at total conversion of alcohol.
d The product is t - butyl hydroperoxide.
Scheme 18.12 Oxidation of alcohols on TS - 1 and Ti,Al - β .
18.5 Oxidation of Alcohol and Other Oxygenated Compounds 729
