Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

680 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
17.3.6
Ar - Adsorption [126, 127]
The heat of adsorption of Ar was also measured for acidity evaluation. In the case
of Ar - TPD, an effect of the probe molecule diffusion in micropores is observed
with some samples, such as zeolites, at high temperature - programmed rates. The
adsorption method is not infl uenced by diffusion of the adsorbed molecule because
the Ar isotherm is measured at static equilibrium. It is also advantageous that the
usual BET apparatus can be used to obtain the adsorption isotherm. In addition,
the adsorption behavior of Ar is of the Henry type at temperatures around room
temperature.
Langmuir ’ s adsorption equation can be converted into the following equation
by the introduction of an approximation at low coverage:
ln ln lnVP HRT b V
()
=−
()
++∆
0m
(17.6)
where V is the volume adsorbed, P is the equilibrium pressure, ∆ H is the heat of
adsorption, R is the gas constant, T is the adsorption temperature, b
0
is a constant,
and V
m
is the volume corresponding to a monolayer. The heat of adsorption ( ∆ H )
is calculated from the gradient of a plot of ln ( V / P ) vs 1/ T . This method of calcula-
tion is known as the H - method.
Experimental results by means of the volumetric method using a conventional
BET system were observed to be Henry type in the temperature range 233 – 313 K
and the pressure range P = 5 – 30 kPa, achieving the condition of small coverage.
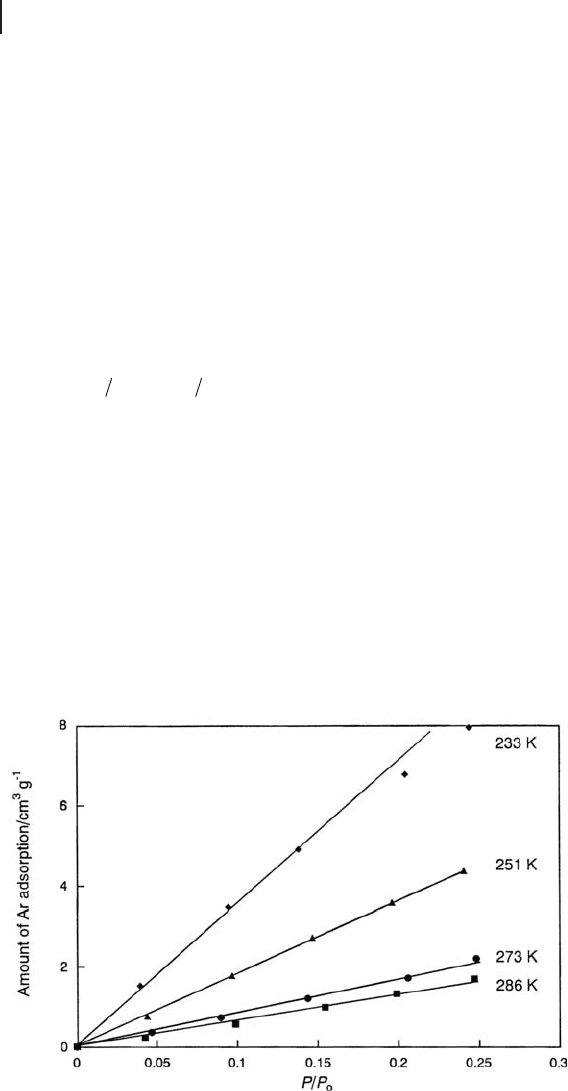
An example adsorption isotherm, for H - mordenite, is shown in Figure 17.3 .
In addition to the above technique, the heat of adsorption can also be determined
from Langmuir - type adsorption isotherms. Langmuir ’ s adsorption equation pro-
Figure 17.3 Adsorption isotherm of Ar on H - mordenite at various temperatures.

vides information regarding the saturated adsorption amount. It is expected that
the number of adsorbed atoms translates into the number of acid sites on the solid
surface. In this case, the heat of adsorption ( ∆ H ) is related to the adsorption con-
stant ( b ) as follows:
bb HRT=−
()
0
exp ∆
(17.7)
and the adsorption heat is calculated from the gradient of a plot of ln b vs 1/ T .
This method of calculation is known as the L - method.
On comparing the two methods of measurement, the advantage of the H -
method is its ease of operation and its shorter experimental time. However, the
adsorption isotherm must be measured with care, as the concentration of adsor-
bate is quite small in the low equilibrium pressure range.
The calculated heats of adsorption are summarized in Table 17.5 [125, 128] . The
heats of adsorption presented are comparable with those obtained by applying the
Henry and Langmuir equations. The relative order of SO
4
/ZrO
2
, mordenite, and
ZSM - 5 is consistent with that of the acid strength evaluated by the heat of ammonia
adsorption [93] . The value of SO
4
/SnO
2
is higher than that of SO
4
/ZrO
2
, indicating
higher acidity for the former, the strongest acidity among the solid metal oxide -
based superacids. For both materials, the heats of adsorption determined by the
H - method are lower than those by the L - method. The heats of adsorption of Ar
obtained from the L - method indicate the highest acid strength. On the other hand,
the H - method provides an average value that includes the weak acid sites, owing
to the wide pressure range used in the measurement. The saturated adsorption
amount is the number of acid sites with high acidity. In general, ammonia cannot
be used as a probe molecule for solid acids containing a component that actively
decomposes ammonia, for example platinum. The table indicates that the present
technique is applicable for metal - containing solid acids.
Table 17.5 Adsorption heats of Ar and acid amounts of various solid acids.
Sample
− ∆ H
H
a)
(kJ mol
− 1
) − ∆ H
L
b)
(kJ mol
− 1
) V
m
b)
(mmol g
− 1
)
SO
4
/SnO
2
23.5 29.6 0.10
SO
4
/ZrO
2
22.4 26.3 0.009
WO
3
/ZrO
2
21.6
SO
4
/Fe
2
O
3
18.9
ZSM - 5 17.3 17.9 0.39
Mordenite 17.3 17.7 1.44
Beta 17.1 0.10
HY 14.8 15.6 0.35
SiO
2
- Al
2
O
3
14.4 14.9 0.35
3 wt% Pt - SO
4
/ZrO
2
22.8
7.5 wt% Pt - SO
4
/ZrO
2
24.6
a Calculated by H - method.
b Calculated by L - method.
17.3 Determination of Acid Strength 681
682 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
17.4
Nature of Acid Sites
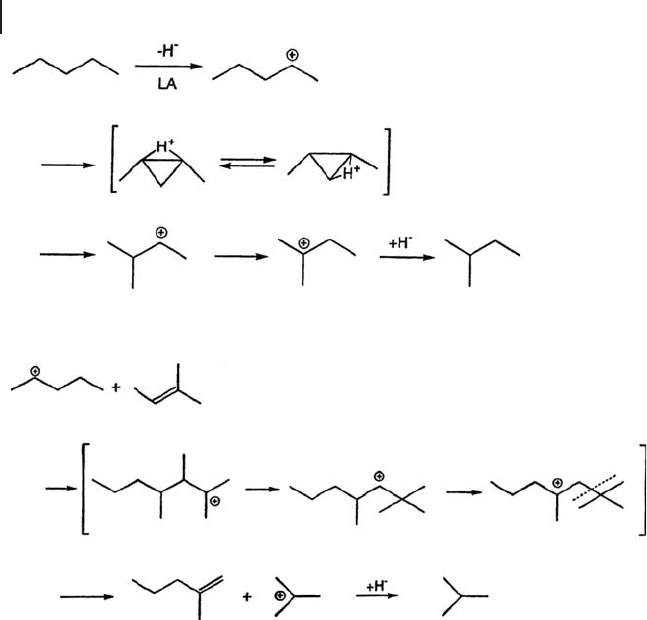
The skeletal isomerization of butane to isobutane is a typical reaction catalyzed by
superacidity. Early in the history of this work, SO
4
/Fe
2
O
3
, SO
4
/TiO
2
, and SO
4
/ZrO
2
,
were termed superacids owing to their ability to isomerize butane at room tem-
perature or below [32, 37, 39] The formation of isobutane from butane, however,
does not necessarily require superacidic strength. A bimolecular reaction pathway
based on the intermediacy of butane is energetically lower than a monomolecular
mechanism [129 – 133] . The monomolecular and bimolecular mechanisms are
shown in Schemes 17.1 and 17.2 , respectively, using pentane as a model.
The processes are the monomolecular reaction through a protonated cyclopro-
pane produced by the abstraction of H
−
over Lewis acid sites and the bimolecular
mechanism where an olefi n takes part in the reaction. The olefi n is produced over
Br ø nsted acid sites. In the case of butane in the monomolecular mechanism, iso-
butane is formed through protonated methylcyclopropane with an activation
energy of 8.4 kcal mol
− 1
followed by the formation of the primary isobutyl cation
with high energy [134] .
Scheme 17.1 Isomerization of pentane via monomolecular intermediate.
Scheme 17.2 Disproportionation of pentane via bimolecular intermediate.
On the Br ø nsted acid sites, H
+
is added to the C
–
H bond of the substrate, fol-
lowed by elimination of H
2
and formation of the secondary butyl cation. The cata-
lyst surface would be in a proton - defi cient condition owing to the elimination of
H
+
as H
2
. As a result, the secondary butyl cation releases H
+
to convert into an
alkene, which is an intermediate of the bimolecular reaction. The thermodynamic
stability of the tertiary cation is higher than that of the secondary cation. The
former cation is easily formed by methyl migration followed by a 1,2 - hydrogen
shift. The tertiary carbenium ion releases H
+
readily, converting into isopentene,
and this would be the intermediate of the bimolecular reaction. The reaction pro-
ceeds via the bimolecular mechanism of oligomerization – cracking involving the
formation of a C
10
intermediate. This is formed from a C
5
alkene and C
5
cation
followed by rearrangement and β - scission to yield isobutane as the fi nal product,
as shown in Scheme 17.2 . Butane is also converted into isobutane in the same
manner.
In order to compare the catalytic action of SO
4
/ZrO
2
with that of H - mordenite
( H - Mor ), the reaction of pentane was performed in a closed recirculation system.
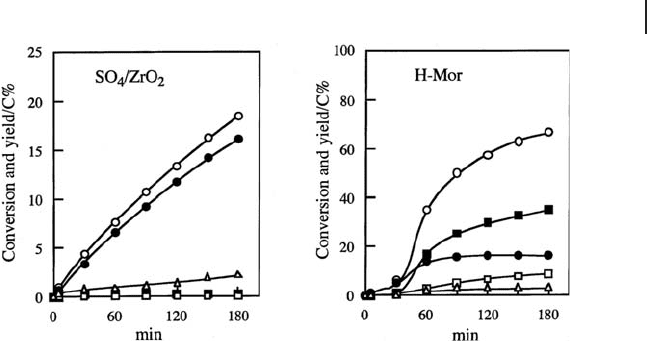
The changes of product yields against reaction time are shown in Figure 17.4 [135] .
The reaction rate with SO
4
/ZrO
2
at 0 ° C is almost constant during the reaction,
and the main product is isopentane, with small amounts of isobutane and hexanes
being detected.
In contrast, the reaction over H - Mor at 200 ° C has different characteristics than
that over SO
4
/ZrO
2
. H - Mor is inactive at 0 ° C, and at 200 ° C a short induction
period is observed for the catalytic activity and product selectivity. Although the
production of isopentane is predominant in the induction period, an increase in
the activity is observed along with the formation of isobutane, butane, and hexanes.
The main product is isobutane after the induction period, highlighting the effect
of Br ø nsted acid sites. The surface alkenes for the bimolecular mechanism
Figure 17.4 Isomerization and disproportionation of pentane
on SO
4
/ZrO
2
at 0 ° C and H - mordenite at 200 ° C; conversion
(䊊 ), butane ( 䊐 ), isobutane ( 䊏 ), isopentane ( 䊉 ), hexanes ( 䉭 ).
17.4 Nature of Acid Sites 683
684 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
are produced on the Br ø nsted acid sites, and alkenes accumulate on the surface
during the induction period. The activity increases with increase in the amount of
the accumulated alkenes. This leads to the conclusion that the monomolecular
reaction proceeds on SO
4
/ZrO
2
and the bimolecular mechanism proceeds on
H - mordenite.
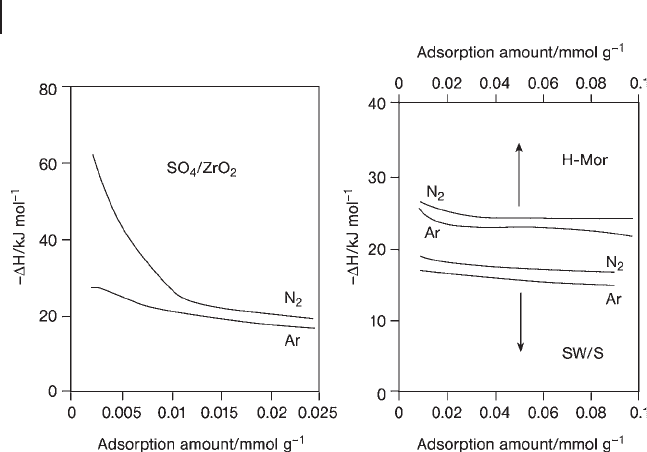
In order to examine the nature of acid sites on SO
4
/ZrO
2
, the heats of adsorption
of N
2
and Ar were measured and compared with those of H - Mor and a heteropoly
acid (H
4
SiW
12
O
40
) supported on SiO
2
( SW/S ). Both heats plotted against the quan-
tity adsorbed are shown in Figure 17.5 [136] . The heat of adsorption of N
2
is larger
than that of Ar, with the difference being 2 – 3 kJ mol
− 1
on H - Mor and SW/S. Both
heats decrease gradually with increasing adsorption amounts, with an almost
constant relationship. On the other hand, SO
4
/ZrO
2
behaves differently. In par-
ticular, a very large heat of adsorption, more than 60 kJ mol
− 1
, is obtained for N
2
adsorption on SO
4
/ZrO
2
, which is much larger than that of H - Mor and the differ-
ence in the adsorption heats of the two gases is more than 30 kJ mol
− 1
.
The nitrogen molecule is strongly adsorbed on Lewis acid sites by interaction
between the 5 σ electron pair and the vacant molecular orbital of the Lewis site
[137] . The stabilization of the N
–
N bond in addition to the stabilization by adsorp-
tion on the acid sites is responsible for the large heat of N
2
adsorption. The acid
sites on SW/S are of Br ø nsted type [138] , and the acidity on H - Mor is also regarded
as Br ø nsted type, though a small number of Lewis sites are generated by treatment
at high temperatures [139] . This is consistent with the formation of isobutane from
pentane. Therefore it is concluded that the acidity of sulfated zirconia is of Lewis
type.
Figure 17.5 Heats of adsorption of Ar and N
2
on SO
4
/ZrO
2
, H - Mor, and SW/S.
17.5
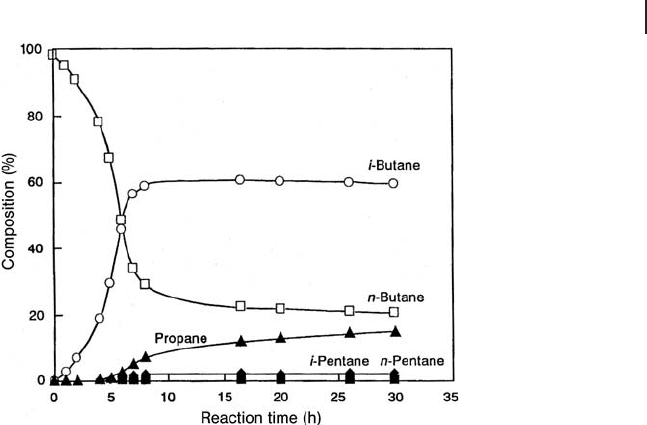
Isomerization of Butane Catalyzed by Sulfated Zirconia [140]
The skeletal isomerization of butane catalyzed by SO
4
/ZrO
2
was carried out in a
closed recirculation system under mild conditions, at 0 ° C for a long reaction
period; the results are shown Figure 17.6 . A long induction period, ∼ 4 h, is
observed with the sole formation of isobutane. At longer times on stream, an
increase in the activity is observed along with the formation of propane and
pentanes. The ratio of butane to isobutane is close to the equilibrium value after
8 h. The apparent activation energy is 13.0 kcal mol
− 1
in the induction period and
8.8 kcal mol
− 1
after that period. The activation energy of the monomolecular
mechanism is higher than that of the bimolecular mechanism. The isomerization
of butane proceeds via a primary carbenium ion in the former mechanism and
via a secondary carbenium ion in the latter. The monomolecular reaction is pre-
dominant on superacidic Lewis sites in the induction period, and the reaction
changes to the bimolecular process on Br ø nsted sites by the formation of surface
alkene intermediates, giving rise to the additional C
3
and C
5
products. The
number of Br ø nsted sites over SO
4
/ZrO
2
is reduced, and the proportion of Lewis
sites is increased with increasing evacuation temperature. On the catalyst pre -
treated in vacuum at 250 ° C, some Br ø nsted acidity remains and causes the bimo-
lecular reaction. However, the activity increase disappears and only isobutane is
obtained on catalysts treated at 450 ° C or higher, which is indicative of the mono-
molecular reaction.
Figure 17.6 Reaction of butane over SO
4
/ZrO
2
at 0 ° C; the
catalyst was pre - treated at 250 ° C in vacuum for 3 h.
17.5 Isomerization of Butane Catalyzed by Sulfated Zirconia 685

686 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
17.6
Isomerization of Cycloalkanes [141]
Cyclohexane is known to be isomerized to methylcyclopentane when catalyzed by
strong acids. In fact, the SO
4
/ZrO
2
catalyst converts cyclohexane into methylcyclo-
pentane and methylcyclopentane into cyclohexane [119, 142, 143] . The reactions
proceed by the monomolecular mechanism via the intermediacy of secondary and
tertiary carbenium ions followed by protonated cyclopropanes.
The isomerization of open - chain alkanes with more than six carbon atoms gives
isobutane as the main product, together with disproportionated materials, even
though the reaction proceeds by the monomolecular pathway [144] . On the other
hand, for cyclic alkanes the monomolecular process with preservation of the cyclic
structure seems to be the most probable, judging from the results for cyclohexane.
The absence of isobutane in the products indicates that the reaction path does not
involve open - chain intermediate species. Therefore, it is of interest to try cycloal-
kanes larger than cyclohexane for clarifi cation of the reaction mechanism along
with the catalytic action of SO
4
/ZrO
2
.
The skeletal isomerization of cycloalkanes with more than six carbon atoms,
that is cycloheptane, cyclooctane, cyclodecane, and cyclododecane, was performed
over SO
4
/ZrO
2
in the liquid phase at 50 ° C.
The results for cycloheptane after 30 min reaction time are shown in Table 17.6 .
Methylcyclohexane is the major product, with a selectivity of 97%, in addition to
small amounts of four dimethylcyclopentanes and ethylcyclopentane. The reaction
of methylcyclohexane under the same conditions also produced the latter fi ve
compounds, and the system reached its equilibrium state after 90 min of
reaction.
The reaction results of cyclooctane are shown in Table 17.7 . The major
product is ethylcyclohexane with a selectivity of 93%. Small amounts of fi ve
dimethylcyclohexanes and methylcycloheptane were formed in addition. The
comparable reaction of ethylcyclohexane produced dimtheylcyclohexanes and
methylcycloheptane.
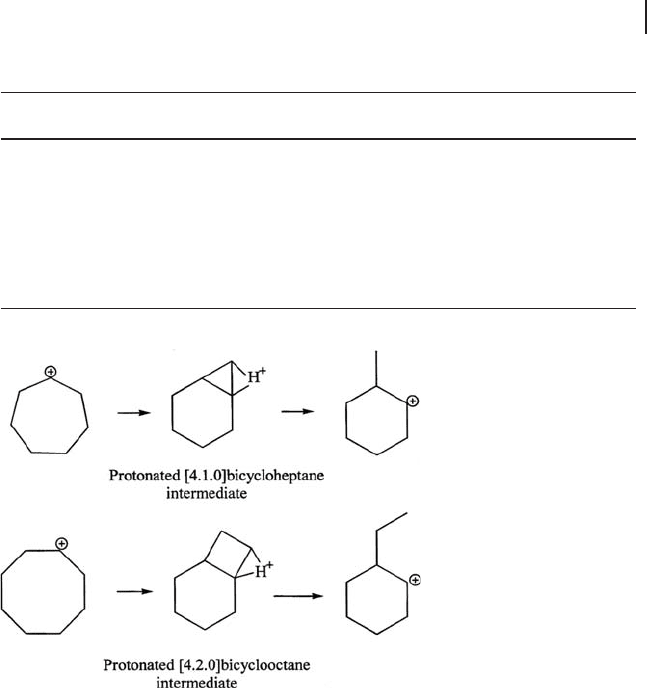
The reaction results of cycloheptane and cyclooctane indicate that the monomo-
lecular pathway is followed, preserving the cyclic structure, without the formation
Table 17.6 Product distribution for the reaction of cycloheptane
at 50 ° C after 30 min reaction time over SO
4
/ Z r O
2
.
Product Yield (%) Selectivity (%)
Methylcyclohexane 53 97
trans - 1,2 - Dimethylcyclopentane 0.7 1.3
trans - 1,3 - Dimethylcyclopentane 0.4 0.7
1,1 - Dimethylcyclopentane 0.3 0.4
cis - 1,3 - Dimethylcyclopentane 0.2 0.3
Ethylcyclopentane 0.2 0.3
of isobutane through protonated cyclopropane and cyclobutane intermediates, as
shown in Scheme 17.3 . The structure of the cyclic hydrocarbon has a large effect
on reaction. No isomerization was observed with cyclodecane, with decalins being
formed by dehydrogenation, and cyclododecane was converted into more than 30
product species by rearrangement, dehydrogenation, and cracking.
17.7
Structure of Sulfated Zirconia
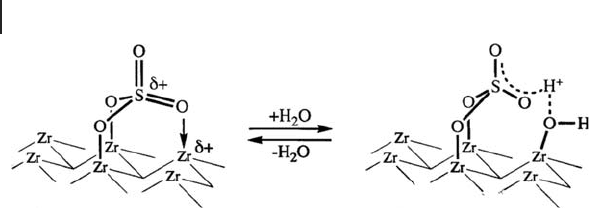
In the very early stages of this work, we studied the catalytic surface using mainly
XPS and IR spectroscopy [32] , and proposed the surface structure to be SO
4
com-
bined with two zirconium species in a bridging bidentate state (refer to Scheme
17.4 ). An analogous model was proposed by Segawa and coworkers [53] , and Bolis
and coworkers [145] .
Table 17.7 Product distribution in the reaction of cyclooctane
at 50 ° C after 30 min reaction time over SO
4
/ Z r O
2
.
Product Yield (%) Selectivity (%)
Ethylcyclohexane 57 93
trans - 1,2 - Dimethylcyclohexane 2.1 3.3
Methylcycloheptane 1.1 1.7
cis - 1,3 - Dimethylcyclohexane 1.0 1.3
trans - 1,4 - Dimethylcyclohexane 0.2 0.3
1,1 - Dimethylcyclohexane 0.1 0.2
trans - 1,3 - Dimethylcyclohexane 0.1 0.2
Scheme 17.3 Intermediates for the isomerizations of
cycloheptane into methylcyclohexane and of cyclooctane into
ethylcyclohexane.
17.7 Structure of Sulfated Zirconia 687
688 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
Later, a number of studies attempted to determine the nature of acid sites in
the catalyst. Also applying XPS and IR spectroscopy, Tanabe and others proposed
a structure of chelating bidentate complexes, in which the sulfate species chelates
to a single Zr atom [102, 146] . This model, a chelating bidentate species, was also
proposed by Ward and Ko [61] .
Morrow and coworkers proposed a structure, on the basis of
18
O exchange using
H
2
18
O combined with IR analysis, in which three oxygens from the sulfate groups
are bonded to Zr species in a tridentate form [147] . They also pointed out the pos-
sibility of the formation of a polysulfate structure at high sulfate loading [148] .
This structure was supported by Morterra and coworkers using IR studies of
adsorbed pyridine [149] .
A monodentate structural model, which contains a bisulfate group, has been
proposed by several workers [103, 104] . The bisulfate OH group is hydrogen -
bonded to an oxygen on the surface of zirconia. A similar model has been proposed
for the surface of sulfated alumina on the basis of NMR studies [150] .
Another bisulfate structure was proposed by Riemer and coworkers using NMR
and Raman spectroscopies, in which two sulfate oxygens are bonded to Zr atoms
in a bridged bidentate state [106] . The same model was also proposed by Lunsford
[151] and Clearfi eld [152] .
Models in which SO
3
species are coordinated with zirconia have also been pro-
posed. One of them, suggested by Vedrine and coworkers [52] , is coordination of
the SO
3
sulfur with lone pairs of the oxygen in ZrO
2
in addition to one of the SO
3
oxygens coordinating with a Zr. Another proposal by White and coworkers [153]
is depicted in such a way that two of the SO
3
oxygens are coordinated with surface
zirconium atoms, leaving a single S
=
O moiety. A thionyl tetraoxide species with
four oxygens bonded to zirconia together with a single S
=
O has been proposed at
low sulfate content [154] .
Finally, sulfated zirconia was investigated by thermal analyses in addition to XPS
in order to provide additional information on the surface. On the basis of the
observations made, we have proposed possible surface structures of sulfated zirco-
nia [155a] . The active species of sulfated zirconia are decomposed over a quite broad
range of temperature, from 700 ° C to > 1000 ° C. XPS data indicates that the S species
comprise SO
4
2−
. An example of the models is shown in Scheme 17.4 , where two
oxygens are bonded to Zr in addition to coordination of an S
=
O group with Zr,
resulting in three grafting bonds in total. The addition of water causes the breakage
Scheme 17.4 A monosulfate structure.
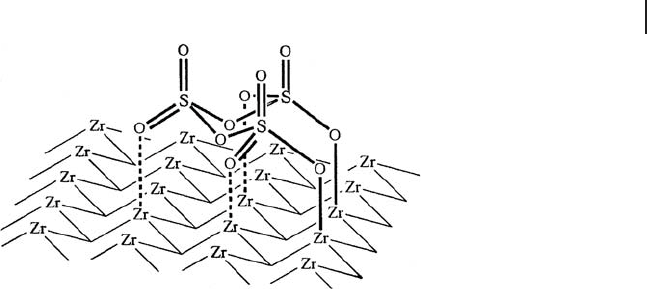
of this coordination, generating Br ø nsted acid sites. Another example, consisting
of a cyclic trimer of SO
3
, is shown in Scheme 17.5 , where two terminal S
=
O anions
are bonded to Zr cations including three coordinations of S
=
O with Zr. These
coordination sites at Zr are also positions for water molecules giving Br ø nsted acid
sites, as shown in Scheme 17.4 in the case of monosulfate species.
The surface is composed of a wide range of coordinated oligomeric species,
predominantly species containing 3 or 4 S atoms, with two ionic bonds between
S
–
O − and Zr being formed, which is identical to the models for monomer and
trimer (Schemes 17.4 and 17.5 ). The active site is not on the metal species, but
rather on the S atoms.
The addition of water causes the breakage of the coordination bonds to yield
Br ø nsted acid sites strengthening Lewis acid sites, as shown in Scheme 17.4 , for
example. Many research groups report the simultaneous existence of Br ø nsted and
Lewis acid sites or the reversible transformation between Br ø nsted and Lewis
acidity upon hydration or dehydration [61, 106, 152] . Fraenkel suggests that in
order to be an effective superacid, sulfated zirconia should contain a critical
amount of moisture [155b] . Several workers propose that the strong acidity requires
the presence of both Lewis and Br ø nsted sites.
17.8
Promoting Effect
17.8.1
Effect of Addition of Metals to Sulfated Zirconia on the Catalytic Activity
A large number of metal - promoted superacids, which are highly active for butane
conversion, have been prepared by the addition of metal salts to SO
4
/ZrO
2
. Metal
promoters include Fe - Mn [156 – 162] : Pt, Pd [163] : Pd [164] : Ga, In, Tl [165] : Pt - Ni
[166] : Ni [167] : Mn [168] : Ce [169] : V, Cr, Mn, Fe, Co, Ni, Cu, Zn [170] : Pt, Pd, Ir
[171] : Al, Ga [172] : Cu [173] : Al [174, 175a – 175d] : Ni - Al [176] : Ga [177 – 181] : Pt, Nb
Scheme 17.5 A structural model of a trimer.
17.8 Promoting Effect 689
