Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


670 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
The gel is obtained as fi ne particles when it is washed with distilled water, and
a large part of the precipitate passes through a conventional fi lter paper, resulting
in diminished yields. This diffi culty can be avoided by washing the gel with
aqueous ammonium acetate solution, which provides a quantitative yield.
17.2.1.3 Preparation of H
4
TiO
4
[30]
A volume of Ti[OCH(CH
3
)
2
]
4
(290 ml) is added to distilled water (2 l) with stirring,
and the white precipitates formed are dissolved by gradually adding conc. HNO
3
(250 ml) with stirring. Ammonia solution (28%, ∼ 300 ml), is added into the aqueous
solution with stirring until pH 8 is attained. The solution is then allowed to stand
for a day. Washing is then undertaken by decantation of a 5 l beaker of deionized
water twice. Finally the resultant material is dried at 100 ° C for 24 h.
Another method of preparing H
4
TiO
4
is by hydrolysis of TiCl
4
as follows. A
volume of TiCl
4
(80 ml) is gradually added to distilled water (2 l) in a 5 l beaker
cooled by ice water, with large amounts of HCl gas being formed. Ammonia solu-
tion (28%) is added at room temperature until a pH of 8 is attained. The resultant
precipitates are washed thoroughly by decantation using 60 l of water until no
chloride ions are detected in the fi ltrate. The aqueous portion might become
cloudy during washing, but the white washings can be decanted.
17.2.1.4 Preparation of Fe(OH)
3
[30]
To a solution of Fe(NO
3
)
3
· 9H
2
O (500 g) dissolved in 2 l of water in a 5 l beaker,
ammonia solution (28%, ∼ 300 ml used, pH 8) is added with stirring to precipitate
Fe(OH)
3
. The aqueous portion is decanted from the precipitate after allowing the
solution to stand. The precipitates are washed by decantation until the liquid
portion becomes cloudy (7 – 8 times), and dried.
17.2.1.5 Preparation of Hf(OH)
4
[35]
HfCl
4
is gradually dissolved in distilled water with care, and the hydroxide is pre-
pared in the manner described above for Zr(OH)
4
.
17.2.1.6 Sulfation, Calcination, and Catalytic Action [30]
The above prepared materials are powdered below 100 mesh and treated with
sulfate ions by exposing 2 g of the hydroxides (gel) in 30 ml of aqueous sulfuric
acid for 1 h, fi ltering, drying in a desiccator at room temperature, and fi nally calcin-
ing [36] . The iron materials are again powdered because of solidifi cation after
drying [36, 37] . The concentration of H
2
SO
4
is 0.5 M for the hydroxides of Zr and
Ti [38, 39] , 3 M for Sn [40, 41] , 0.25 M for Fe [37] , and 1 M for Hf [35] . A recent
study shows that the optimum concentration for Zr is 0.25 N [42] .
After calcination of the sulfate - adsorbed materials in air, the substances are
catalytically active for the skeletal isomerization of butane to isobutane at room
temperature. The activities are dependent on the calcination temperature. The
maximum activity is observed with calcination at 575 – 650 ° C for the Zr catalyst
[32] , 500 – 550 ° C for Sn [34, 41] , 525 ° C for Ti [43] , 500 ° C for Fe [36, 37] , and 700 ° C
for Hf [35] .

17.2 Preparation 671
All the catalysts are calcined in Pyrex glass tubes in air for 3 h and sealed in
ampoules while being hot, to minimize exposure to humidity until use.
17.2.1.7 Preparation of Sulfated Silica [44]
Silica gel is obtained by hydrolyzing Si(OC
2
H
5
)
4
(100 ml) with water (100 ml) and a
few drops of HNO
3
. The mixture is stirred until gel formation. The precipitates are
obtained by evaporation of excess water and ethanol, formed by hydrolysis of
Si(OC
2
H
5
)
4
, followed by drying at 100 ° C, and powdering. The silica (3 g) is exposed
to SO
2
Cl
2
for 1 h followed by evacuating HCl evolved by the reaction of surface OH
group with SO
2
Cl
2
and excess SO
2
Cl
2
in vacuum, and calcining in air at 400 ° C.
17.2.1.8 Preparation of Sulfated Alumina
In the case of the sulfate - treated superacids of Zr, Sn, Ti, Fe, Hf, and Si, superacid
sites are not created by the treatment of sulfate ion on the crystallized oxides but
rather on the amorphous forms, followed by calcination to crystallization. The
superacid of Al
2
O
3
is prepared from the crystallized oxide, γ - Al
2
O
3
[45, 46] .
A highly active catalyst is obtained by hydrolysis of aluminum isopropoxide [47] .
Distilled water is added with stirring to a solution of Al[OCH(CH
3
)
2
]
3
(10 g) dis-
solved in isopropanol (300 ml) to precipitate Al(OH)
3
, followed by washing the
precipitates, drying, and calcining for crystallization at 500 ° C for 3 h. The crystal-
lized materials are then hydrated before sulfation. The sample (5 g) is suspended
in water (5 l) at 80 ° C for 3 h, fi ltered, and dried at 100 ° C. SO
4
/Al
2
O
3
is obtained by
treatment with 2.5 M H
2
SO
4
and calcination at 600 – 650 ° C for 3 h.
17.2.1.9 Property and Characterization
Properties of the sulfated materials thus prepared are summarized as follows
[43, 48] .
1. Superacidity is generally created by adsorbing sulfate ions onto amorphous
metal oxides followed by calcination in air to convert to the crystalline forms.
However in the case of Al
2
O
3
, a superacid is prepared from the crystallized
oxide.
2. Specifi c surface areas of the catalysts are much larger than those of the oxides
without the sulfate treatment except for the Al
2
O
3
. A particularly large increase
in the area is observed on the highly active and acidic catalysts. The main reason
for the increase in surface area is the retardation of crystallization by sulfate
treatment. The areas of the Al
2
O
3
catalysts are smaller than those of the oxides
without the sulfate treatment.
3. By XRD analysis the degree of crystallization of the sulfated oxides is much
lower than that of the oxides without the sulfate treatment. Temperatures of
the crystallization or phase transformation for SO
4
/ZrO
2
and SO
4
/TiO
2
are circa
150 and 200 ° C higher than those for pure ZrO
2
and TiO
2
, respectively; the XRD
pattern of SO
4
/ZrO
2
(650 ° C) and SO
4
/TiO
2
(525 ° C), whose superacidities are
highest, are pure tetragonal and anatase forms, respectively.

672 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
4. The sulfate samples have IR spectra that are different from those of metal
sulfates; the materials show absorption bands at 980 – 990, 1040, 1130 – 1150,
and 1210 – 1230 cm
− 1
, which are assigned to the bidentate sulfate coordinated to
metal ions.
5. XPS spectra of SO
4
/ZrO
2
and SO
4
/Fe
2
O
3
show that the surface is not Zr(SO
4
)
2
and Fe
2
(SO
4
)
3
, but composed of ZrO
2
and Fe
2
O
3
with SO
4
2−
, respectively. On the
other hand, the spectra of SO
4
/TiO
2
and SO
4
/Al
2
O
3
are consistent with the
presence of surface Ti(SO
4
)
2
and Al
2
(SO
4
)
3
, respectively.
6. The IR spectra of pyridine adsorbed on SO
4
/ZrO
2
and SO
4
/TiO
2
show the facile
conversion of Lewis sites to Br ø nsted sites by water molecules. Lewis and
Br ø nsted sites are easily interchangeable by adsorption or desorption of water
molecules [49 – 55] .
7. Upon dehydration in N
2
at 375 ° C, a new IR peak centered at 1370 cm
− 1
appears;
the band is assigned to an S
=
O stretching vibration and is observed for the
catalytically active material [56, 57] .
8. The sulfated materials also show oxidizing action at elevated temperatures. In
particular the superacids of Fe
2
O
3
and SnO
2
demonstrate strongly oxidizing
potential at temperatures above 100 ° C [58, 59] .
9. Catalysts obtained by treatment with sulfuric acid are usually more active than
those obtained with ammonium sulfate treatment. However, a recent study
shows that the analogous activity is generated on SO
4
/ZrO
2
by the kneading
method with ammonium sulfate [60] .
17.2.1.10 One - Step Method for Preparation of SO
4
/ZrO
2
[61 – 65]
Ward and Ko investigated preparation of sulfate - zirconia aerogels in a one - step
synthesis by the sol – gel method followed by supercritical drying [61] . Sulfuric
acid is mixed with zirconium n - propoxide (16.2 ml of 70 wt% in propanol) in n -
propanol (30 ml) and reacted with water (1.3 ml) and nitric acid (1.9 ml of 70%
w/w) to form a zirconia - sulfate co - gel; supercritical drying with carbon dioxide
removes the alcohol solvent forming a high surface area aerogel. The properties
and catalytic activities are similar to those of the compounds we have prepared.
A single - step sol – gel method has also been used for the preparation of SO
4
/TiO
2
[66] .
17.2.1.11 Commercial Gels for Preparation of SO
4
/ZrO
2
and SO
4
/SnO
2
Commercial zirconia - gels are now supplied by chemical companies such as MEL,
Nakarai, and Daiichi Kigenso. For instance, XZO631 and 632 are the gels, and
XZO682 is a sulfate - adsorbed gel from MEL. According to our experience, com-
mercial gels have often led to catalysts superior to those prepared in the laboratory
[67, 68] . Similar observations have been made with SO
4
/SnO
2
. A commercial gel
prepared from meta - stannic acid gives satisfactory results for this type of catalysis
[69] . SO
4
/SnO
2
was prepared using meta - stannic acids (SnO
2
· H
2
O, commercial
17.2 Preparation 673
grade) supplied by Kojundo Kagaku, Ltd. and Yamanaka & Co. Ltd. The activity
for the acid - catalyzed conversion of methanol into dimethyl ether was higher than
for catalysts obtained by hydrolysis of SnCl
4
and much higher for the isomerization
of butane.
17.2.1.12 Effect of Drying and Calcination Temperatures on the Catalytic Activity
of SO
4
/ZrO
2
[68]
The catalytic activity of SO
4
/ZrO
2
varies with the type of zirconia gel and the drying
and calcination conditions. The calcination temperature showing the maximum
activity and acidity often varies with the type of prepared gel. For instance, the
maximum activity for the conversion of butane to isobutane is observed with cal-
cination at 575 and 650 ° C, respectively, for the materials prepared from ZrO(NO
3
)
2
and ZrOCl
2
as starting reagent [32] .
The activity also depends on the drying temperature of the gel before sulfation.
For the gels obtained by hydrolysis of ZrO(NO
3
)
2
followed by drying at 100 ° C and
300 ° C, the difference in the calcination temperature showing the maximum activ-
ity for the butane conversion was 50 ° C, and the difference in maximum activity
was a factor of two.
Several sulfated zirconias were prepared by changing the drying temperature of
gel in the range 100 – 400 ° C and the fi nal calcination temperature in the range
475 – 700 ° C. It was found that the drying temperatures exhibiting the highest activ-
ity for the butane conversion are not always fi xed, for instance 200 ° C for one and
300 ° C for another.
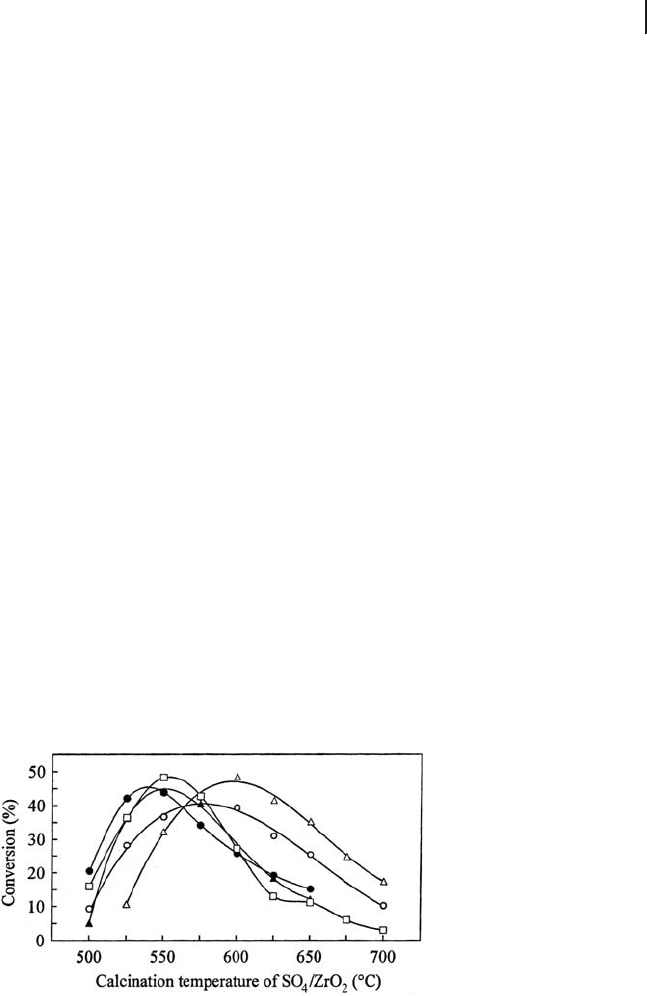
Figure 17.1 summarizes the effect of the calcination temperature on the catalytic
activity for butane isomerization over sulfated zirconias prepared from different
zirconia gels dried at the optimum temperatures. The fi gure indicates that the
maximum activities are approximately the same for different catalysts even though
Figure 17.1 Activities for reaction of butane at 180 ° C over the
SO
4
/ZrO
2
catalysts prepared from various Zr gels: MEL 631
dried at 300 ° C ( 䊐 ), MEL 632 dried at 200 ° C ( 䉱 ), Nakarai
dried at 300 ° C ( 䊉 ), and the gels prepared by hydrolysis of
ZrO(NO
3
)
2
( 䉭 ) and ZrOCl
2
( 䊊 ) followed by drying at 300 and
200 ° C, respectively.

674 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
the temperatures to give maximum activity are different. The calcination tempera-
tures required to give maximum activity for different catalysts fall within a tem-
perature range < 50 ° C. Residual species such as Cl
−
and NO
3
−
in the gel result in
differences in the optimum drying and calcination temperatures owing to differ-
ences in the state of dehydration of the zirconia gel or to reactivity of the zirconia
support with the sulfate species. However, selection of the optimum drying and
calcination temperature generate the same ultimate catalytic activity. The present
results point out that the optimum temperature for drying the Zr gel and the fi nal
calcination should be determined according to the type of zirconia gel.
17.2.2
Tungstated, Molybdated, and Borated Metal Oxides
17.2.2.1 Preparation of WO
3
/ZrO
2
and MoO
3
/ZrO
2
[30]
A sample of Zr(OH)
4
(10 g), obtained from ZrOCl
2
, is heated at 300 ° C, the gels
are impregnated with aqueous ammonium metatungstate [(NH
4
)
6
(H
2
W
12
O
40
] · n H
2
O,
50 wt% WO
3
, 3.8 g] and water (15 ml) in a 100 ml beaker followed by evaporating
water at room temperature, drying, and calcining in air at 800 ° C for 3 h. The con-
centration is 15 wt% W based on the hydroxide, and 13 wt% W after calcination at
650 – 950 ° C. The analogous material is also formed by the kneading method with
tungstic acid (H
2
WO
4
) which is insoluble in water; a wet mixture of Zr(OH)
4
(10 g)
and H
2
WO
4
(2 g) with a little water is kneaded for 3 h [70, 71] .
After heating Zr(OH)
4
(10 g) at 300 ° C the sample is impregnated with molybdic
acid (H
2
MoO
4
, 2.5 g) dissolved in ammonium hydroxide (28%, 2 ml) and water
(15 ml) followed by evaporating water at room temperature, drying, and calcining
at 800 ° C in air for 3 h. The concentration is 5 wt% Mo metal based on the hydrox-
ide [72] .
17.2.2.2 Preparation of WO
3
/SnO
2
, WO
3
/TiO
2
, and WO
3
/Fe
2
O
3
[30]
After the hydroxides of Sn, Ti, and Fe, prepared by hydrolysis of SnCl
4
, TiCl
4
, and
Fe(NO
3
)
3
, respectively, are dried at 300 ° C, the gels are impregnated with aqueous
ammonium metatungstates [(NH
4
)
6
(H
2
W
12
O
40
)] followed by evaporating water,
drying, calcining in air for 3 h at 1000, 700, and 700 ° C for the Sn, Ti, and Fe
materials, respectively. The concentration is 15 wt% W based on the hydroxides
(11 – 13 wt% W after calcination) [73, 74] .
WO
3
/SnO
2
is also prepared from a commercial gel, used after drying meta -
stannic acid of Kojundo Kagaku, Ltd. at 100 ° C [69] . Calcination at 1000 ° C after
impregnation of the tungstate generates the highest activity for acid - catalyzed
reactions. High temperatures such as 1000 ° C for calcination do not discriminate
precursor stannia gels, though the calcination temperature giving the highest
activity for SO
4
/SnO
2
differs according to the gel.
17.2.2.3 Preparation of B
2
O
3
/ZrO
2
[75, 76]
Zirconium hydroxide is impregnated with aqueous boric acid followed by evapo-
rating water and calcining in air at 650 ° C (3 wt% B). The same catalyst is obtained

by suspending the hydroxide in 2 - propanol solution of trimethyl borate followed
by adding water to hydrolyze the borate.
17.2.2.4 Property and Characterization
Properties of the catalysts thus prepared are summarized as follows [43, 48] .
1. Superacid sites are not created by impregnation on the crystallized oxides, but
on the amorphous forms whose calcination then converts them to the crystalline
forms. Recent work shows that a WO
3
/ZrO
2
catalyst prepared by impregnation
of the crystalline zirconia (65% tetragonal, 35% monoclinic) exhibits comparable
behavior [77, 78] .
2. WO
3
/ZrO
2
(800 ° C) and MoO
3
/ZrO
2
(800 ° C), whose activities are highest, are
observed to be 100% tetragonal ZrO
2
by XRD, with the TiO
2
in WO
3
/TiO
2
(700 ° C)
being the anatase polymorph [74] .
3. Specifi c surface areas of the WO
3
and MoO
3
catalysts are much larger than
those of the oxides without tungsten and molybdenum oxides, pure metal
oxides.
4. XPS spectra of the WO
3
and MoO
3
supported catalysts show their surface to be
WO
3
or MoO
3
and ZrO
2
, SnO
2
, TiO
2
or Fe
2
O
3
[73] .
17.3
Determination of Acid Strength
Several methods have been used to determine the surface acidity of solid acids,
but each method has its limitations. Common methods are titration with the
Hammett indicators, temperature - programmed desorption ( TPD ) , adsorption
microcalorimetry, catalytic test reactions, and IR and NMR spectroscopies. These
techniques exhibit several advantages and disadvantages.
Titration with a variety of Hammett indicators is one of the most widely used
techniques to determine the distribution of acid strengths on the solid surface [79] .
However, many arguments have been raised in the past against the use of Hammett
indicators for evaluation of solid acidity [80 – 83] .
A commonly used technique is TPD of adsorbed bases such as ammonia and
pyridine [84 – 86] . However, there are critical questions in this method [87 – 89] . The
NH
3
molecule is well known to interact with both the acidic OH group and the
basic oxygen [90] . Thus, NH
3
gives information on dual acid – base sites.
Instead of TPD, microcalorimetry of adsorption shows the heat evolved during
the adsorption of probe molecules, usually ammonia, on acid sites [91 – 94] . This
measurement can determine the distribution of adsorption enthalpies but cannot
differentiate between adsorption on Lewis and Br ø nsted acid sites.
Catalytic activity can be used to rank solid acidity, and activity for the skeletal
isomerization of butane is often used to indicate very strong acidity, in particular
superacidic strength [95] . A comparative study using the isomerizations of butane
17.3 Determination of Acid Strength 675

676 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
and pentane as test reactions gave good correlations between their rate constants
and the Hammett acid strengths [96] . These test reactions do not differentiate
between the acid strength and the number of acid sites. The mechanism of butane
or pentane isomerization, however, has been shown to be bimolecular, so rates of
alkane isomerization alone cannot be used to compare acidities [97 – 100] . One
paper indicates that the isomerization of α - pinene enables solid acids to determine
the Br ø nsted acid strength; superacidity promotes the formation of limonene over
camphene [101] .
A comparative study using IR spectroscopy can rank solid acidity by determining
the frequency shift of the adsorption band of pyridine [102] , the band shift of OH
groups due to the adsorption of benzene or CD
3
CN [103, 104] , and the shift of the
adsorbed CO on Lewis sites [105] . These IR techniques, however, are not widely
used for evaluation of solid acidity.
Solid - state NMR spectroscopy enables site - specifi c characterization of solid acids
such as protonated zeolites [106, 107] . Attempts have been made to relate the acid
strength of solids to the
1
H chemical shift of surface OH groups, the shift brought
by the adsorbed bases such as CD
3
CN and CCl
3
CN, and the
31
P shift of the
adsorbed
31
P(CH
3
)
3
[108 – 113] . A disadvantage of NMR spectroscopy is the compli-
cated chemical shift due to hydrogen bonding.
17.3.1
Hammett Indicators
The acid strength of a solid is defi ned as the ability of the surface to convert an
adsorbed neutral base into its conjugate acid. The strength is expressed by the
Hammett acidity function, H
0
, as explained in the Introduction. The color of suit-
able indicators adsorbed on a surface can give a measure of acid strength. If the
color is that of the acid form of the indicator, then the value of the H
0
function of
the surface is equal to or lower than the p
BH
K
+
of the conjugate acid of the indica-
tor. Lower values of H
0
correspond to greater acid strength.
The acid strengths shown in Table 17.3 were examined by the visual color
change method using the Hammett indicators shown in Table 17.1 [43, 48] . The
indicator dissolved in solvent was added to the sample in powder form in a non -
polar solvent, sulfuryl chloride [38] or cyclohexane [40] . The strength of colored
materials such as SO
4
/Fe
2
O
3
and MoO
3
/ZrO
2
was estimated from their catalytic
activities in comparison with those of the catalysts determined by the Hammett -
indicator method.
Determination of the acid strength of solid catalysts using Hammett indicators,
however, has been criticized frequently because of the heterogeneity of the solid
surface [81, 104, 110, 114 – 116] . The principle of the Hammett acidity function is
based on the equilibrium equation in a homogeneous solution, and its application
to the heterogeneous condition is subject to severe criticism. In addition, the color
change of the adsorbed indicators on solids as determined by the naked eye is
subjective. The effects of interactions between the solvent and the solid surface
has also been raised [9] .

17.3.2
Test Reactions
Owing to the heterogeneity of solid superacids, accurate acidity measurements are
diffi cult to perform and interpret. The most simple and useful way to estimate the
acidity of a solid catalyst is to test its catalytic activity in acid - catalyzed reactions.
We usually compare activity with those of aluminosilicates such as silica - aluminas
and zeolites. These materials have strong enough acidities to cause the Friedel –
Crafts reactions, and their acidities are known to be in the range of superacidity
[117] . The Hammett - indicator method indicates that the acid strength of the SiO
2
-
Al
2
O
3
used is in the range of − 12.70 < H
0
≤ − 11.35, whose acidity is H
0
= ∼ − 12 and
superacidic [37] . The acidity and catalytic activity of zeolites are generally higher
than the silica - aluminas, with mordenites being highest among them ( H
0
= ∼ − 14)
(ZSM - 5: H
0
= ∼ − 13) [67] .
In order to confi rm the acidity results measured using the indicators shown in
Table 17.1 , we have investigated as many acid - catalyzed reactions as possible. The
reactions are summarized in Table 17.4 [43, 48, 118, 119] . Among them, the skel-
etal isomerization of light paraffi ns, in particular butane and pentane, has been
the most widely applied. The isomerization of butane at room temperature was a
well known test reaction for superacidity at the beginning of this work [43, 48,
118] . The activity for many of the reactions tested correspond to the acidities as
determined by use of the Hammett indicators.
17.3.3
Temperature - Programmed Desorption ( TPD )
TPD using base adsorbents such as ammonia and pyridine has been one of the
common techniques to evaluate the amount and strength of acid sites on solid
acids. However, the desorption temperature of ammonia and pyridine adsorbed
on solid superacids is so high (500 ° C and above) owing to the strong interaction
that the adsorbed compounds are decomposed or oxidized before desorption. The
surface structure can even be destroyed by reactions with probe molecules
[120 – 122] .
Table 17.4 Acid - catalyzed reactions.
Dehydration of alcohol → MeOH, EtOH, i - PrOH
Decomposition of alkylbenzene → Ph - Me, Ph - Et, Ph -
i
Pr
Friedel – Crafts acylation → acetylation, benzoylation, and so on
Isomerization of paraffi n → open - chain C
1
– C
7
, cyclic C
6
– C
12
Esterifi cation → AcOH + MeOH, EtOH, and so on
C
8
OH + phthalic acid, and so on
Cationic polymerization → Me, Et,
i
Bu vinyl ether
Oligomerization → β - pinene, 1 - octene, 1 - decene
Others → aldol condensation, and so on
17.3 Determination of Acid Strength
677

678 17 Preparation of Superacidic Metal Oxides and Their Catalytic Action
TPD using pyridine was attempted for estimating the acidity of superacidic
materials, even though this method suffers a major drawback. An examination of
the termination temperature of pyridine desorption was made using TG - DTA
[123] . When the adsorbed compound is decomposed or oxidized to destruction
before desorption, its temperature must be below that of the real desorption. The
temperatures of pyridine desorption obtained were generally proportional to the
H
0
values of the highest acid strength determined by the Hammett method or
estimated by catalytic activity, except that of SO
4
/SnO
2
. All of the temperatures for
the superacids were higher than those of Al
2
O
3
, ZrO
2
- TiO
2
, SiO
2
- ZrO
2
, and SiO
2
-
Al
2
O
3
, whose acidities are below superacidic, and whose oxidative action is quite
weak. In the case of SO
4
/SnO
2
whose temperature was unexpectedly low, oxidation
of the adsorbed pyridine occurred on the catalyst surface at a temperature below
that expected from the acid strength by the Hammett method.
17.3.4
Temperature - Programmed Reaction ( TPR a )
Another method similar to TPD, is temperature - programmed reaction ( TPRa )
using furan as a probe molecule [118] . Furan is resinifi ed on the surface of solid
acids when heated at a temperature that depends on the acid strength. Solids with
weak acidity give rise to the reaction at elevated temperatures, and the reaction
temperature is dependent on the surface acidity. A relationship is observed between
decrease of the temperature and increase of the acid strength. The resinifi cation
of furan adsorbed on the surface of catalyst is exothermic, and TPRa using DTA
is a technique for estimating the acid strength of superacids.
The initial temperatures of resinifi cation were compared with the H
0
values of
superacids as determined by the Hammett method, and a linear relationship was
observed. The TPRa results gave the H
0
value of SO
4
/SnO
2
as − 18; the value
was not determined by the Hammett method because − 16.12 relates to 1,3,5 -
trichlorobenzene indicator which has the lowest p
BH
K
+
value among the Hammett
indicators used to date (Table 17.1 ).
17.3.5
Ar - TPD [124, 125]
A new method has involved the use of Ar as a probe atom. We tried Ar as a probe
TPD and found that Ar - TPD could be applied to the evaluation of the acid strength
of solid superacids.
Noble gases such as Ar and Xe interact only with the acidic sites, giving infor-
mation on these sites. Hence these atoms are useful as probes of the intrinsic
nature of the acidic sites. For noble gases, polarization and dispersion are domi-
nant. Ar shows an acid – base - like interaction in a polarized state with acid sites at
low temperature owing to its induced dipole.
The acid strength was fi rst evaluated as an activation energy of Ar desorption.
The activation energy was calculated by the following equation:
2ln lnTbERT
mdm
const−= +
(17.5)
Here T
m
is the peak temperature of desorption, b the rate of temperature
increase, and E
d
the activation energy. A linear plot is obtained between 2 ln
T
m
− ln b and 1/ T
m
, and E
d
is determined from the gradient.
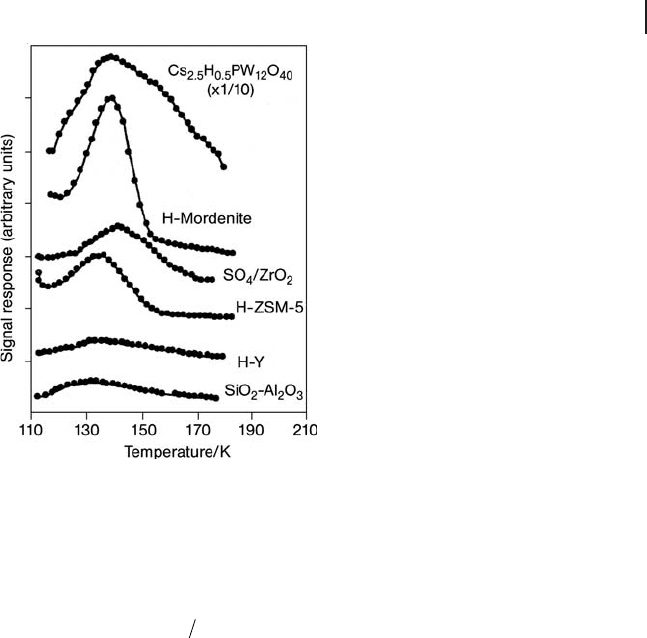
The Ar - TPD experiments were performed in the temperature range 113 – 223 K
at the programmed rate of 2 – 5 K min
− 1
using a cooling system with N
2
gas bubbled
through liquid N
2
along with an electric heater regulated by a temperature control-
ler. The profi les at the rate of 2 K min
− 1
are shown Figure 17.2 . The activation
energies of Ar desorption are calculated from the estimated values of T
m
to be 5.5,
6.0, 6.6, 6.7, 7.6, and 9.3 kJ mol
− 1
for SiO
2
- Al
2
O
3
, zeolites of H - Y, H - ZSM - 5, and
H - Mordenite, heteropoly acid (Cs
2.5
H
0.5
PW
12
O
40
), and SO
4
/ZrO
2
, respectively. The
value for SO
4
/SnO
2
is determined to be 10.6 kJ mol
− 1
[33, 34] .
The heat of adsorption of ammonia correlates with the Hammett acidity func-
tion, H
0
. Similarly, the activation - energies of SiO
2
- Al
2
O
3
, three zeolites, and SO
4
/
ZrO
2
relate well with their H
0
values determined by the Hammett method and
represented by their highest acid strengths, with a linear plot being obtained. By
extrapolation of the linear plot to the activation energy 9.3 kJ mol
− 1
for SO
4
/ZrO
2
,
the H
0
value is estimated to be − 19 [68] , which agrees with that estimated from
the heat of adsorption of ammonia [93] . The value of 10.6 kJ mol
− 1
found for SO
4
/
SnO
2
is estimated to correspond to H
0
= − 21.
Figure 17.2 Ar - TPD profi les of solid acids; temperature programmed rate: 2 K min
− 1
.
17.3 Determination of Acid Strength 679
