Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.

covalent, and therefore the coordination numbers is lower than 6. The typical
example of this type is BaGeO
3
. In spite of a t value close to 1, i.e., ideal ionic
size combination, BaGeO
3
crystallizes not in the perovskite structure but in the
silicate-related one. This difference occurs because the preferred coordination
number of Ge is 4. On the other hand, due to the progress in high-pressure
technology, the synthesis of new Ge-based perovskite oxides has been reported
[5]. As the coordination number of Ge increases with the pressure, perovskite
structures with higher coordination numbers are preferred, and a typical exam-
ple of this is CaGeO
3
. Another group of interest ing perovskite compounds is
oxynitrides, i.e., LaWO
3–x
N
x
, LaTiO
2
N, etc. Therefore, the value of t, which is
determined by the ionic size, is an important index for the stability of perovskite
structures; however, the contribution of the chemical nature, such as the coor-
dinating number of the constituent elements, needs to be taken into account.
The formation of superstructures in the perovskites is discussed next. If a
B-site cation is progressively replaced by a dopant, a large difference in ionic
radii tends to lead to the form ation of the superstructures rather than random
arrangements of the two kinds of ions. The typical case of this is Ba
2
CaWO
6
,
which is regarded as Ba
2
(CaW)O
6
. Similarly, in compounds with the general
formula Ba
3
MTa
2
O
9
, there is random distribution of M and Ta ion in the
octahedral positions when M is Fe, Co, Ni, Zn, or Ca, whereas formation of
a superstructure with hexagonal lattice is observed in Ba
3
SrTa
2
O
9
. Another
interesting type of superstructure observed in the perovskite is the ordering of
cation vacancies located on A sites: e.g., MNb
3
O
9
(M ¼La, Ce, Pr, Nb) and
MTa
3
O
9
(M ¼La, Ce, Pr, Nd, Sm, Gd, Dy, Ho, Y, Er). In these oxides, there
is an octahedral framework of the ReO
3
type with incomplete occupancy of
the 12-fold-coordinated A sites. Figure 1.4 shows the structure of LaNb
3
O
9
.
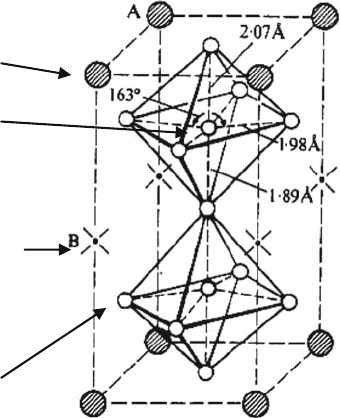
A site
deficient
O
B
A
Fig. 1.4 Structure of
LaNb
3
O
9
, A site-deficient
perovskite oxide
1 Structure and Properties of Perovskite Oxides 5
The B sites of the perovskite structure are occupied by Nb ion, and two-thirds of
the A sites remain vacant.
Other typical polymorphs of the perovskite structure are Brownmillerite
(A
2
B
2
O
5
)andK
2
NiF
4
structures. Brownmillerite (A
2
B
2
O
5
) is an oxygen-
deficient type of perovskite in which the oxygen vacancy is ordered. The unit
cell contains BO
6
and BO
4
units in an ordered arrangement. Because of the
oxygen deficiency, the coordination number of A-site cations decreases to 8.
The lattice parameter of the Brownmillerite structure relates to the cubic lattice
parameter (a
p
) of the ideal perovskite as a ¼b ¼
p
2a
p
,c¼4a
p
. Cu-based oxides
or Ni-based oxides tend to adopt these oxygen-deficient structures because of
the large amou nt of oxygen defects.
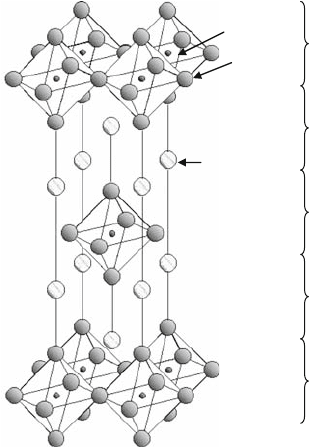
A combination of ordered B sites and oxygen defects is seen in K
2
NiF
4
structures, which is well known as it shows superconducting properties. The
K
2
NiF
4
structures consist of two units, the KNiF
3
perovskite unit and the KF
rock salt unit (Fig. 1.5), which are connected in series along the c-axis. As the
rock salt structure is embedded into the c-axis direction, the K
2
NiF
4
compound
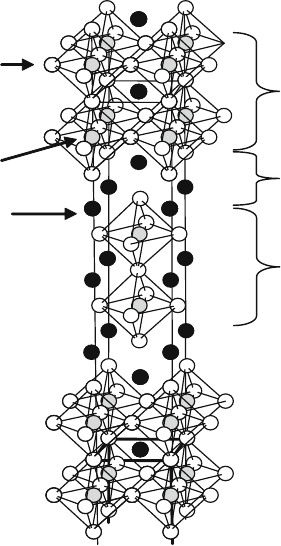
shows strong two-dimensional properties. Based on the intergrowth of the
different numbers of KNiF
3
and KF units, there are many structures called
Ruddelsden-Popper compounds with the general formula (ABO
3
)
n
AO
(Fig. 1.6); i.e., Sr
3
Ti
2
O
7
(n ¼2), Sr
4
Ti
3
O
10
(n ¼3). It is interesting to compare
the isostructural Sr
2
TiO
4
or Ca
2
MnO
4
with SrTiO
3
or CaMnO
3
, which crystal-
lize in the perovskite structures. Two different A cations forming the perovskite
and the rock salt units are also possible, and LaO
nSrFeO
3
is the typical
example of this arrangement. Another interesting variant of these K
2
NiF
4
structures occurs when two different anions occupy the two building blocks
A ion
B ion
O ion
Perovskite
Rock salt
Perovskite
Rock salt
Perovskite
Fig. 1.5 K
2
NiF
4
structure, a
perovskite-related structure
6 T. Ishihara
exclusively, i.e., SrFeO
3
SrF or KNbO
3
KF. In any case, it is evident that per-
ovskite oxides comprise a large family of oxides. As a result, a variety of crystal
structures and properties is expected in these compound s. For further detailed
discussion on the perovskite-related oxides, the reader is referred to references [6–9]
1.3 Typical Properties of Perovskite Oxides
Because of the variety of structures and chemical compositions, perovskite
oxides exhibit a large variety of properties. Well-known pro perties of the
perovskite oxides are ferroelectricity in BaTiO
3
-based oxides and superconduc-
tivity in Ba
2
YCu
3
O
7
, etc. In a ddition to these well-known properties, several
perovskite oxides exhibi t good electrical conductivity, which is are close to that
of metals, and ionic conductivity, as well as mixed ionic and electronic con-
ductivity. Based on these variations in electrical cond ucting property, perovs-
kite ox ides are chosen as the components for SOFC. It is also well known that
several perovskite oxides exhibit high catalytic activity with respect to various
reactions, in particular, oxidation reactions [10]. Table 1.2 provides examples of
the typical propert ies of perovskite oxides. In this section, several typical
properties of the perovskite oxides, namely, ferroelectricity, magnetism, super-
conductivity, and catalytic activity, are briefly discussed.
A
B
O
Perovskite
Perovskite
Rock salt
Fig. 1.6 Ruddelsden-
Popper structure, another
type of perovskite-related
structure
1 Structure and Properties of Perovskite Oxides 7
Dielectric properties: Ferroelectricity, piezoelectricity, electrostriction, and
pyroelectricity are special properties inherent to diel ectric materials and are
important properties of electroceramics. The best known property of perovs-
kite oxides is ferroelectric behavior, where BaTiO
3
,PdZrO
3
, and their doped
compounds are representative example s. The study of ferroelectricity in
BaTiO
3
has a long history, and many detailed reviews have been published.
Furthermore, because the ferroelectric behavior of BaTiO
3
has a strong
relationship with the crystal structure, detailed studies of crystal structure
have been reported for BaTiO
3
.BaTiO
3
undergoes mainly three-phase trans-
formation, that is, from monoclinic, to tetragonal, and to cubic, as the
temperature increases. Above 303 K, BaTiO
3
crystallizes in the cubic perovs-
kite structure, which does not show ferroelectric behavior. The high dielectric
constant observed in BaTiO
3
can b e explained on the basis of the anisotropy
of the crystal structure. Figure 1.7 shows the crystal structure of BaTiO
3
using
Table 1.2 Typical properties of perovskite oxides
Typical property Typical compound
Ferromagnetic property BaTiO
3
, PdTiO
3
Piezoelectricity Pb(Zr, Ti)O
3
, (Bi, Na)TiO
3
Electrical conductivity ReO
3
, SrFeO
3
, LaCoO
3
, LaNiO
3
, LaCrO
3
Superconductivity La
0.9
Sr
0.1
CuO
3
, YBa
2
Cu
3
O
7
, HgBa
2
Ca
2
Cu
2
O
8
Ion conductivity La(Ca)AIO
3
,CaTiO
3
, La(Sr)Ga(Mg)O
3
,BaZrO
3
, SrZrO
3
,BaCeO
3
Magnetic property LaMnO
3
, LaFeO
3
,La
2
NiMnO
6
Catalytic property LaCoO
3
, LaMnO
3
, BaCuO
3
Electrode La
0.6
Sr
0.4
CoO
3
,La
0.8
Ca
0.2
MnO
3
Ba
E
z
= 4.6 × 10
9
E
z
= –7.5 × 10
9
E
z
= 1.1 × 10
10
E
z
=4.1×10
10
δz = 0.003Å
δz = –0.05Å
δz = 0.06Å
δz = –0.09Å
Ti
BaTiO
3
O (3)
O (2)
Fig. 1.7 Crystal structure of
BaTiO
3
using Ewald method
and local density of charge
8 T. Ishihara
the Ewald method as well as local charge density [11]. It is seen that the large
negative potential is localized on the O
3
oxygen atom. When the electric field is
applied, B a
2þ
and Ti
4þ
cations move to the direction opposite to that of the
oxygen atom. Thus, a net dipole moment is created in the unit cell. According
to the Slater theory [11], the electrostatic field is strongly affected by the atoms
locatedinO
3
sites; thus, a large dipole moment is generated in BaTiO
3
.
Electrical conductivity and superconductivity: One of the most well known
properties of perovskite oxides is superconductivity. In 1984, superconductivity
was first reported by Bednorz and Mu
¨
ller in La-Ba-Cu-O perovskite oxide [12].
After their report, much attention was paid to new types of high-temperature
oxide superconductors, mainly Cu-based oxides. As a result, several supercon-
ducting oxides with different A-site cations have been discovered. However, the
presence of Cu on the B site was found to be essential for superconductivity to
occur. High-temperature oxide superconductors of the YBa
2
Cu
3
O
7
system [13]
and the Bi
2
Sr
2
Ca
2
Cu
3
O
10
system [14] were reported in 1987 and 1988, respec-
tively, and currently the critical temperature of the superconducting transition
(T
c
) has been further increased to 130–155 K in the HgBa
2
Ca
2
Cu
3
O
8+d
system
[15]. As all high-temperature superconducting oxides are cuprites (Cu-based
oxides), superconductivity is clearly related to the Cu-O layers. The critical
temperature for superconductivity, T
c
, is related to the number of Cu-O layers
in the crystal structure:
One Cu-O layer: T
c
30 K
Two Cu-O layers: T
c
90 K
Three Cu-O layers: T
c
110 K
Four Cu-O layers: T
c
120 K
It is expected that further increase in the number of Cu-O layers may result
in higher values of T
c
. However, because of the low chemical stability, synthesis
of five or more Cu- O layered compounds has not been successful so far.
YBa
2
Cu
3
O
7
is one of the most important superconductor systems with high
T
c
, and detailed studies of its crystal structure have been performed. Also,
the content of oxygen nonstoichiometry is an important factor for high T
c
.
When the value of d is smaller than 0.5, YBa
2
Cu
3
O
7–d
crystallizes in an orthor-
hombic structure, which is superconductive, whereas for d > 0.5, YBa
2
Cu
3
O
7–d
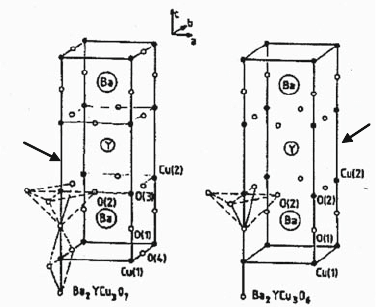
has a tetragonal structure, which does not exhibit superconductivity. Figure 1.8
shows the crystal structures of both oxygen-deficient phases in YBa
2
Cu
3
O
7–d
.
The main difference between the tw o structures is that the incorporation of
oxygen in the lattice expands the b lattice parameter to a greater extend than the
a lattice parameter. Those changes in crystal structure are related to the oxygen
content, which is determined by the annealing temperature and oxygen partial
pressure during postannea ling treatment. As discussed, superconductivity in
high T
c
oxides is also dependent on the crystal structure; thus, the high chemical
stability of the perovskite crystal structure could be effective in achieving high
values of T
c
.
1 Structure and Properties of Perovskite Oxides 9
In addition to supercond uctivity, there are many perovskite oxides showing
high electronic conductivity, which is close to those of metals such as Cu. The
typical examples of such perovskite oxides are LaCoO
3
and LaMnO
3
, which is
now commonly used as a cathode in SOFC. These perovskite oxides shows
superior hole conductivity, which is as high as s ¼100/S/cm. Doping of alio-
valent cation on the A site is also highly effective in enhancing the electrical
conductivity because of the increased number of mobile charge carriers gener-
ated by the charge compensation.
Catalytic activity: Because of the variety of component elements and their
high chemical stability, perovskite oxides have be en also extensively studied as
catalysts for various reactions. Two types of research trends clearly emerged
from these characteristics. The objective of the first trend is the development of
oxidation catalysts or oxygen-activated catalysts as an alternative to catalyst
containing precious metals, whereas the second trend regards perovskite as a
model for active sites. The stability of the perovski te structure allows prepara-
tion of compounds with an unusual valence state of elements or a high extent
of oxygen deficiency. Table 1.3 summarizes the reactions studied by using
perovskite oxides as catalysts. Evidently, the high catalytic activity of perovs-
kite oxides is based partially on the high surface activity to oxygen reduction
ratio or oxygen activation resulting from the large number of oxygen vacancies
present.
Among the various catalytic react ions studied, those applicable to envir-
onmental catalysis (e.g., automobile exhaust gas cleaning catalyst) attract
particular at tention. Ini tially, it was reported that perovskite oxide consist-
ing of Cu, Co, Mn, or Fe exhibited superior activity to NO direct decom-
position at higher temperatures [16–18]. The direct NO decomposition
reaction (2NO ¼N
2
þO
2
) is one of the ‘‘dream reacti ons’’ in the catalysis
field. In this reaction, the ease of removal of surface oxygen as a product of
Orthorhombic Tetragonal
Oxygen
deficient
layer
Oxygen
deficient
layer
Fig. 1.8 Orthorhombic and
tetragonal crystal structures
of BaY
2
Cu
3
O
7
, an oxygen-
deficient perovskite
10 T. Ishihara
the reaction plays an important role, and due to the facil ity of oxygen
deficiency present, per ovskite oxides are active with res pect to this reaction
at high temperatures. It is pointed out that doping is highly effective in
enhancing N O decomposition activity. Under an oxygen-enri ched atmo-
sphere (up to 5%), a relatively high NO decomposition activity was reported
for Ba(La)Mn(Mg)O
3
perovskite [19].
Recently, another interes ting application of perovskite oxides as autom o-
bile catalysts has been reported, namely, the so-called int ellig ent cata lysts [20].
Up to now, three- way Pd-Rh-Pt catalysts have been widely used for the
removal of NO, CO, and uncombus ted hydrocarbons. T o decrease the amount
of precio us metals, a catalyst consistin g of fine particles with high surface-to-
volume ratio is required. Howeve r, these fine particles are not stable under
oper ating conditions and easily sinter, resulting in deactivati on of the catalyst.
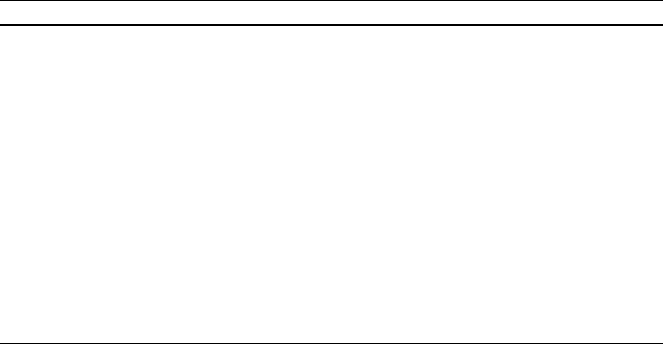
To maintain a high dispersion state, the redox property of perovskite oxides
has been proposed; i.e., under oxidat ion conditions, palladium is oxidized and
exists as LaFe
0.57
Co
0.38
Pd
0.05
O
3
, and under reducing conditions, palladium is
deposited as fine metallic partic les with a radi us of 1–3 nm. This cycling of the
catalyst t hrough oxidizing and reducing conditions results in the partial sub-
stitution of Pd into and deposition fr om the perovskite framework, thus
maintaining a high dispersion state of Pd. This method was found to be highly
effective in improving the long-term stability of Pd during removal of pollu-
tants from exhaust gas (Fig. 1.9). The high dispersion state of Pd can be
recovered by exposing the catalys t to an oxidation and reduction environment.
As a result, this catalyst is called an intelligent catalyst. This unique property
also originates from the high stability of the perovskite crystal structure in
complex oxides.
Table 1.3 Main catalytic reactions studied by using perovskite oxides
Catalytic reaction Example
Oxidation CO, lower hydrocarbon, Methanol
Catalytic combustion
LaCoO
3
, LaMnO
3
deNOx Selective reduction LaAIO
3
, SrTiO
3
NO decomposition BaMnO
3
, SrFeO
3
,
YBa
2
Cu
3
O
7
NO absorption LaAIO
3
, BaCeO
3
, BaFeO
3
Hydrogenation C
2
H
4
hydrogenation LaCoO
3
CH
4
coupling Oxidative CH
4
coupling BaTiO
3
,
Ba
0.5
Sr
0.5
Fe
0.2
Co
0.8
O
3
Oxygen electrode Oxygen reduction (alkaline solution)
Oxygen generation (alkaline solution)
Cathode for Solid Oxide Fuel Cell
Oxygen sensor
LaCoO
3
, LaMnO
3
LaCoO
3
, LaFeO
3
LaCoO
3
, LaMnO
3
LaCoO
3
, LaMnO
3
Gas sensor Oxygen sensor, Humidity sensor,
Alcohol Sensor
SrTiO
3
, BaSnO
3
,
LaCr(Ti)O
3
, GdCoO
3
1 Structure and Properties of Perovskite Oxides 11
1.4 Preparation of Perovskite Oxide
Because the perovskite structure is stable at high temperatures and also stable in
terms of thermodynamic equilibrium, the perovskite oxides form only at a
temperature typically higher than 1273 K. The most simple and popular
method for preparat ion of perovskite oxides is the so-called solid-state reaction
method, when the starting compounds (often simple oxides and carbonates) are
calcined at temperatures higher than 1273 K. However, because of the high
temperature of the calcination, the Burumauer-Emmott-Teller (BET) surface
area of the resulting perovskite powders is generally small, usually less than 10
m
2
/g. The preparation of perovskite oxide powders with a large surface area,
namely, fine particles, is strongly demanded in various fields, in particular, for
catalyst and electrode application not only for solid oxide fuel cells (SOFC) but
also for batteries and/or electrolysis. To obtain fine particles of perovskite
oxides, some advanced synthetic methods that general ly involve the use of
organic compounds have been developed. However, the preparation of perov s-
kite oxide powders with a large surface area is quite a difficult subject, and the
BET surface area is generally smaller than 50 m
2
/g. This restriction is easily
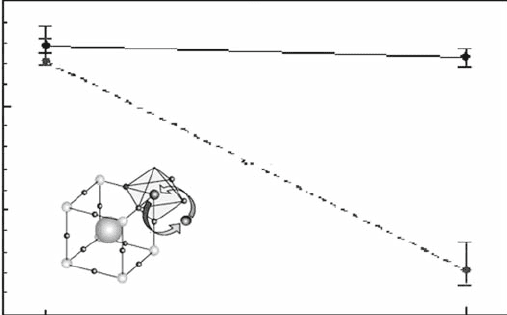
understood by considering a sim ple relationship between the specific surface
area (S) and the diameter of a spherical particle (D) [21]:
S ¼ 6=ðrDÞ (1:1)
where r is the density of the sample. Figure 1.10 shows the relationship between
the geometrical surface area (S) of a spherical body and radius (D): the density
Removal of pollutant/%
Operation at 900°C/h
Conventional catalyst
(Pd/Al
2
O
3
)
Intelligent catalyst
LaFe
0.57
Co
0.37
Pd
0.05
O
3
oxidation
reduction
A
B
O
Pd
0
85
90
95
100
100
Fig. 1.9 Structure of ‘‘intelligent catalyst’’ and comparison of the catalytical activity of the
intelligent catalyst and the conventional Al
2
O
3
-supported one for the removal of pollutants in
exhaust gas
12 T. Ishihara
of LaCoO
3
perovskite oxide is much lower than that of a general single oxide
such as MgO or Al
2
O
3
. Therefore, for the purpose of obtaining a high surface
area, such as 100 m
2
/g, the required particle size of the perovskite oxide must be
smaller than 10 nm, which is quite difficult to achieve.
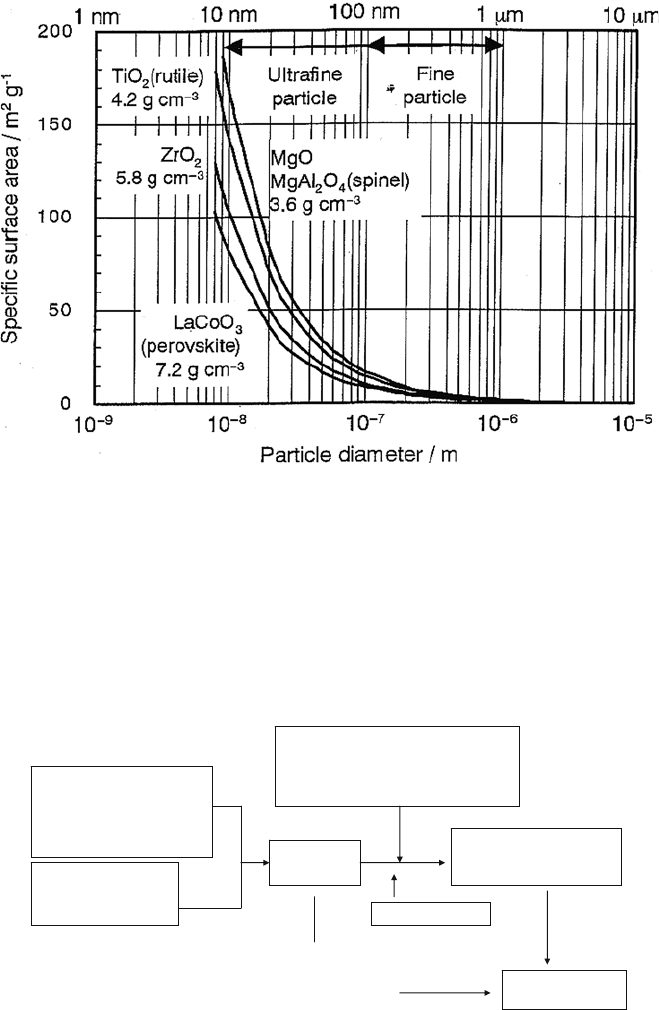
Figure 1.11 summarizes the general procedure of the liquid-phase synth-
esis method used in the preparation of perovskite oxides with a large surface
Fig. 1.10 Relationship between geometrical surface area (S) of a spherical body and radii (D)
Starting material
(Metal salt, metal
Alkoxide,
metal organic compound)
Solvent
(Water, organic one)
Solution
Precipitating agent
Gel formation agent
Complex formation agent
Evaporation
Precursor
(precipitate, gel, etc.)
heating
Final Oxide
Unique reaction
condition
(Hydrothermal,
Supercrytical etc.)
Fig. 1.11 General procedure of the liquid-phase synthesis method
1 Structure and Properties of Perovskite Oxides 13

area. In this method, atomic-level dispersi on of the c omponent elemen ts in
the precursor solution is essential. Based on the dispersion method, the
proposed liquid-phase preparation method could be classified into three
groups (Table 1.4). The techniques classified into group I use energy such as
ultrasonic vibration or supercritical conditions to achieve a high dispersion
state. The application of microwave heating to a precursor containing
BaCl
2
, Ti isopropoxide, and KOH has been employed during the synthesis
of BaTiO
3
fine pa rticles. It has been reported that BaTiO
3
perovskite
powder with a particle size of 20–30 nm was successfully prepared [22]. On
the other hand, group II focuses on the usage of micelles, which limit the
space for the perovskite precursor. LaMnO
3
prepared by using reverse
micelles has been repor ted to possess high electrode a ctivity when used at
the anode of a metal-air battery. Finally, techniques in group III involve the
usage of organic compounds for achieving atomic-level dispersion in the
precursor solutions. In the most popular cases, the addition of a mmonia is
used to obtain uniform precipitates of perovskite precur sors. However,
because of the difference in the precipitation rates, it is difficult to obtain
a precursor with uniform distribution of constituent elem ents at the atomic
level.
Teraoka et al. reported the use of organic coordination compounds for the
preparation of perovskites [23]. They found that addition of acetic acid or
maleic acid is useful for obtaining finely powdered perovskite oxides by decreas-
ing the crystallization temperature. Figure 1.12 shows the C
3
H
8
oxidation rate
of LaMnO
3
perovskite oxide prep ared by various methods and compositions
plotted against the BET surface area. It is evident that the C
3
H
8
oxidation rate
increases monotonically with increasing the BET surface area of LaMnO
3
, and
it can be easily understood how the preparation method is important for
improving the surface activity of perovski tes.
Table 1.4 Proposed liquid phase synthesis method for perovskite oxides
Category Method
Group I
(Controlled evaporation or
reactant decomposition rate)
Spray pyrosis, Spray (mist, aerosol) thermal
decomposition, Freeze dry, Combustion synthesis,
Microwave assisted method, Supercritical water
Group II
(Usage of designed micro pore) Antimicelle
Group III
(Designed precursor) Hy droxide precursor; Uniform precipitation, Sol gel
method another precursor; Cyanide decomposition,
Oxalic Acid method, EDTA-citrate complexing
method, Pechini method
14 T. Ishihara
