Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.

essentially based on simulation and experimental work, which has been per-
formed independently in 1995 by Tuckerman et al. and Agmon [7, 8]. Essential
features of the suggested mechanism are that (i) intermolecular proton transfer
occurs through rather short hydrogen bonds; (ii) this transfer is highly coupled
to hydrogen bond-breaking and bond-forming processes in the weaker
bonded second hydration sphere of the protona ted species (H
3
O
þ
or H
5
O
2
þ
);
and (iii) the position of the protonic charge follows the center of symmetry of
the hydrogen bond pattern [9]. In other words, proton transfer and structural
reorganization, which together form an uninterrupted trajectory for proton
translocation, take place in different parts of the hydrogen-bonded structure.
In this way, both pro ton transfer and structural reorganization, the two essen-
tial ingredients of ‘‘structure diffusion’’ may take place at very high rates [9].
Later, a similar mechanism has been found for proton conduction in imidazole
[10], an amphoteric molecular compound, which shows high intrinsic proton
conductivity in the liquid state. As in the case of phosphoric and phosphonic
acid, this is also the result of the high degree of self-dissociation, which is low in
the case of water.
Albeit the proton conduction mechanisms in hyd rogen-bonded networks
comprise the interactions of many species, proton conduction mechanisms in
oxides are usually less complex ; this has to do with the fact that protons are
usually present at low concentrations as part of noninteracting [11], positively
charged point defects (generally hydroxide ions residing on oxide ion sites,
OH
O
). These defects show significant hydrogen bond interaction with neigh-
boring oxide ions, and it is the local dynamics of this hydrogen bonding that
turned out to be the clue to the understanding of proton conduction mechan-
isms in perovskite-type oxides. Only in very few perovskite-type oxides, such as
Ba
2
YSnO
5.5
[12], very high proton content may add some complexity to the
proton conduction mechanism.
In the first part of this short chapter, it is discussed where protons find
energetically favored sites in oxides with the perovskite structure, before the
mechanisms of proton diffusion via these sites are described in detail. While this
discussion is restricted to structure and dynamics of protonic defects in ideal
(cubic) perovskites, the following section addresses complications such as sym-
metry reduction and the effect of the presence of dopants, before, finally, a few
implications for the development of proton-conducting electrolytes for fuel cell
applications are discussed.
13.2 Proton Sites
The possible positions for protons in perovskite-type oxides are strongly con-
strained through the strong binding interaction with the oxygen, i.e., the most
electronegative species in this type of compound, with which protons form
hydroxide ions. In other words, protons are localized within the valence
262 K.D. Kreuer
electron density of the oxygen, which attains some H1s ch aracter. The orienta-
tion of the hydroxide ion is then mainly determined by the hydrogen bonding
with other, neighboring oxide ions, which is an attractive interaction, and the
interaction with the cations of the structure, which, of course, is repulsive.
Although clear signatures of hydrogen bonding were found in the OH-stretch-
ing vibration [13–18], i.e., typica l red-shift ed continua, there have been dif fer-
ent suggestions for the orientation of these bonds. Within the simple cubic
perovskite structure, i.e., a framework of corner-sharing undistorted oxygen
octahedra, each oxygen has eight nearest oxygen neighbors with separations
corresponding to the edge length of the octahedra, and four next nearest
neighbors, which reside on the vertices of the four neighboring octahedra in
thesameplane.
A proton jump width of 170 pm obtained from quasi-elastic neutron scatter-
ing (QNS) spectra of Y-doped SrZrO
3
was interpreted as the separation
between proton sites on the edges of neighboring octahedra corresponding to
linear hydrogen bonding of the hydroxide ions to their nearest oxygen neigh-
bors [19] Similar positions have been favored by first-principles molecular
dynamics simulations of protons in Sc-doped SrTiO
3
[20, 21]. From a neutron
and X-ray powder diffraction study on deuterated Ba
3
Ca
1+y
Nb
2–y
O
9–d
, how-
ever, the deuterons were refined to point toward the four next nearest oxygens
[22]. These positions actually do not violate the empirical Badger–Bauer rule
[23], which is excluding hydrogen bonding within the same coordination poly-
hedron. The uncertainty of the proton sites is actually reflecting the locally flat
free energy surfaces, especially in the case of perovskites with small lattice
constants such as SrTiO
3
. In the very first quantum molecular dynamics
study of protons in BaCeO
3
[24], the trace of the proton at T ¼ 900 K was
found to be confined to a kind of ‘‘donut’’ with the hydroxide oxygen defining
the center. This ‘‘donut’’ actually contains all the sites just discussed, and from a
careful analysis of the proton probability density function for different perovs-
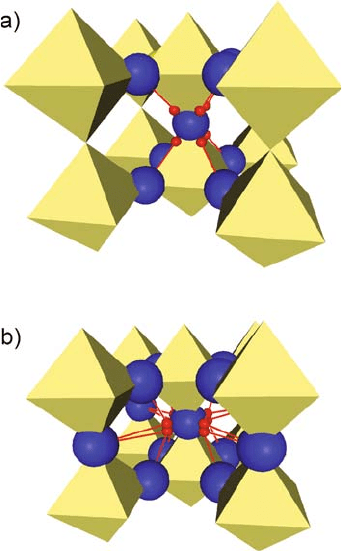
kite-type oxides, proton sites have been obtained [25, 26]. For perovskites with
large lattice constants such as BaCeO
3
and BaZrO
3
, sites close to the octahedra
edges were identified (Fig. 13.1a), whereas in perovskites with small lattice
constants such as SrTiO
3
and CaTiO
3
, the proton sites are closer to the plane
formed by the hydroxide ion and its four next nearest oxygen neighbors
(Fig. 13.1b). As illustrated in Fig. 13.1, there are always 8 sites per hydroxide
ion (24 per unit cell), but for large lattice constants the only hydrogen bond
interaction is within octahedra, while for small lattice constants, also hydrogen
bonding to oxygen of neighboring octahedra appea rs to be possible. Hydrogen
bonding is actually indicated by a small contraction (a few pm) of the average
separation between the hydroxide ion and its eight nearest and four next nearest
oxygen neighbors (e.g., [27]); it is most likely the repulsion with the highly charged
B-site cation (Ti
4þ
) and the attraction from the next nearest oxygen, which is
pushing the proton out of the octahedron edge. The resulting degree of hydrogen
bond bending (for the average configuration) surely has severe implications on
the proton conduction mechanisms, which are discussed in the next section.
13 Mechanisms of Proton Conduction in Perovskite-Type Oxides 263
13.3 Mechanisms of Proton Conduction (Undoped, Cubic
Perovskites)
The elementary reactions underlying the mobility of protonic defects have been
investigated in great detail, experimentally as well as by num erical simulations
from quite different points of view. It is near at hand to even consider proton
tunneling, but as in any fast proton conductors tunneling effects are anticipated
to be negligible [1]. Even without taking lattice relaxation (polarization) effects
around the proton into consideration, proton tunneling is estimated to become
significant at extremely low temperature (T < 14 K) only [28].
Apart from the strong localization of the proton within the hydroxide ion,
the proton self-lo calization also involves a slight contraction of the average
OH/O separation (see above) and an expansion of the separation between the
hydroxide ion and the two neighboring B-site cations (e.g., Ce
4þ
; Fig. 13.1)
[24–26]. Any proton conduction mechanism must at least partially compensate
for these relaxation effects; i.e., it must involve some host lattice adjustment in
the transition state configuration. In a first attempt to relate lattice dynamics to
proton transfer between adjacent oxide ions, the coupling of proton transfer to
the O–B–O bending mode was investigated numerically [29]. The rate of proton
Fig. 13.1 Illustration of
proton sites (small spheres )
as obtained from molecular
dynamics simulations for
cubic perovskite-type oxides
with large lattice constants
(a) and small lattice
constants (b) [24, 25]. Only
the oxygens (dark)
interacting with the proton
are shown. Possible
hydrogen bond interactions
are indicated by grey lines
264 K.D. Kreuer
transfer was found to increase, and the H/D isotope effect was decreasing with
increasing anharmonicity of this mode. The influence of the hydrogen bonding
on the local dynamics had been neglected, and only proton transfer had been
treated in this early study, but the importance of the oxide ion dynamics had
already been clearly recognized. Generally speaking, the local lattice relaxation
may be treated as a diffusing small polaron, which the proton is following; this
is nothing but structure diffusion (see Introduction), whi ch was treated analy-
tically by several authors [30, 31, 32]. However, these approaches are either
phenomenological or they make severe assumptions on the relevant modes
(e.g., O–B–O bending). In particular, they do not give any specific information
on chemical interactions and how they determine the evolution of configura-
tions involved in the proton conduction mechanism.
Our current more detailed understanding of proton conduction mechanisms
in perovskite-type oxides actually started to emerge from independent quantum
molecular dynamics simulations [23], quasi-elastic neutron scattering [33], and
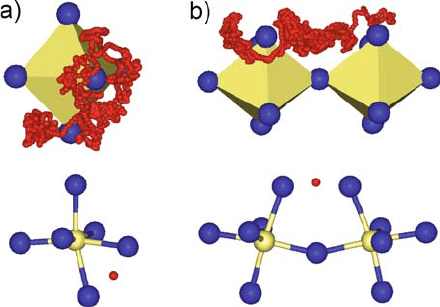
m-SR experiments [34, 35]. They all clearly show rapid rotational diffusion of
the proton within a ‘‘donut’’ around the oxygen with which the proton is making
a covalent bond (Fig. 13.2). In this local dynamics the strong covalent bond
remains intact, and it is only the orientation of the hydroxide ion that is
changing. Because of the free energy barriers separating the different orienta-
tions, this dynamics is stochas tic, i.e., diffusional in nature. Of course, it
involves forming and breaking of hydrogen bonds (see red lines in Fig. 13.1),
which obviously costs very little excitation, which is explained later. These early
Fig. 13.2 Proton traces sampled by quantum molecular dynamics simulations involving intra-
octahedron transfer (a) and inter-octahedra proton transfer (b) [24, 25]. The transition state
configurations for proton transfer are shown for both cases: In the case of intra-octahedron
transfer, this is characterized by B–O bond elongations and strong contraction of the OH–O
separation; in the case of inter-octahedra transfer, severe tilting of the participating octahedra
is involved (see text)
13 Mechanisms of Proton Conduction in Perovskite-Type Oxides 265
results also indicate that it is the proton transfer from a hydroxide ion to a
neighboring oxide ion that is rate-limiting, long-range pro ton transpo rt.
Considering the large average oxygen separations (e.g., compared to hydro-
gen bonding in water) of typically 290–320 pm, this appears to be reasonable,
but it seemed to be at odds with the strongly red-shifted OH-stretching absorp-
tions in the IR spectra ([12] and references therein), indicating strong hydrogen
bond interactions, which favor fast proton transfer reactions rather than fast
reorientation processes, the latter requiring the breaking of such bonds. As the
structural oxygen separation is larger than 290 pm in most perovskite-type
oxides and strong hydrogen bonds may only be formed for significantly lower
separations, the free energy the system gains by hydrogen bond formation is
competing with the free energy required for the lattice distortion involved in the
OH/O contraction. A reanalysis of a quantum-MD simulation of a protonic
defect in cubic BaCeO
3
[25, 36, 37] demonstrates that these two free energy
contributions are almost balanced for a wide range of oxygen separations
(approximately 250–300 pm). In this way, short oxygen separations, which
favor proton transfer, and large oxygen separations, which allow rapid bond
breaking invo lved in rotational diffusion, correspond to similar free energies of
the entire system and, therefore, have similar probabilities of occurring. Indeed,
the simulation finds the protonic defect to form short but transient hydrogen
bonds with all eight nearest oxygen neighbors. In the time-averaged picture seen
in diffraction experiments, this leads only to a slight reduction of the structural
OH/O separations (see Fig. 13.1), but in most instant configurations one of the
eight OH/O separations is reduced to about 280 pm as a result of hydrogen
bonding [24]. Although the hydrogen bond interaction has a stabilizing effect
of about 0.5 eV on this configuration [38], the bond is a soft high-energy
hydrogen bond with extended bond length variations ; this also leads to config-
urations where the protonic defect behaves almost like a free OH with small OH
stretching amplitudes compared to the extended stretching vibrations in the
hydrogen-bonded state.
From the thermodynamics of such ‘‘dynamical hydrogen bonds’’ one may
actually expect an activation enthalpy of long-range proton diffusion not more
than 0.15 eV provided that the configuration O–H...O is linear, for which the
proton transfer barrier vanishes at O/O separations less than about 250 pm.
However, the mobility of protonic defects in cubic perovskite-type oxides has
activation enthalpies of the order of 0.4–0.6 eV [35], which raises the question of
which interactions are controlling the activation enthalpy of proton transfer.
As already pointed out, in the average configuration the hydrogen bonds are
bent to some extent (see Fig. 13.1). This change suppresses proton transfer for
two reasons: there is a remaining proton transfer barrier even at low oxygen
separations, and any proton transfer requires both energy and momentum
transfer in this bent configuration. An analysis of a few transition state config-
urations obtained from molecular dynamics simulations showed that the B–O
bonds are elongated, which reduces the repulsion between the B-site cation and
the proton and allows for the formation of an almost linear, short hydrogen
266 K.D. Kreuer
bond in the transition state complex [Fig. 13.2(a)]. The proton transfer in this
configuration probably occurs over some remaining barrier, as indicated by the
experimentally observed H/D isotope effects [28, 39]. Although the H/B repul-
sion is redu ced in this configuration, major contributions to the activation
enthalpy arise from the B–O bond elongation and the proton transfer barrier.
The importance of the H/B repulsion is also evidenced by the finding that the
activation enthalpies of proton mobility in cubic perovskites with pentavalent
B-site cations (I–V perovskites) are significantly higher than for perovskites
with tetravalent B-site cations (II–IV perovskites) [36]. On this background,
proton mobility in III–III perovskite may be even higher, provided the oxide
shows cubic symmetry.
So far, we have just considered the mechanism of proton conduction in
perovskite-type oxides with large lattice constants, and these are commonly the
ones with potentially high concentrations of protonic defects and therefore also
high proton conductivity [35], but perovskite-type oxides with small lattice con-
stant (e.g., SrTiO
3
,CaTiO
3
) may also exhibit high proton mobilities. In these
cases, hydrogen bonding even to the next nearest oxygen, between the vertices of
the octahedra, becomes possible [Fig. 13.1(b)], opening another proton transfer
path as observed in MD simulations [20, 24]. The transition state complexes then
involve a tilting of neighboring octahedra between which the transfer takes place
(Fig. 13.2). While only 25% of the observed proton transfers are occurring
between octahedra (inter-octahedra transfer) in the case of SrTiO
3
, proton transfer
in CaTiO
3
is observed to be dominated by inter-octahedra transfers (70%). It
should be noted that for the latter the highest proton mobility has been found in
the simulations [25], but small concentration of protonic defects under common
conditions prevents titanates from showing high proton conductivity [37].
The mechanisms just described provide a qualitative explanation for the
empirical finding that the highest proton conductivities are observed in oxides
with the cubic perovskite structure [40]. The framework of corner-sharing BO
6
octahedra shows high coordination numbers for both cation sites (12 for the A
site and 6 for the B sit e). There is only 1 oxygen site in the ideal perovskite
structure, with each oxygen surrounded by 8 nearest and 4 next nearest oxygens.
Generally speaking, the high coordination numbers lead to low bond strengths
and low angles between the bonds, which is in favor of the above-described
dynamics. For example, the rotational diffusion of the protonic defect corre-
sponds to a dynamical hydrogen bonding of the OH with the 8 or 12 oxygen s,
forming the ‘‘reaction cages’’ (see Fig. 13.1). The angles between the possible
orientations are small enough that the effective barriers for bond-breaking and
bond-forming processes are usually very low, and the equivalence of all oxygens
guarantees the absence of any extra contribution to the activation energy that
may originate from differences in site energies. The latter is especially important
for the proton trans fer reaction, which may be biased to a particular oxygen
site, which then may act as a kind of proton trap. Also, the high number of
equivalent transfer paths directly enters into the pre-exponential factor of
proton mobility.
13 Mechanisms of Proton Conduction in Perovskite-Type Oxides 267
13.4 Complications (Symmetry Reduction, Doping, Mixed Site
Occupancy)
From these considerations, it is anticipated that any perturbation of the highly
symmetric perovskite structure may lead to a decrease of the rate of long-range
proton transport. This may not only be brought about by the reduction of the
symmetry of the crystallographic (time/space-averaged) structure, but also
locally by the presence of point defects (e.g., acceptor dopants, oxide ion
vacancies), mixed site occupations, or higher dimensional defects, such as
dislocations or grain boundaries.
The effects of the tilting and twisting of the BO
6
octahedra, i.e., the reduction
of the crystallographic symmetry, has been investiga ted in detail by comparing
structural and dynamical features of protonic defects in Y-doped BaCeO
3
and
SrCeO
3
[41]. The large orthorhombic distortion of SrCeO
3
has tremendous
effects on the arrangement of the lattice oxygen, which leads to the appearance
of shorter and longer O/O separations and also changes the chemical character
of the oxygen. The cubic oxygen site splits into two sites with probabilities of 1/3
(O1) and 2/3 (O2). As a result of different chemical interactions with the cations,
especially the strontium on the A site, the oxygens on these sites show distinctly
different electron densities (basicities) and therefore different binding energies
for the proton. Although in SrCeO
3
the most basic oxygen is O1, it is O2 in
BaCeO
3
. Assuming that protons are associated with these sites for most of the
time, they may show long-range proton transport via the more frequent O2 sites
in BaCeO
3
, wher eas isotropic long-range proton transport in SrCeO
3
must
involve transfer be tween chemically different O1 and O2 sites. Much more
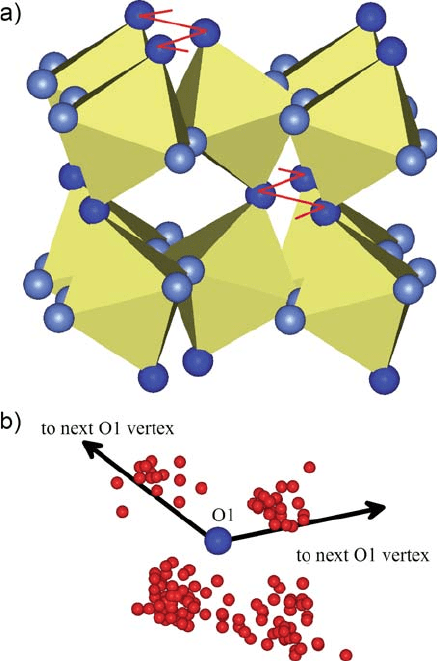
rapid transport is anticipated to occur between neighboring O1 oxygens. How-
ever, they just form one-dimensional arrays as a result of octahedra tilting
[Fig. 13.3(a)], and transport along these paths is expected to be very sensitive
to any perturbation. Even for the fast rotational diffusion step, the orthorhom-
bic distortion is clearly inducing a biasing of the hydroxide orientation toward
the more basic oxygen neighbors, O1 [Fig. 13.3(b)]. All these observations are
thought to be the reason for the higher activation enthalpy and lower conduc-
tivity in SrCeO
3
compared to BaCeO
3
. Similar simulations were later carried
out on orthorhombic CaZrO
3
[42], and also in this case octahedra tilting is
opening the proton transfer between the vertices of neighboring octahedra,
whereas no intra-octahedron proton transfer was detected in the simulation.
The mobility of protons is not only very sensitiv e tow ard reduction of the
crystallographic symmetry but also toward local structural and chemi cal per-
turbations induced by the acceptor dopant or by mixed occupancy on the B site.
To allow for the formation of protonic defects by dissociative absorption of
water, perovski te-type oxides are commonly doped with aliovalent ions (accep-
tor dopants) matching the ionic radius of the B-site cation (e.g., In
3þ
for Zr
4þ
).
Indeed, this simple concept has been proven successful, e.g., for oxide ion
conductors (Sc-doped zirconia shows higher oxide ion conductivity than
268 K.D. Kreuer
Y-doped zirconia). But when it comes to pro ton conductivity in oxides, this
approach clearly fails. Although Sc
3þ
and In
3þ
are matching Zr
4þ
with respect
to their ionic radii, BaZrO
3
shows much lower proton mobility when doped
with Sc or In compared to Y as an acceptor dopant with a significantly higher
ionic radius [39]. Only for the latter, the proton mobility and its activation
enthalpy are virtually independent on the dopant concentration. Although Y on
the Zr site expands the lattice locally and on the average and even leads to
tetragonal dist ortions at concentrations above 5 mol%, it actually leaves the
acid–base properties of the coordinating oxygen almost unchanged [24].
Obviously, this chemical match of the dopant makes it so to say ‘‘invisible’’ to
the diffusing proton. The most common observation, however, is a decreasing
proton mobility and an increasing activation enthalpy with increasing dopant
concentration, as observed in Y-doped BaCeO
3
, for example [11, 37], for which
the local distortions around the acceptor dopant has recently been studied
experimentally [43].
Fig. 13.3 Proton diffusion
path in orthorhombic
SrCeO
3
as obtained from a
quantum molecular
dynamics simulations [40].
The proton rotational
diffusion around O1 is
distinctly biased toward the
neighboring O1 (b), which is
part of a one-dimensional
proton diffusion path
formed by O1 oxygen only
(a). Proton transfer between
O1 (dark) and the less basic
and more frequent O2 (grey)
is anticipated to control
long-range, isotropic proton
diffusion (see text)
13 Mechanisms of Proton Conduction in Perovskite-Type Oxides 269
In an attempt to calculate association energies for the formation of clusters
of protoni c defects and different kinds of acceptor dopants in SrCeO
3
-based
proton conductors [44], the lowest energies have been found for Y and Yb, the
most commonly used dopants for this type of compound. For CaZrO
3
, the most
favorable dopants are anticipated to be Ga, Sc, and In [45] Even interaction of
protonic defects with residual oxide ion vacancies has been anticipated to
influence the local proton dynamics in SrTiO
3
[20].
With this background, it is not surprising that also cation ordering on the B
site of complex perovskites may become disadvantageous for proton mobility.
For example, in Ba
3
Ca
1+x
Nb
2–x
O
3–d
, proton conductivity is higher with lower
activation enthalpy when chemically and geometrically different Ca and Nb are
randomly distributed on the B site. Ca/Nb ordering occurring after annealing
significantly reduces the conductivity [46]. This observation is in line with the
results of an investigation of proton conductivity in the system SrTiO
3
–Ba-
TiO
3
–SrZrO
3
–BaZrO
3
[39]. Although Sr/Ba mixing on the A site actually left
some space for a materials optimization, Zr/Ti mixing on the B site led to a
significant suppression of the proton conductivity. The highest proton mobi-
lities in this system were actually found for the end members SrTiO
3
and
BaZrO
3
, both showi ng the simple cubic perovskite structure.
13.5 Implications for the Development of Proton-Conducting
Electrolytes for Fuel Cell Applications
The requirements for the use of proton-conducting oxides as separator material
in solid oxide fuel cells (SOFC) not only comprise a high mobility of protonic
defects but also a high concentration of such defects and stability under fuel
cell operating conditions. At the moment, only electrolytes based on highly
Y-doped BaZrO
3
seem to combine these properties in a unique way [35]. Surpris-
ingly, the development of this material required very little compromising.
BaZrO
3
is actually the cubic perovskite with the highest lattice constant. The
high symmetry is essential for the high solubility limit of protonic defects and
for the high isotropic proton mobility. The high lattice constant (a > 420 pm for
Y-doped samples [39]) goes along with a high stability of protonic defects, and the
covalency of the Zr–O bond reduces the Zr/H-repulsive interaction and, there-
fore, the activation enthalpy of the mobility of protonic defects. Only
Ba
3
CaNb
2
O
9
has a similarly favorable lattice constant, but Ca/Nb ordering on
the B site leads to a symmetry reduction. Less densely packed BaCeO
3
already
shows a significant orthorhombic lattice distortion.
Another key feature is the availability of a nearly perfect acceptor dopant
(i.e., a dopant that leaves the oxygen basicity almost unchanged). Although in
all other reported cases the increase of the acceptor dopant concentration leads
to a reduction of the proton mobility and an entropic destabilization of proto-
nic defects, both the proton mobility and the thermodynamics of hydration are
270 K.D. Kreuer
practically unchanged for dopant levels up to 20% Y in BaZrO
3
[37]. High
proton mobility and entropically stabilized protoni c defects even at high dopant
concentrations and the high solubility limit lead to the high proton conductivity
of this material. For temperatures below about 7008C and a water partial
pressure of 23 hPa, this exceeds the oxide ion conductivity of the best oxide
ion conductors. Although the bulk conductivity of Y-doped BaZrO
3
is even
slightly higher than the proton conductivity of BaCeO
3
-based oxides, the
chemical stability is by far more advantageous, as expected from the higher
electronegativity of Zr compared to Ce and the higher covalency of the Zr–O
bond. For the CO
2
partial pressure of air (38 Pa corresponding to 380 ppm),
pure BaZrO
3
is stable above 3008C, which is only slightly higher than for
BaTiO
3
and SrTiO
3
, which are known for their superior stabilities [12].
High bulk proton conductivity, high stability, and a wide ionic domain [47]
therefore make Y-doped BaZrO
3
an interesting parent compound for the
development of proton- conducting electrolytes for SOFC applications. Unfor-
tunately, the unfavorable brit tleness, the grain boundary impedance, and the
increasing phase instability with increasing Y-dopant level remain problems to
be solved. The addition of small amounts of BaCeO
3
or a compromise in the
choice of the kind of dopant may help to reduce these problems.
In any case, the potentially very high proton mobility in perovskite-type
oxides, which was discussed in detail in this chapter, may become a clue to the
reduction of the unfavorably high ope ration temperature of conventional
SOFCs.
Acknowledgments I thank R. Merkle for reading the proofs and U. Traub for preparing the
figures.
References
1. K.D. Kreuer, Chem. Mater. 8, 610–641 (1996)
2. K.D. Kreuer, W. Weppner, A. Rabenau, Solid State Ionics, 3/4, 353–358 (1981)
3. K.D. Kreuer, A. Rabenau, W. Weppner, Angew. Chem. Int. Ed. Engl. 21, 208 (1982)
4. S.F. Fischer, G.G. Hofacker, M.M. Ratner, J. Chem. Phys. 52, 1934 (1970)
5. R.A. Marcus, J. Chem. Phys. 24, 966 (1956)
6. C.P. Flynn, A.A. Stoneham, Phys. Rev. 1, 3966 (1970)
7. M.E. Tuckerman, D. Marx, M.M. Klein, M. Parrinello, J. Chem. Phys. 103, 45 (1995)
8. N. Agmon, Chem. Phys. Lett. 244, 456 (1995)
9. K.D. Kreuer, Solid State Ionics 136–137, 149–160 (2000)
10. W. Mu
¨
nch, K.K. Kreuer, W. Silvestri, J. Maier, G. Seifert, Solid State Ionics, 145, 437
(2001)
11. K.D. Kreuer, W. Mu
¨
nch, M. Ise, A. Fuchs, U. Traub, J. Maier, Ber. Bunsenges. Phys.
Chem. 101, 1344 (1997)
12. P. Murugaraj, K.K. Kreuer, T. He, T. Schober, J. Maier, Solid State Ionics 98, 1 (1997)
13. K.D. Kreuer, Solid State Ionics 97, 1 (1997)
14. H. Yugami, Y. Shibayama, T. Mattori, M. Ishigame, Solid State Ionics 79, 171 (1995)
15. T. Scherban, Y.Y. Baikov, E.E. Sharkova, Solid State Ionics 66, 159 (1993)
16. S. Shin, H.H. Huang, M. Ishigame, Solid State Ionics 40/41, 910 (1990)
13 Mechanisms of Proton Conduction in Perovskite-Type Oxides 271
