Ishihara T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Fuel Cells and Hydrogen Energy)
Подождите немного. Документ загружается.

8.3 Perovskite Chemistry
Perovskite oxide materials possess the general stoichiometry ABO
3
. Convention-
ally, the A cation is larger than the B cation. In the archetype, the A cation has an
oxidation state of +2 and the B cation has the oxidation state +4. These materials
comprise three different ionic species, each with its own equilibrium defect con-
centration due to three different activation energies for defect formation, which,
combined with the constraint of electroneutrality, make for diverse and potentially
useful defect chemistry, particularly when considering electronic, hole, and ionic
conduction under atmospheres of different oxygen partial pressures [13].
Perovskite oxides generally exhibit excellent thermal and mechanical stability.
They remain stable well above 10008C, and therefore the temperatures of SOFC
operation do not present a problem. This condition is in contrast with the Ni-
cermet, where nickel sintering and agglomeration are potential hazards. It is also
important that the anode exhibits good physical and mechanical compatibility
with the dense electrolyte layer onto which it is deposited. The electrolyte choice is
usually YSZ, although there is interest in alternative electrolyte materials such as
lanthanum gallate. Examination of unit-cell parameters and thermal expansion
coefficients reveals that perovskites generally show good compatibility with the
electrolyte material. Several perovskites have also been shown to be stable in the
kind of reducing environment encountered at the fuel electrode, and specific
perovskites have exhibited the levels of ionic and electronic conductivity neces-
sary to be realistically viable as an SOFC anode.
Perovskites containing a transition metal are of particular interest because of
the availability of multiple oxidation states, which facilitate elect rocatalytic
processes and provide mechanisms for electronic conductivity. For example,
under reducing atmospheres the transition metal ions cha nge to lower oxidation
states, effectively freeing up electrons to pass current. Typical examples of such
species are titanium, niobium, and vanadium. SrNbO
3
has an electronic con-
ductivity of 10
4
Scm
1
under reducing conditions [14], and Petric reported a
conductivity of 10
3
cm
1
for SrVO
3
under similar conditions [15]. Unfortu-
nately, it was also found that these compo unds could not be fabricated in air,
ELECTROLYTE/ANODE INTERFACE
CH
4
CH
4
O
2–
O
2–
O
2–
e
–
e
–
CO
2
CO
2
H
2
O
H
2
O
*
*
*
*
*
*
*
*
*
*
*
*
*
*
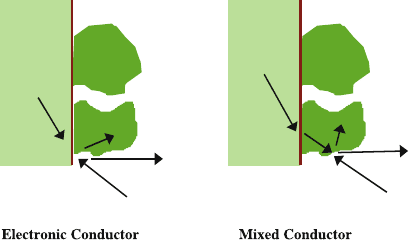
Fig. 8.1 Enhancement of
electrode reactive surface
area for fuel oxidation in an
SOFC anode by mixed
conducting materials
8 Perovskite Oxide Anodes for SOFCs 169
and their stability under fuel electrode conditions was questionable. As well as
the increased electronic conductivity, there may also be an increase in the oxide
ion contribution to conductivity due to the formation of oxygen vacancies on
reduction, according to Eq. (8.2):
O
X
O
$ 2e
0
þ
1
=
2
O
2
þ V
O
(8:2)
By raising the intrinsic oxygen vacancy concentrationinthismanner,thereisan
increase in available hopping sites. The increase in vacant sites facilitates oxygen
transport through the crystal and hence raises the potential for oxide ion conduc-
tivity. The coordination of these oxide vacancies to the B-site ion is particularly
important in determining mobility. Generally the B-site ion is six coordinate,
octahedral. If fivefold or fourfold coordinates are also well known for a particular
transition metal ion, then an obvious mechanism for oxide ion conduction exists;
however, this would not be the case for B-site ions such as Cr(III), which strongly
prefer sixfold coordination; this is illustrated in the chart shown in Fig. 8.2.
8.4 Doping, Nonstoichiometry, and Conductivity
Defect concentrations and hence defect chemistry of perovskites can be con-
trolled and tailored significantly by dopin g. Figure 8.3 shows schematically the
various possibilities.
MO
5
V
V
V
V
Cu
II
Cu
II
MO
4
tet
MO
4
Sq
?
??
Fuel ?
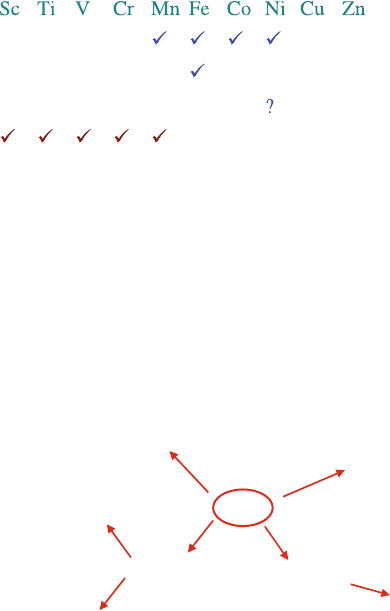
Fig. 8.2 Comparison between different perovskite B-site ions comparing stability under fuel
conditions and ability to reduce coordination number to allow vacancy oxide ion conductivity
of transition metals in perovskite oxides
ABO
3
ABO
3
–δ
ABO
3+
δ
A
n
B
n
O
3n+1
A
1–x
BO
3
A
2
BO
4
…..
A
0.6
BO
3
A
0.3
BO
3
Fig. 8.3 Possible domains of
perovskite
nonstoichiometry
170 J.T.S. Irvine
By substitution of parent cations with similar-sized cations of different
valence, defects can be introduced into the structure. Oxygen ion vacancies or
interstitials can be generated by substitution of B-site ions with cations of
lower or higher valence, respectively, producing compounds of stoichiometry
AB
(1–x)
B
0
x
O
(3 –/+d)
. A-site vacancies can be introduced by substitution of A-site
ions with cations of higher valence, giving compounds of stoichiometry of
A
(1–x–)
A
0
x
BO
3
. Substitution of A-site ions with lower-valence cations results in
oxygen vacancy formation giving compounds of stoichiometry A
(1–x)
A
0
x
BO
(3–d)
.
The effects of such extrinsic defect concentrations on both carrier concentration
and conductivity mechanism are discussed next.
Oxygen ion vacancy concentrations can be increased by partial substitution
of the tetravalent B-site ions with lower-valency cations, as shown in Eq. (8.3):
O
X
O
þ B
X
B
þ MO $ M
00
B
þ V
O
þ BO
2
(8:3)
M
B
00
is a divalent cation, and V
O
is an induced oxygen ion vacancy. It is
expected that these additional vacant sites facilitate oxygen transport through
the crystal by increasing the number of potential carriers.
The most important example of a perovskite that exhibits high oxide ion
conductivity when doped is lanthanum gallate, LaGaO
3
. Slater et al. [16] per-
formed neutron diffraction and conductivity studies on La
0.9
Sr
0.1
Ga
0
.
8
Mg
0.2
O
2.85
and found it to exhibit significantly different structure and properties when com-
pared with the undoped compound. In this case, both the A-site and the B-site have
been doped to create oxygen vacancies, the resulting material possessing an oxide
ion conductivity of 6.6 10
2
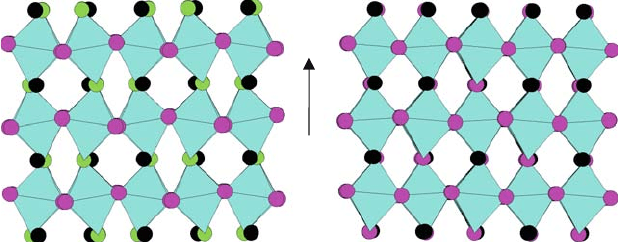
at 1000 K. Figure 8.4 shows schematically structural
changes that may account for the dependence of activation energy on temperature
involving the tilting of GaO
6
octahedra. The oxide ion conductivity attained is
25°C 1000°C
O1
O2
[001]
p
Fig. 8.4 Comparison between structure of La
0.9
M
0.1
Ga
0.8
Mg
0.2
O
2.85
at ambient temperature
and 10008C illustrating significant changes in octahedral tilting with temperature: views down
[110]
p
, La atoms in black, O atoms, and MO
6
octahedra shown [16]
8 Perovskite Oxide Anodes for SOFCs 171
higher than YSZ at the same temperature and has sparked considerable ongoing
research into its application as a SOFC electrolyte [16–18].
Strontium titanate is an archetypal example of perovskite and exhibits a wide
range of defect chemistry that aptly illustrate the different factors that may
influence electronic conductivity. The effects of changing oxygen partial pres-
sure upon undoped and differently doped strontium titanates are shown in
Fig. 8.5. Important aspects are the extended p-type behaviou of the B-site-
doped sample at higher P
O2
, the p-type behavior of the undoped sample at
higher P
O2
, and the very high n-type conductivity of the A-site-deficient sample
at lower P
O2
values. Equation 8.4 shows how such a doped material is likely to
exhibit p-type conductivity at the expense of ionic conductivity in high oxygen
partial pressures. The ambient oxygen atoms fill the positively charged vacan-
cies, generating a pair of holes in accordance with electroneutrality.
1
=
2
O
2
þ V
O
$ O
X
O
þ 2h
(8:4)
Equations (8.3) and (8.4) combine to give Eq. (8.5):
B
X
B
$ M
00
B
þ 2h
(8:5)
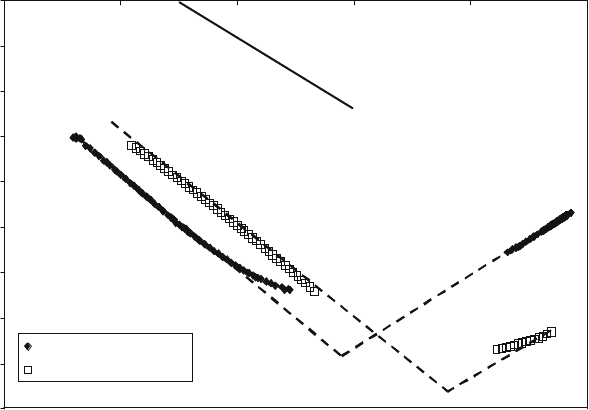
The p-type behavior of the undoped sample at high P
O2
has various possible
explanations, the most likely being simply that equilibrium intrinsic oxygen
–4.50
–4.00
–3.50
–3.00
–2.50
–2.00
–1.50
–1.00
–0.50
0.00
0.00–5.00–10.00–15.00–20.00–25.00
log (P
O2
)
log (conductivity)
5% Bsite mg @ 835°C
undoped @ 930°C
Slope = – 0.210
Slope = –0.246
Slope = 0.201
La
0.4
Sr
0.4
TiO
3
Fig. 8.5 SrTiO
3
, conductivity variation for different doping scenarios with oxygen partial
pressure at 9308C [13]
172 J.T.S. Irvine
vacancies are filled by ambient oxygen atoms generating holes. The high n-type
conductivity at low P
O2
seen for the A-site-deficient sample can be explained
with reference to the equilibria described by Eqs. (8.6) and (8.7).
2e
0
þ
1
=
2
O
2
þ V
O
$ O
X
O
(8:6)
O
X
O
þ A
X
A
$ AO þ V
O
þ V
00
A
(8:7)
The large value of V
A
00
achieved by doping reduces the number of intrinsic
Schottky defects pushing Eq. (8.7) to the left and hence reducing V
O
. For a
given P
O2
, Eq. (8.6) shifts left to oppose the change, facilitating the removal of
lattice oxygen by hydrogen and the associated generation of free electrons.
Figure 8.6 shows the temperature dependence of the n-type conduction in a
strontium titanate, which indicates metallic behavior [12].
8.5 Perovskite Anode Materials
Perovskite oxides can accommodate a large content of oxygen vacancies; hence,
some perovski tes are good oxygen ionic conductors. The small B site in the
perovskite allows first-row transition elements to be introduced in the lattice.
These elements exhibit multivalency under different conditions, which may be a
source of high electronic conductivity. Good ionic and mixed conductivity is
thus found in several perovskite oxides. As alread y mentioned, such mixed
conductivity is beneficial to electrode performance. P-type perovskite materi-
als are widely considered for SOFC and other applications [19]. Mixed con-
ducting perovskites, such as La
1–x
Sr
x
MnO
3
with modest oxide ionic conduc-
tivity or La
1–x
Sr
x
Co
1–x
Fe
x
O
3
with quite high oxide ionic conductivity, have
been used as SOFC cathode materials [8, 20]. La
1–x
M
x
CrO
3
(M ¼Ca, Sr), a
purely electronic conductor, has also been widely used as the interconnector
for SOFCs [8].
0
0
0.05
0.1
0.15
0.2
0.25
1000800600400200
temperature (C)
Resistivity
Fig. 8.6 Resistivity (in
ohm-cm) vs. temperature for
A-site-deficient
Sr
0.875
Ti
0.75
Nb
0.25
O
(3–d)
exhibiting metallic
conductivity [12]
8 Perovskite Oxide Anodes for SOFCs 173
Perovskites have also been widely investigated as potential SOFC anode
materials. Among these materials, chromites and titanates are promising [21,
22]. Interesting results have been obtained with lanthanum strontium titanates
[23] and especially cerium-doped lanthanum strontium titanate [24]; however, it
is now thought that the cerium-doped anodes are in fact two phases consisting
of a ceria–perovskite assemblage [24].
It was also reported that Y-doped SrTiO
3
exhibits high electrical conduction
under SOFC anodic conditions [25–27]. For example, the optimized composition
of Sr
0.86
Y
0.08
TiO
3–d
exhibits a conductivity of 82 S/cm at a P
O2
of 10
–19
atm at
8008C. However, the sample was pre-reduced in pure argon or 7% H
2
/Ar at
14008C before cond uctivity measurements. It is supposed that the conductivity
of the materials would be significantly lower if the sample were only reduced below
10008CinthiscaselessTi
4þ
was reduced to Ti
3þ
, which is the source of the high
electronic conductivity. The high-temperature pre-reduction process for such tita-
nates makes it diff icul t to co-fir e the anode and cathode. The conductivity of
Sr
0.86
Y
0.08
Ti
0.9
Sc
0.1
O
3
is only about 1–2 S/cm when reduced in situ in 5% H
2
at
9008C [28]. No phase changes were found for a mixture of Y-doped SrTiO
3
(SYT)
with YSZ or LSGM on calcining at 14008C for 10 h, indicating good chemical
compatibility between the SYT and electrolyte materials. The conductivity of
SrTiO
3
in a reducing atmosphere can also be improved by replacing titanium
with some niobium. For charge compen sation, the strontium content at the A
site should decrease. Good electrical conductivity was observed for Sr
1–x
Ti
1–x/
2
Nb
x
O
3–d
(x 0.4) [29] on reduction in low oxygen partial pressure, with a
maximum for the sample with x ¼0.25, s ¼5.6 S/cm at 9308C(P
O2
¼10
18
atm).
Lanthanum strontium titanates are usually treated in the literature as simple
cubic perovskites, although the presence of extra oxygen beyond the ABO
3
stoichiometry plays a critical role in both the structure and the electrochemical
properties, as summarized in Fig. 8.1. The lower members of the La
4
Sr
n-4
Ti
n
O
3n+2
series, n < 7, are layered phases, having oxygen-rich planes in the
form of crystallographic shears joining consecutive blocks. These planes become
more sporadic with increasing n (i.e., decreasing the oxygen content) until they
are not a crystallographic feature, rendering local oxygen-rich defects randomly
distributed within a perovskite framework, n > 11 [30, 31]. The presence of
such disordered defects appears to strongly affect the redox characteristics of
the oxide, as indicated by marked effects on conductivity induced by mild
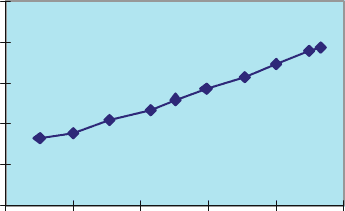
reduction (Fig. 8.7). Unfortunately, although the materials from this lantha-
num strontium titanate oxygen excess series are much easier to reduce, and
hence exhibit much higher electronic conductivity than their oxygen stoichio-
metric analogues, they do not exhibit very good electrochemical performance
[32]. This detriment is attributed to the inflexibility of the coordination
demands of titanium, w hich strongly prefers octahedral coordination in the
perovskite environment.
To make the B-site co ordination more flexible and hence to improve electro-
catalytic performance, Mn and Ga wer e introduced to replace Ti in La
4
Sr
8
Ti
12
O
38–z
-based fuel electrodes. Mn supports p-type conduction in oxidizing
conditions and has been previously shown to promote electroreduction under
174 J.T.S. Irvine
SOFC conditions [33]. Furthermore, Mn is known to accept lower coordination
numbers in perovskites [34], especially for Mn
3þ
, and thus it may facilitate
oxide ion migration. Similarly, Ga is well known to adopt lower coordination
than octahedral in perovskite-related oxides. The possibility of mixed ionic/
0.25
4
5
6
n
8
10
12
∞
δ
0.20
0.15
0.10
0.05
0.00
0.00
–5.00
0.1 0.2 0.3 0.4 0.50
–4.00
–3.00
–2.00
–1.00
1/n
Log σ\Scm
–1
Sc series
Local
defects
Extended defects
Layered
n>12
n<12
n
= 12
5nm
3
2
1
4nm
4nm
Fig. 8.7 High Resolution Transmission Electron Microscopy (HRTEM) images (top)ofsam-
ples from the ‘‘La
4
Sr
n–4
Ti
n
O
3n+2
’’ series varying from ordered extended planar oxygen excess
defects (1; n ¼5) through random layers of extended defects (2; n ¼8) to disordered extended
defects (3; n ¼12). The middle picture shows the location of these phases on the composition
map with 1/n plotted against oxygen excess, d, in perovskite unit ABO
3+d
.Thebottom diagram
shows defect electronic conductivity (s/cm
1
) of grain component as determined by ac
impedance spectroscopy on samples quenched from 13008C in air, also plotted against d [31]
8 Perovskite Oxide Anodes for SOFCs 175
electronic condu ction is very important because it would allow the electro-
oxidation process to move away from the three-phase electrode–electrolyte–gas
interface onto the anode surface, with considerable catalytic enhancement.
The anode polarization resistance was measured using three-electrode geo-
metry. By optimization of electrode microstructure, polarization resistances in
wet H
2
were 0.12 O cm
2
in wet H
2
, 1.5 O cm
2
in wet 5% H
2
, and a remarkably
low value, 0.36 O cm
2
, in wet CH
4
, at 9508C. These polarization resistances were
attained after about 24 h in fuel conditions; initial polarisation resistances were
two to three times higher. This long time period to achieve equilibration is fairly
typical for donor-doped strontium titanates that are not cation vacancy com-
pensated, and we attribute this to reorgani zation of a complex defect structure.
The open circuit voltages (OCVs) matched the value predicted by the Nernst
equation, 0.97 and 1.13 V at 9508C, for wet 5% H
2
and wet H
2
, respectively. The
OCVs in wet CH
4
, for a one-layer 50:50 YSZ:LSTMG anode, were: 1.39 V at
9508C, 1.32 V at 9008C, and 1.36 V at 8508C. These values were reproducible
after 2 days of testing in wet 5% H
2
, wet H
2
and wet CH
4
[35].
Although SrVO
3
shows excellent electronic conductivity of 1000 S/cm at
8008C and an oxygen partial pressure of 10
20
atm, it is unstable under a more
oxidizing atmosphere [36]. The conductivity in a reducing atmosphere may
drop rapidly if strontium at the A site is partially or completely replaced by
lanthanum, and neither is the stability in oxidizing condition improved. A
material that has both adequate high-temperature conductivity in a reducing
atmosphere and redox stability has not been found in the vanadates. In addi-
tion, the reduction process of SrVO
3
is rather slow once the material is oxidized.
The perovskite oxide La
0.6
Sr
0.4
Co
0.2
Fe
0.8
O
3
was proposed as an SOFC
anode at intermediate temperatures (5508–7008C) [37]. The stability of these
materials under fuel atmosphere is in doubt, however, even at such low tem-
peratures. SrFeCo
0.5
O
x
exhibits both high electronic and ionic conductivities in
air and is applicable for ceramic membrane used for gas separation [38]. It has
been reported that mixed conductors SrFeCo
0.5
O
x
, SrCo
0.8
Fe
0.2
O
3–d
and
La
0.6
Sr
0.4
Fe
0.8
Co
0.2
O
3–d
may be used as SOFC anode materials. Although
there remain some questions about the exact structure of SrFeCo
0.5
O
x
, these
are generally thought to be related perovski te/brownmillerite intergrowths of
the Grenier type [39]. The performance was not ideal when using only these
mixed conductors as an anode; however, the performance was impr oved when
these mixed conductors were used as an interlayer between an Ni-YSZ anode
and YSZ electrolyte. The long-term stability would again be a problem because
SrFeCo
0.5
O
x
is unstable under anodic conditions [40].
As stated above, LaCrO
3
-based materials have been investigated as inter-
connect materials for SOFCs [8]; however, they are also potential anode mate-
rials for SOFCs due to their relatively good stability in both reducing and
oxidizing atmospheres at high temperatures [41]. The reported polarization
resistance using these materials is too high for efficient SOFC operation,
although significant improvements have been achieved using low-level doping
of the B site. As no significant weight loss was observed when LaCrO
3
was
176 J.T.S. Irvine
exposed to a reducing atmosphere (P
O2
¼10
21
atm at 10008C) [42], this
indicates that chromium strongly retains its sixfold coordination. Indeed,
Cr
III
is well known to strongly prefer sixfold coordination in its chemistry;
thus, it is difficult to introduce the oxygen vacancies that are required for
oxygen ion conduction into the LaCrO
3
lattice.
When the B sites are doped by other multivalence trans ition elements that do
tolerate reduced oxygen coordination, such as Mn, Fe, Co, Ni, and Cu, oxygen
vacancies may be generated at the B-site dopa nts in a reducing atmosphere at
high temperature. Thus, a significant degree of B-site dopant is required to
generate a percolation path for oxygen vacancies to achieve high oxygen ion
conductivity. Quite a lot of attention has been focused on 3% replacement of Cr
by V, and although methane cracking seems to be avoided [43], the polarization
resistance is still of the order of 10 O cm [2, 21, 38, 44]. The introduction of other
transition elements into the B site of La
1–x
Sr
x
Cr
1–y
M
y
O
3
(M ¼Mn, Fe, Co, Ni)
has been shown to improve the catal ytic properties for methane reforming [45].
Of the various dopants, nicke l seems to be the most success ful, and the lowest
polarization resistances have be en reported for 10% Ni-substituted lanthanum
chromite [46]; however, other workers have found nickel evolution from 10%
Ni-doped lanthanum chromites in fuel conditions [47]. Certainly nickel oxides
would not be stable in fuel atmospheres, and although the nickel may be
stabilized by the lattice in the higher oxidation state, there will always be the
suspicion that the activity of nickel-doped perovskites is the result of surface
evolution of nickel metal and hence questions about long-term stability. A
composite anode of 5% Ni with a 50:50 mixture of La
0.8
Sr
0.2
Cr
0.8
Mn
0.2
O
3
and Ce
0.9
Gd
0.1
O
1.95
was successfully used for SOFCs with different fuels [48].
The combination of both titanium and chromium at the B site of a perovskite
has also been investigated [49, 50]. The highest conductivity in 5% H
2
/Ar of 5 S/
cm at 10008C was observed with composition La
0.4
Ca
0.6
Cr
0.2
Ti
0.8
O
3–d
; how-
ever, the catalytic effect of these materials for oxidization of hydrogen at the
anode is possibly not ideal, because a large anode polarization resistance was
observed when La
0.7
Sr
0.3
Cr
0.8
Ti
0.2
O
3
was applied as the SOFC anode [49].
8.6 A(B,B
0
)O
3
Perovskites
As perovskites with one cation occu pying the B site have not generally yielded
good enough properties for efficient anode operation in SOFCs, an extensive
series of studies has considered the possibility of enhancing performance by
using two different B-site ions, both with concentration in excess of percolation
limit (i.e., >30%). The objective is to obtain complementary functionality from
appropriate cation combinations, hopefully without seriously degrading the
good properties induced by the individual ions. Not surprisingly, many of the
tested combinations did compromise properties, but in some important
instances good complementary functionality has been achieved.
8 Perovskite Oxide Anodes for SOFCs 177
Early studies focused on double perovskites with niobium and a first-row
transition metal or main group ion occupying the B site. With Nb and Mn
occupying the B site, electronic conductivity is fairly low, probably reflecting
the rock salt-type ordering of the B cations [51]. Using Cu and Nb somewhat
improves conductivity in air, but in reducing conditions copper metal is
exolved and conductivity is impaired, as the resultant perovskite is more
resistive and the copper does not form a conducting network [52]. Using Ga
with Nb again results in an ordered superstucture that impairs electronic
conductivity [53].
Improved performance has been obtained with complex perovskites based
upon Cr and Mn at the B sites forming compositions (La,Sr)Cr
1–x
M
x
O
3–d
[54].
Previous workers have focused upon doped lanthanum chromite, where doping
is used in the solid-state chemical sense of up to 20% dopant on the B site,
usually 5% or 10%. (La
0.75
Sr
0.25
)Cr
0.5
Mn
0.5
O
3
(LSCM) exhibits comparable
electrochemical performance to Ni–YSZ cermets. The electrode polarization
resistance approaches 0.2 O cm
2
at 9008C in 97% H
2
/3% H
2
O. Very good
performance is achieved for methane oxidation without using excess steam . The
anode is stable in both fuel and air conditions and shows stable electrode
performance in metha ne. Thus, both redox stability and operation in low-
steam hydrocarbons have been demonstrated, overcoming two of the major
limitations of the current generat ion of nickel zirconia cermet SOFC anodes.
Catalytic studies of LSCM demonstrate that it is primarily a direct oxidation
catalyst for metha ne oxidation as opposed to a reforming catalyst [55], with the
redox chemistry involving the Mn–O–Mn bonds [56]. Although oxygen ion
mobility is low in the oxidized state, the diffusion coefficient for oxide ions in
reduced LSCM is comparable to yttria-stabilized zirconia [57].
Another important double perovskite is Sr
2
MgMoO
6–d
, which has recently
been shown to offer good performance, with power densities of 0.84 W/cm
2
in
H
2
and 0.44 W/cm
2
in CH
4
at 8008C, and good sulfur tolerance [58]. The
molybdenum-containing double perovskite was initially prepared at 12008C
in flowing 5% H
2
and then deposited on top of a lanthanum ceria buffer layer
before testing [59].
8.7 Tungsten Bronze Anode Materials
Tungsten bronze-type materials have also been investigated as potential SOFC
anodes. The tungst en bronze structure can be obtained from the perovskite by
rotation of some of Ti/NbO
6
octahedra: 40% of the A sites (A2 sites) are
increased in size from tetracapped square prisms to pentacapped pentagonal
prisms, 40% remai n essentially unchanged, and the remaining 40% of the sites
is de creased in size (Fig. 8.8). The formula may be written as A
0.6
BO
3
when the
small-size A sites are left empty. The distortion of the octahedra means that
some B–O bonds are extended and some are shorter than the average. The
178 J.T.S. Irvine
