Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

298 Luo et al.
2. Spin at 14,000g for 15 s, then remove supernatant to waste.
3. Repeat (1) and (2) twice.
4. Wash 2: Add 1000 µL stringent wash buffer B to each pellet and rotate at RT for 30 min.
5. Spin at 14,000g for 15 s, then remove supernatant to waste.
6. Wash 3: Add 250 µL of stringent wash buffer C to each pellet and rotate at RT for 15 min.
7. Spin at 14,000g for 15 s, then remove supernatant to waste.
8. Carefully and completely remove all liquid from step 7.
9. Elution: Add 50 µL of elution buffer to each pellet and rotate at RT for 1 h.
10. Spin at 14,000g for 15 s.
11. Collect 50 µL of supernatant into a new tube and take 2 µL for radioactive β-monitoring.
The eluate is ready for electrophoresis by SDS-PAGE or other process.
12. Store samples at –20°C.
3.4. Identification of Target Proteoglycans
3.4.1. SDS-PAGE
1. Transfer 20 µL of medium eluate (or 10 µL lysis eluate) to a 1.5-mL boiling tube.
2. Add 5 µL 5× sample loading buffer (non-DTT for nondenatured condition or DTT-con-
taining for disulfide cleavage condition).
3. Boil tube at 100°C in a water bath for 5 min.
4. Cool tube using running water (not ice water).
5. Spin at 14,000g for 10 s.
6. Load sample into an individual well of the SDS gel.
7. Run the gel at constant 38 mA (set voltage to highest, 500 V) for about 90 min. Monitor
the gel to avoid sample overrun.
3.4.2. Processing of SDS-PAGE and Radiography
8. Transfer gel to 300 mL of fixing solution in a glass container.
9. Place the container onto a shaker and shake at 62.5 rpm for 30 min.
10. Drain solution and replace with a fresh 300 mL of fixing solution and shake at 62.5 rpm
for 30 min.
11. Transfer gel to a square Petri dish.
12. Add 15 mL of Entensify Enhancer Part A to dish.
13. Shake on shaker at 125 rpm for 45 min.
14. Add 15 mL of Entensify Enhancer Part B
15. Shake on shaker at 125 rpm for 45 min.
16. Dry gel in a Gel Dryer at 60°C for 120 min, until completely dry.
17. Place the dried gel on a radiogram cassette and expose an X-ray film to the gel in a
dark room.
18. Place the cassette in a –80°C freezer for hours to days (depending on the intensity of
radioactivity) (see Note 4).
19. Develop the film in an X-OMAT (Kodak) machine. Alternatively, bands in the gel can be
quantified on an Instant Imager (Packard). The methionine-labeled (Pro-Mix) proteins
are shown in Fig 2.
3.5. Characterization of Expressed Proteoglycans
1. Transfer 30 µL of eluate (from Subheading 3.3.3., step 11) to a 1.5-mL boiling Eppendorf
tube (For a 60-µL digest reaction).

Expression and Characterization of Engineered PGs 299
2. Add 3 µL of 20× Chondroitinase ABC digest buffer, 5 µL of 10% Triton X-100, 5 µL of
0.1 M NEM, and 2 µl of H
2
O.
3. Add 15 µL of Chondroitinase ABC solution (0.3 Units of Chondroitinase ABC).
4. Mix well, incubate at 37°C for 120 min.
5. Run samples in a SDS-PAGE gel, comparing to nondigested eluate (same as Subheading
3.4.). The Chondroitinase ABC-digested samples (sulfate labeled) are shown in Fig 3.
3.6. Detection of Proteoglycan Interaction
with Heat-Shock Proteins Part I
1. (Steps 1–7 at 4°C) Combine spent medium (or cell lysate) with protein A beads and
Hsp25 antibody, rotating for 5 h.
2. Spin at 14,000g for 15 s, then transfer supernatant to waste.
3. Add 1000 µL of washing buffer D to each tube and rotate for 60 min.
4. Spin at 14,000g for 15 s, then transfer supernatant to waste.
5. Repeat steps 3 and 4 twice.
Fig. 2. Methionine labeling experiment. (A) Spent medium: autoradiography of empty
vector (control, lane 1) and aggrecan G3 construct (lane 2) from wild-type CHO cells (1). Note
that the diffuse band in lane 2 shows a typical proteoglycan pattern (bracket). (B) Cell lysates:
autoradiography of empty vector (lane 1) and aggrecan G1 construct (lane 2) from wild-type
CHO cells (1). Note the intracellular G1 core protein (arrow, lane 2).
Fig. 3. Sulfate labeling experiment and GAG chain characterization. (A) Autoradiogra-
phy of sulfate-labeled products of empty vector (lane 1) and aggrecan G3 construct (lane 2)
from wild-type CHO cells (1). Note that only chondroitin/heparan sulfate GAGs are labeled
(not the core protein). (B) Autoradiography of sulfate-labeled products of aggrecan G3 con-
struct in Chondroitinase buffer (lane 1) and G3 digested by Chondroitinase ABC (lane 2). Note
that the enzyme digestion almost completely eliminates the diffuse band.
300 Luo et al.
6. Spin at 14,000g for 15 s.
7. Carefully and completely remove all liquid from step 6.
8. (From here on, at RT) Add appropriate amount (e.g., 30 µL) of 1× loading buffer with or
without DTT.
9. Boil tube at 100°C in a water bath for 5 min.
10. Cool tube using running water.
11. Spin at 14,000g for 10 s.
12. Load supernatant onto SDS-PAGE.
13. Run the gel and process the gel as under Subheading 3.4.
3.7. Detection of Proteoglycan Interaction
with Heat-Shock Proteins Part II
1. Transfect and label cells as under Subheading 2. (before sample collection).
2. (Steps 2–5 in cold room) Wash labeled cells (live on dish) twice with 5 mL of washing
buffer E.
3. Lyse cells with 1 mL of digitonin lysis buffer (containing DTSSP), then scrape cells
off dish.
4. Transfer lysate to a 1.5-mL Eppendorf tube and incubate on ice for 30 min.
5. Add 200 µL of 10 mM glycine to sample (to deactivate excess cross-linker), then incu-
bate on ice for 10 min.
6. Ni chromatography as under Subheading 3.3.
7. Electrophorese samples with dithiothreitol loading buffer (to release cross-linked pro-
teins) as under Subheading 3.4.
4. Notes
1. All ingredients are sterilized, molecular biology grade.
2. A PCR hot-start program typically contains one denature cycle (94°C for 4 min, 72°C for
2 min and 55°C for 1 min) and 30 cycles of amplification (94°C for 45 s, 55°C for 45 s
and 72°C for 3 min). Annealing temperature and elongation temperature need to be
adjusted for different oligo lengths and templates. See a PCR publication for details.
3. Low speed spin for 15 s can be used to collect all liquid in the tube after inversion.
4. Stringent washes are necessary to eliminate background proteins and for clear visibility of
the candidate proteins. Stringent washing buffer (wash 1) can be increased with higher
molar concentrations of urea (utmost 8 M). If more stringency is needed, radioimmune
precipitation (RIPA) components can be added to the buffer and subsequent washes. A
repeat wash 3 is recommended if RIPA is added to wash 2 and wash 3, since deoxy cholate
will allow different charges on the proteins, converting in one regular band into two bands
on a gel (thereby making it hard to judge whether the correct proteins are expressed).
5. Film exposure time has to be adjusted (from 1 h to several days) so that the appropriate
exposure is obtained. The criterion is that you can see the desired bands clear enough. Or
you can use an imager to obtain the appropriate exposure through computer imaging.
References
1. Luo, W., Kuwada, T. S., Chandrasekaran, L., Zheng, J., and Tanzer, M. L. (1996) Divergent
secretory behavior of the opposite ends of aggrecan. J. Biol. Chem. 271, 16,447–16,450.
2. Zheng, J., Luo, W., and Tanzer, M. L. (1998) Aggrecan synthesis and secretion: a para-
digm for molecular and cellular coordination of multiglobular protein folding and intrac-
ellular trafficking. J. Biol. Chem. 273, 12,999–13,006.

Intracellular Localization of Engineered PGs 301
301
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
28
Intracellular Localization of Engineered Proteoglycans
Tung-Ling L. Chen, Peiyin Wang, Seung S. Gwon, Nina W. Flay,
and Barbara M. Vertel
1. Introduction
Proteoglycans undergo numerous synthetic and processing events as they progress
through the exocytic pathway. In cells that express genetically engineered constructs
encoding proteoglycans, immunolocalization is a useful approach in identifying
specific intracellular compartments involved in their processing and trafficking.
2. Materials
2.1. Expression of Target Proteoglycan (Cell Culture and Transfection)
1. Proteoglycan constructs packaged into vectors suitable for expression in target cells (1,2).
In our experiments, pcDNA3 and its derivatives (Invitrogen) were used. FLAG and 6xHis
epitope tags were engineered at the C-terminus.
2. Mammalian cell lines: Chinese hamster ovary (CHO) cells, COS-1 cells, and HeLa cells
were used.
3. Culture medium: Ham’s F12 medium (for CHO, Life Technologies) or Dulbecco’s Modi-
fied Eagle’s Medim High Glucose Pyruvate (HG-DMEM, for COS-1 and HeLa, Irvine
Scientific) with 10% fetal bovine serum (Atlanta Biologicals) and 1% antibiotic-antimycotic
solution (Life Technologies).
4. Trypsin-EDTA solution (Sigma, cell culture grade).
5. Tissue culture dishes.
6. Sterilized cover slips: No. 1 cover slips were prepared by soaking in 95% ethanol for at least
10 min, followed by rinsing with sterile distilled water.
7. SuperFect transfection reagent (Qiagen).
8. Opti-MEM (Life Technologies).
9. Phosphate buffered saline (PBS).
2.2. Immunofluorescence Localization of Expressed Proteoglycan
1. Fixatives: 100% methanol, stored at –20°C or 2–4 % paraformaldehyde in PBS.
2. Nonidet P40 (NP-40), 0.1% in PBS.
302 Chen et al.
3. Normal goat serum (NGS).
4. Primary antibody: M2 anti-FLAG antibody (Eastman Kodak), Penta-His or Tetra-His
antibody (Qiagen).
5. Secondary antibody: FITC-conjugated goat IgG anti-mouse IgG (Jackson Labs).
6. PBS.
7. Mounting medium (3): 15% Vinol 205 polyvinyl alcohol (w/v, see Note 1), 33% glycerol
(v/v), 0.1% azide, pH 8.5. Dissolve 20 g of Vinol 205 polyvinyl alcohol in 80 mL of 0.1
M Tris, 0.1 M NaCl, pH 8.5, for 16 h with stirring. Add 40 mL of glycerol and 1.2 mL of
10% Na azide with continued stirring for another 16 h. Pellet undissolved particles at
20,000g for 20 min. Aliquot the viscous supernatant and store at –20°C. Use a defrosted
aliquot stored at 4°C as your working solution.
2.3. Ultrastructural Localization of Expressed Proteoglycan
1. Paraformaldehyde-lysine-periodate fixative (4):
a. Solution A: 0.1 M lysine–0.05 M phosphate buffer, final pH = 7.4. Dissolve 1.827 g
of lysine HCl in 50 mL of distilled water. Adjust to pH 7.4 with 0.1 M Na
2
HPO
4
.
Bring the final volume to 100 mL with 0.1 M phosphate buffer, pH 7.4. Osmolarity
should be approx 300 mo. Store at 4°C.
b. Solution B: 20% paraformaldehyde. Mix 10 g of paraformaldehyde in 50 mL of distilled
water, heating in a 60°C water bath with stirring. Slowly add 1–3 drops of 1 N NaOH
until the solution clears. Store at –20°C. Spin or filter to remove debris before use.
To make 10 mL of fixative, mix 7.5 mL of solution A with 1 mL of solution B and 1.5 mL
of distilled water. Add 21.4 mg of NaIO
4
. Final composition is 0.01 M NaIO
4
, 0.075 M
lysine, 0.0375 M phosphate buffer, 2% paraformaldehyde. Upon mixing solutions A and
B, the pH will decrease from 7.4 to approx 6.2. The fixative is used at this lower pH and
needs to be made fresh directly before use.
2. Wash and permeabilization solution: 0.05% saponin in PBS.
3. Blocking solution: 10% NGS/0.06% glycine/0.05% saponin/PBS.
4. Primary antibody: M2 anti-FLAG, diluted 1/100 in PBS with 5% NGS and 0.05%
saponin.
5. Rabbit IgG anti-mouse IgG, diluted 1/25 in PBS with 5% NGS and 0.05% saponin.
6. Peroxidase-conjugated goat IgG anti-rabbit IgG Fab fragments (Jackson Labs), diluted
1/50 in PBS with 5% NGS and 0.05% saponin.
7. Diaminobenzidine (DAB): Tare a 15-mL polystyrene tube, weigh out DAB, and dissolve
in DAB buffer to generate a 0.2% solution—e.g., weigh out 10 mg of DAB and dissolve
in 5 mL of DAB buffer. (see Note 2.)
8. DAB buffer: 0.05 M Tris-HCl, pH 7.4.
9. Hydrogen peroxide (30% solution, Sigma).
10. Sucrose-containing cacodylate buffer: Mix 0.2 M Na cacodylate, pH 7.4, and 60% sucrose
to make 0.1 M Na cacodylate containing 6% sucrose.
11. Glutaraldehyde (grade I, 25% aqueous solution, Sigma). (see Note 2.)
12. Osmium tetroxide (2% aqueous solution, Electron Microscopy Sciences) (see Note 2).
13. Potassium ferrocyanide [K
4
Fe(CN)
6
. 3H
2
O, Sigma]. (see Note 2.)
14. Ethanol.
15. Hydroxypropyl methacrylate (HPMA, Electron Microscopy Sciences).
16. Epon with catalyst:
a. tEpon 812 (Tousimis Research Corp.).
b. Nadic methyl anhydride (NMA, Tousimis Research Corp.).
Intracellular Localization of Engineered PGs 303
c. Dodecenylsuccinic anhydride (DDSA, Tousimis Research Corp.).
d. Tri-dimethylamino methyl phenol (DMP-30, Tousimis Research Corp.).
To make 50 mL of mixture, pour 21 mL of NMA into a 100 mL plastic beaker with a volume
marker, add 3 mL of DDSA using a syringe, then add tEpon 812 to the 50-mL mark. Stir
with a wood stick for 15 min, taking caution to avoid bubbles. Add 0.75 mL of DMP-30
with a syringe, and stir well for another 10 min. Unused portions may be stored in syringes
at –20°C for later use. (see Note 2.)
17. Lead citrate: The lead citrate solution is prepared and used according to Reynolds (5). All
glassware must be washed with 3% HCl and thoroughly rinsed with double-distilled (dd)
water in advance. Fill a 50-mL volumetric flask with 30 mL of dd water. Add 1.33 g of
Pb(NO
3
)
2
and 1.76 g of trisodium citrate. Shake continuously for 1 min. The contents will
appear milky. Let stand for an additional 30 min with intermittent shaking. This is necessary
for the complete conversion of reagents into lead citrate. Adjust to pH 12 by the slow
addition (with inversion of the flask) of 1.0 N NaOH. The proper pH is reached just at the
point when the solution clears (after the addition of about 8 mL of 1.0 N NaOH). Adjust to
the final volume of 50 mL using dd water. Filter with Whatman’s #2 filter paper. Store the
solution at room temperature in a tightly capped amber glass bottle. The solution should not
be jarred; allowing it to stand undisturbed enables small particles to settle to the bottom
and improves the quality of the stain.
3. Methods
3.1. Expression of Target Proteoglycan (Cell Culture and Transfection)
1. Seed 1.1 × 10
6
CHO cells onto a 100-mm culture dish containing sterilized cover slips
36–40 h before transfection (see Notes 3 and 4). For ultrastructural studies, seed 1 × 10
5
cells onto a 35-mm culture dish.
2. Incubate in a 37°C incubator with 5% CO
2
. For CHO cells, the dishes should be 80%
confluent on the day of transfection.
3. In a polystyrene tube, dilute 5 µg of DNA into 150 µL of Opti-MEM (contains no serum or
antibiotics). Mix solution.
4. Add 10 µL of SuperFect transfection reagent to the DNA solution (see Note 5). Mix
solution.
5. Incubate the samples for 5–10 min at room temperature (20–25°C) to allow complex
formation.
6. While complex formation takes place, set up 35-mm dishes with 1 mL of Opti-MEM and
transfer one cover slip with attached cells to each dish.
7. Add 1 mL of complete medium containing serum and antibiotics to the reaction tube contain-
ing the transfection complexes. Mix by pipetting up and down twice, and immediately
transfer the total volume to the cover slips in the 35-mm dishes.
8. Incubate the cells with the complexes for 2 h at 37°C in an atmosphere of 5% CO
2
.
9. Remove medium containing the remaining complexes from the cells. Wash cells once
with PBS.
10. Add new complete medium. Incubate cells for additional hours as each experiment requires
(usually 24–48 h).
3.2. Immunofluorescence Localization of Expressed Proteoglycan
1. Prior to fixation, wash cells with PBS 2 times.
2. Fix with cold methanol for at least 20 min, or with room-temperature paraformaldehyde
for 15 min.
304 Chen et al.
3. Permeabilize the paraformaldehyde-fixed cells with 0.1% NP-40 for 15 min. (Skip this
step if the cells were fixed with methanol.)
4. Rinse with PBS.
5. Block the fixed samples with 15% NGS at 37°C for 15 min.
6. After removing NGS, add primary antibody to the sample (anti-FLAG was used at 0.04 mg/mL,
Tetra-and Penta-His were used at 0.01 mg/mL) and incubate for 2 h at 37°C.
7. Wash with PBS for 1 h with 5–6 changes.
8. Incubate with FITC-conjugated goat IgG anti-mouse IgG (0.2 mg/mL) for 1 h at 37°C.
9. Wash with PBS for 1 h with 5–6 changes.
10. If double-fluorescence localization is desired, repeat steps 4–7 using selected rabbit
polyclonal antibodies and Texas Red-conjugated goat IgG anti-rabbit IgG (see Note 6).
11. Mount samples on microscope slides with mounting medium.
12. Observe samples with a microscope equipped with phase-contrast (or differential inter-
ference-contrast) and incident-light fluorescence optics (see Notes 7 and 8). Document
with conventional photographic camera or capture images using digital camera (see
Figs. 1 and 2).
3.3. Ultrastructural Localization of Expressed Proteoglycan
1. Wash cells with PBS 2 times.
2. Fix with paraformaldehyde-lysine-periodate fixative for 45 min at room temperature (see
Notes 9 and 10).
3. Wash with PBS 2 times.
4. Incubate 15 min with 0.05% saponin/PBS to permeabilize cells.
5. After most of the wash solution is removed, use Kimwipes to dry the edge of culture dish.
This step generates surface tension around the central area of the dish so that as little as
50 µL of blocking reagents (and later, antibody solutions) is required for covering the
area during incubation. Incubate in blocking reagent for 20 min.
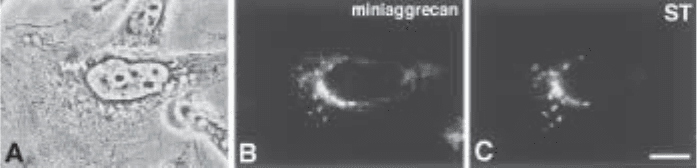
Fig. 1. Immunofluorescence localization of expressed “miniaggrecan” (containing the
chicken aggrecan N-terminus with the signal sequence and G1 domain, a segment of the chon-
droitin sulfate attachment region, the G3 domain, and a 6xhis epitope tag) and the Golgi en-
zyme, ST. Transfected CHO cells exhibit miniaggrecan localization within the perinuclear
Golgi complex (B). The region is further identified as the Golgi complex by the co-transfection
and localization of the ST Golgi enzyme (C). The corresponding phase micrograph shown in
(A) contains an expressing cell that exhibits positive immunofluorescence localized to the Golgi
subcellular compartment, and nonexpressing cells that serve as useful negative controls.
Miniaggrecan was detected using the mouse monoclonal Tetra-His Antibody and FITC-goat
IgG anti-mouse IgG. ST was localized using polyclonal anti-ST antibodies and Texas Red-goat
IgG anti-rabbit IgG. The calibration bars of Figs. 1 and 2 represent 10 µm.
Intracellular Localization of Engineered PGs 305
6. Wash with 0.05% saponin/PBS 2 times before adding primary antibody.
7. Run Kimwipes around edge of culture dish to prepare the surface for antibody addition
and incubate in primary antibody for 1.5 h at 37°C.
8. Wash 6–7 times over 45 min with 0.05% saponin/PBS.
9. Run Kimwipes around edge of culture dish to prepare the surface for antibody addition and
incubate in rabbit IgG anti-mouse IgG for 1.5 h at 37°C.
10. Wash 6–7 times over 45 min with 0.05% saponin/PBS.
11. Run Kimwipes around edge of culture dish to prepare the surface for antibody addition and
incubate in peroxidase-conjugated goat anti-rabbit FAB for 1.5 h at 37°C.
12. Wash 6–7 times over 45 min with 0.05% saponin/PBS.
13. Wash 3 times over 5 min in DAB buffer.
14. During wash, prepare DAB solution.
15. Add 1.3 mL of DAB solution per dish and incubate 10 min on a shaker.
16. Add 1.5 µL of hydrogen peroxide for each dish, cover the dish, and incubate for an addi-
tional 5–10 min. Monitor color change using an inverted microscope and when reaction is
sufficient, stop by removing DAB solution (see Note 11).
17. Wash 3 times over 10 min with DAB buffer.
18. Wash for 10 min with 1/1 mixture of DAB buffer and sucrose-containing cacodylate buffer.
19. Wash with sucrose-containing cacodylate buffer 2 times, 10 min each.
20. Fix with 2% glutaraldehyde in 0.1 M cacodylate buffer containing 6% sucrose for 30 min
at room temperature.
21. Wash with sucrose-containing cacodylate buffer 2 times, 10 min each.
22. Fix with 1% OsO
4
/1.5% KFe
4
(CN)
6
in 0.1 M cacodylate buffer containing 6% sucrose for
45 min in the dark and cold.
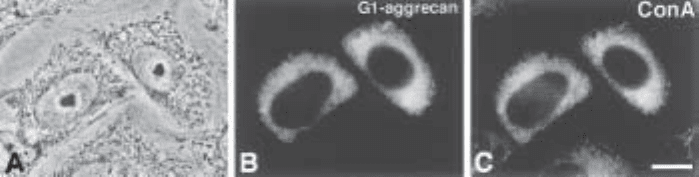
Fig. 2. Colocalization of G1-aggrecan (containing the chicken aggrecan N-terminus with
the signal sequence and G1 domain, a segment of the chondroitin sulfate attachment region,
and a 6xhis epitope tag) and concanavalin A. CHO cells transfected with G1-aggrecan exhibit
protein localized principally in regions throughout the cytoplasm (B). Identification of these
cytoplasmic regions as the ER is established by the colocalization of FITC-concanavalin A, a
lectin that recognizes mannose-rich oligosaccharides in the ER (C). Two expressing cells and
segments of several nonexpressing cells are revealed in the corresponding phase micrograph
(A) and by the concanavalin A localization (C). The nonexpressing cells serve as negative
controls for G1-aggrecan localization, which was accomplished using the mouse monoclonal
Penta-His Antibody and Texas Red-goat IgG anti-mouse IgG. Reproduced with permission
from Wang, P. W., Chen, T.-L., Luo, W., Zheng, J., Qian, R., Tanzer, M. L., Colley, K., and
Vertel, B. M. (1999) Immunolocalization of 6xHis-tagged proteins in CHO cells with QIA
express Anit-His Antibodies. Qiagen News 1, 3–6. (6). Used with permission.
306 Chen et al.
23. Wash with sucrose-containing cacodylate buffer 4 times, 15 min each.
24. Fix with 1% tannic acid in 0.1 M cacodylate buffer containing 6% sucrose for 15 min.
25. Wash with sucrose-containing cacodylate buffer 2 times, 10 min each.
26. Wash with water 3 times over 10 min.
27. Dehydrate through 50% ethanol, 10 min; 75% ethanol, 10 min; 90% ethanol, 10 min; 90%
HPMA, 3 times over 15 min; 95% HPMA, 15 min; 97% HPMA, 15 min (see Note 12).
28. Exchange through a series of HPMA/tEpon solutions: 2/1 HPMA/tEpon, 15 min; 1/1
HPMA/tEpon, 30 min; 1/2 HPMA/tEpon, 30 min. Add HPMA/tEpon mixture to dish,
cover, and mix continuously.
29. Exchange into Epon with catalyst over 30 min with 3 changes.
30. Drain off excess Epon with catalyst to leave just enough to cover the cell layer.
31. Infiltrate overnight at 37°C in oven with dessicant. Be sure dishes in the oven are flat, and
cover them with a single layer of foil into which small holes have been poked to allow
residual dehydrating agents to evaporate off.
32. Transfer dishes to 60°C oven for polymerization, keeping the dishes flat to maintain a uni-
form depth of Epon polymer on the cells. Two days are required for complete polymerization.
33. Upon completion of polymerization, break the culture dish to recover the embedded cells
in a thin layer of polymerized Epon (see Note 13). Cut out a small piece (<1 mm each
side) to be mounted (using epoxy or Krazy Glue) on a block suitable for sectioning. A
light microscope can be used to help in selecting optimal areas for mounting.
34. Ultrathin sections are collected onto electron microscope grids. Cells may be counter-
stained briefly with lead citrate before viewing under the electron microscope. For lead
citrate counterstaining, prepare a Petri dish chamber with parafilm on the bottom. Add
moist NaOH pellets to the chamber in order to sequester CO
2
and prevent the formation
of lead carbonate precipitate. For counterstaining, drop the lead citrate solution from a
syringe with a filter onto the parafilm. Float each grid, sample side down, onto separate
drops. Cover the chamber with aluminum foil to protect against light and CO
2
contamina-
tion. Remove excess stain from the grids by dipping them through a series of double-
distilled water rinses and allow the grids to dry before viewing under the electron
microscope.
4. Notes
1. Vinol 205 polyvinyl alcohol (also called Airvol 205) is available as a sample on request
from Air Products and Chemicals, Inc. (Allentown, PA). Defrosted working solutions are
stable for 6 mo at 4°C.
2. Toxic compounds used in this procedures include DAB, cacodylate, osmium tetroxide,
glutaraldehyde, and the dehydration solutions and nonpolymerized embedding materials.
Handle the solutions in the hood with gloved hands. Dispose as chemical waste. DAB can
be detoxified by treatment with chlorox.
3. The initial cell density required to seed the cover slips varies among cell types used for trans-
fection. The major factors to be considered are cell growth rate, the time required for cells to
attach to the cover slips, and the survival rate after transfection. For example, CHO cells can
grow directly on the glass surface of cover slips, but require 36–40 h to attach and spread well,
while COS and HeLa cells attach and spread better on gelatinized carbon-coated cover slips,
and do so by 24 h. The COS and HeLa cells are less sensitive to SuperFect transfection reagent,
and so 70% confluence is the optimal density at the time of transfection. Cell density adjust-
ments may need to be made to allow for differences in the toxicity of SuperFect lots.
4. Gelatinized carbon-coated coverslips can be used for cells that do not attach well to
uncoated glass surfaces. A carbon coat is applied to glass cover slips using carbon rods
Intracellular Localization of Engineered PGs 307
(Ted Pella) in a vacuum evaporator (Denton, DV502) run under standard conditions. Car-
bon-coated cover slips are stored in 95% ethanol and treated with 1% gelatin before use.
5. The optimal volume (µL) of SuperFect reagent and the ratio of SuperFect volume to the
quantity of DNA (µg) may vary depending on the specific cell type and DNA construct.
6. Compartment-specific antibodies and fluorophore-conjugated lectins can be used to
help identify intracellular compartments involved in trafficking of proteoglycans. For
example, concanavalin A, a lectin that reacts with mannose-rich oligosaccharides
added co-translationally to glycoproteins in the endoplasmic reticulum (ER), can be
used as a marker for the ER. Co-transfection with a construct that encodes the Golgi
enzyme sialyltransferase (ST) has been used in our experiments to identify the Golgi
complex (6). In this case, expressed ST was localized with polyclonal antibodies
against ST. Alternatively, antibodies specific for Golgi complex proteins, such as
TGN38, may be used to identify the trans-Golgi network.
7. When observing immunostained cells under the fluorescence microscope, it is important
to distinguish real signals from nonspecific background. An overall high level of cellular
fluorescence usually suggests a background problem. In transfection experiments, the
nontransfected (therefore, nonexpressing) cells serve as a convenient internal negative
control for immunostaining (see Figs. 1 and 2).
8. The background problem in immunostaining reactions can usually be reduced by diluting
the primary and/or secondary antibody concentrations and by modifying incubation times.
9. If the final concentration of paraformaldehyde used in the fixative is increased to improve
ultrastructure, the effect on antigenicity must also be determined.
10. The protocol for preembedment immunoperoxidase localization is a modification (7) of
the method described by Brown and Farquhar (8).
11. DAB color reaction should be stopped when the reaction becomes saturated and starts to
extend into peripheral structures.
12. The protocol for embedding monolayer cell cultures is according to Brinkley et al. (9).
13. For ultrastructural localization studies, the monolayer of cells is best cultured in dishes
with thin walls because these plastic dishes can be pried off easily to release the polymer-
ized Epon-containing embedded cells.
Acknowledgements
Research support was provided by National Institutes of Health grants DK28433,
AR45909, and grants from the Arthritis Foundation. We are grateful to Drs. Marvin
Tanzer and Wei Luo (Univ. Connecticut Health Center) for providing aggrecan
constructs and Dr. Karen Colley (Univ. Illinois College of Medicine, Chicago) for
providing antibodies and constructs for sialyltransferase.
References
1. Zheng, J., Luo, W. and Tanzer, M. L. (1998). Aggrecan synthesis and secretion. A paradigm
for molecular and cellular coordination of multiglobular protein folding and intracellular
trafficking. J. Biol. Chem. 273, 12,999–13,006.
2. Luo, W., Kuwada, T. S., Chandrasekaran, L., Zheng, J., and Tanzer, M. L. (1996).
Divergent secretory behavior of the opposite ends of aggrecan. J. Biol. Chem. 271,
16447–16450.
3. Dahdal, R. Y. and Colley, K. L. (1993) Specific sequences in the signal anchor of the
galactoside-2,6-sialyltransferase are not essential for Golgi localization. J. Biol. Chem.
268, 26,310–26,319.
