Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

when S. cerevisiae is injected into laboratory mammals. Chitin is a /3(l-4-)-linked
polymer of jV-acetylglucosamine. It confers enormous mechanical strength. In S.
cerevisiae a ring of chitin is formed at the mother-bud junction (see Fig. 2.2). This ring
persists after cell separation and is referred to as the 'bud scar'. Chitin is readily stained
with the optical brightener Calcofluor White, and all of the bud scars on a cell can
easily be visualized. Thus, it is possible to determine both the 'age' of a cell (by counting
the number of bud scars) and the ploidy of the cell (by observing the pattern of bud
scars, because, as explained earlier, haploids and diploids have different patterns of
bud formation). Indeed, ageing research is also possible in this organism (Kennedy &
Guarente 1996). In spore walls, the outermost layers contain a special polymer which
is based upon dityrosine (Briza et al. 1990).
Candida albicans
Pharmaceutical and clinical significance
Candida albicans is a dimorphic organism which is part of the normal body flora of
humans. For the majority of normal healthy individuals it will never cause any problems.
However, in a number of settings it can cause severe disruption to lifestyle or even
death. In the USA it is now the third most frequent cause of nosocomial (hospital
acquired) infections. Post-operative infection arises typically where a patient has been
in intensive care for weeks and has undergone several cycles of bacterial infections
and high dose antibacterial therapy. In excess of 50% of deep-seated Candida infections
are lethal. Persons suffering from AIDS, transplant patients and other immuno-
compromised individuals are at even greater risk. The azole family of antifungal
compounds (see Chapter 5) is frequently deployed but several of these block the
metabolism of cyclosporins (which are administered for chronic immunosuppressive
therapy) and thereby increase immunosupressivity. A possible alternative antifungal
drug is amphotericin B, but this interacts with cyclosporins to give increased nephro-
toxicity. Catheterized patients can become infected with C. parapsilosis which causes
problems by virtue of its ability to form biofilm. In many apparently normal women,
C. albicans causes vaginal thrush which can be so extreme as to incapacitate. Some
denture-wearers and malnourished children can develop thrush in the mouth; in the
case of the latter this can extend to large portions of the face.
It would be reasonable to imagine that the cell wall would be a focus for attacking
this organism. In reality, whilst there have been studies of the biosynthesis of cell wall
materials, the precise molecular organization within the cell walls is largely unknown.
jS-glucans and chitin form a skeleton for the mannoproteins which are found both at the
outer surface and throughout the entire cell wall. By using wheat germ agglutinin it has
been shown that chitin is concentrated in the cross-walls between mother and daughter
cells, but is also distributed throughout the whole of the cell wall. It has not been
possible to examine the distribution of glucans using plant lectins because there is no
known lectin which reacts specifically with glucans. However, the use of a monoclonal
antibody that reacts with (l,6)-/5-glucan has enabled the linkages which connect (1,6)-
/3-glucan to mannoproteins and the distribution of (l,6)-/?-glucan in the cell walls to be
studied (Sanjuan et al. 1995). In S. cerevisiae, the synthesis of (l,6)-/3-glucan begins
early in the secretory pathway whereas in C. albicans it is apparently incorporated at a
later stage. In both yeasts, (l,6)-/3-glucan is located within an inner layer of the cell
wall which can be rendered accessible with tunicamycin.
In the yeast form, C. albicans could be confused with S. cerevisiae upon simple
microscopic inspection. However, major differences exist which would soon become
apparent even to one not familiar with yeast taxonomy. Candida albicans is diploid
and has no sexual cycle. This means that classical genetic methods like those described
for S. cerevisiae are not possible with this organism. Attempts to isolate mutants by the
use of mutagenic agents are almost bound to fail because of the improbability of
producing mutations in both copies of a given gene and nowhere else in the genome.
Naturally occurring mutants do, of course, exist. The more recent developments of
molecular genetics are now being applied to Candida, but the reader should not conclude
that this organism is understood to anything like the extent of S. cerevisiae. The isolation
of C. albicans genes homologous to those in S. cerevisiae by means of complementation
of S. cerevisiae mutants has been particularly useful as have techniques such as 'Ura
blasting' in which first one copy of a given Candida gene is disrupted with the coding
sequence of the URA3 gene and then the second copy is treated likewise. This process
can be applied sequentially to produce a strain with defined mutations in known genes
(Gow et al. 1993).
3.2 Alternative morphologies
One spectacular difference between S. cerevisiae and C. albicans is the ability of the
latter to switch to a hyphal pattern of proliferation (Gow 1994). A variety of different
factors and conditions have been described which can elicit this switch. These include
serum, neutral pH, certain temperature profiles, the addition of yV-acetylglucosamine
and many more (Odds 1988). In germ tube formation, a protuberance develops from
the cell and thereafter growth remains highly polarized (Fig. 2.7). The cell which
forms the tip of the developing germ tube remains polarized throughout the cell cycle.
It must be emphasized that this is hyphal growth where cell division is symmetric,
but in contrast to pseudohyphal development (where the next cell cycle is started
synchronously), in this case growth is asynchronous with the result that the apical cell
becomes progressively longer (see Fig. 2.5). It has not been shown that the virulence or
pathogenicity of C. albicans are uniquely due to either the yeast or hyphal form.
Nonetheless, the ability to be able to interconvert between the distinct morphologies
must surely be to its advantage. The hyphal form is considered to be specialized for
foraging (Kron & Gow 1995). Even if this is not the correct conclusion, it certainly
conveys the ability to penetrate tissue, whilst the yeast form would seem to be more
effective for dispersal, e.g. through the blood system. One of the justifications for
studying morphological switching in C. albicans is that this may reveal a unique target
for therapy or prophylaxis.
Another form of switching is well-known in C. albicans. This is the phenomenon
of 'phenotypic switching' (Soil 1992) whereby colony morphologies vary dramatically
(e.g. white, opaque, fuzzy, wrinkled.) These may seem trivial to the pharmacist or
physician, but the variability extends far beyond the mere appearance of the colonies.
It can encompass a vast array of biochemical alterations, antigenicities and drug
Yeasts and moulds 45
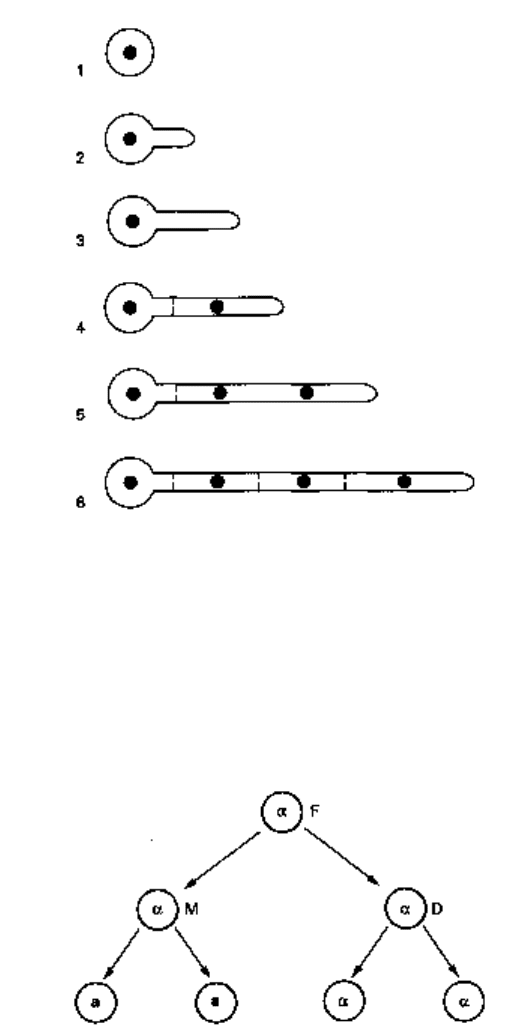
Fig. 2.7 Germ tube formation by
Candida albicans. For simplicity
the diagram merely illustrates the
nuclear content of the parental
yeast cell and the developing
germ tube. In real life there is a
complex rearrangement of
cytoplasmic constituents which
results in all parts except the
apex becoming highly
vacuolated.
sensitivities. Furthermore, it is documented that patients who have suffered from repeated
vaginal candidosis have yielded the same strain which has presented an alternative
phenotype on each occasion (Soil et al. 1989). Thus, this phenomenon seems to represent
a mechanism which has evolved to enable C. albicans to escape destruction by the
immune system. Phenotypic switching is so-called because it has been assumed that a
mutational event could not be responsible due to the high frequencies (up to 10%)
Fig. 2.8 Mating type switching in Saccharomyces cerevisiae. The founder cell (F) is a virgin (i.e. has
not formed a bud previously), carries the HO gene and is mating type a. After the first cell cycle there
will be the mother cell (M) and the daughter (D), both of which are mating type a. Mother cells which
carry HO are able to switch mating type whilst in the Gl phase of the cell cycle. Assuming that M
switches to mating type a, the progeny which result from M (a mother and a daughter) will thus both
be mating type a. Daughters cannot switch mating type, hence D will produce two cells of mating
type a. Note that two of the cells present at the four cell stage are mothers and hence capable of
switching mating type before entering the next budding cycle.
46 Chapter 2
observed. However, there clearly has to be a genetic basis to such variation. In S.
cerevisiae, mating type switching can occur (Herskowitz et al 1992) due to the presence
of the HO gene. This gene confers on a haploid strain of either mating type the ability
to switch to the opposite mating type. The opportunity to switch is only available to
mother cells which are in the Gl phase of the cell cycle. Thus, it is quite easy to calculate
that a single cell that was (say) mating type a could give rise after two complete cell
cycles to a colony that comprised two cells of mating type a and two of mating type a
(Fig. 2.8.) Hence, one could say that mating type switching in S. cerevisiae has a
frequency of approximately 50% in appropriate strains, i.e. even higher than the
frequency of phenotypic switching in C. albicans. The molecular basis of phenopic
switching remains unclear at the time of writing.
4 Cryptococcus neoformans
Cryptococcus neoformans is an encapsulated yeast which causes cryptococcosis, a
subacute or chronic infection of the central nervous system. In extreme cases, tissue
damage can also occur in the skin, bones and internal organs. Cryptococcal meningitis
is very frequent in AIDS patients. Despite this, it is not a newly discovered organism
and its pathogenic capabilities have been known for many years. For example, in 1955
it was known that Cr. neoformans was the causative agent in 10% of all fatal human
mycoses in the USA (Emmons 1955). It does not form a pseudomycelium, neither does
it develop hyphae. Surely this yeast provides adequate proof that neither is necessary
to be pathogenic to any warm-blooded animal! The yeast cells are almost spherical and
in conditions of high osmolarity they produce a much reduced capsule, such that the
overall size is less than 5fm\ in diameter. This renders them small enough to remain as
dust in the atmosphere and be inhaled. This is reckoned to be the route of all infections.
The yeast is inhaled and carried to the alveoli of the lungs. When in the warm, moist
alveoli, the yeast cells regenerate their thick capsules with the consequent release of
polysaccharides and glycoproteins into the host's bloodstream, both of which serve as
virulence factors (Murphy 1996). The capsule products cause neutrophils to lose their
surface L-selectin, which is required for leukocytes to attach to endothelial cells before
moving from the blood system to the tissues. Since the leukocytes are not sent to the
site of infection in the tissue, the yeast escapes. The immune system is further confused
by yeast cell products in the bloodstream due to the induction of suppressor T
lymphocytes which attenuate immune responses. It is believed that the release of melanin
from the yeast also helps it to evade the immune system. Melanin is thought to act as an
antioxidant which thereby protects cryptococci from oxidative killing. With such an
armoury of virulence, the reader will appreciate why rapid identification of this organism
is so important.
5 Neurospora crassa
Neurospora crassa is a filamentous pink mould. It became famous to scientists due to
the work of Beadle and Tatum in the 1940s when they developed the 'one gene—one
enzyme' hypothesis. Its life cycle is shown in Fig. 2.9. Unfortunately, the full force of
fungal nomenclature comes into play when considering this organism. Aerial hyphae
Yeasts and moulds 47
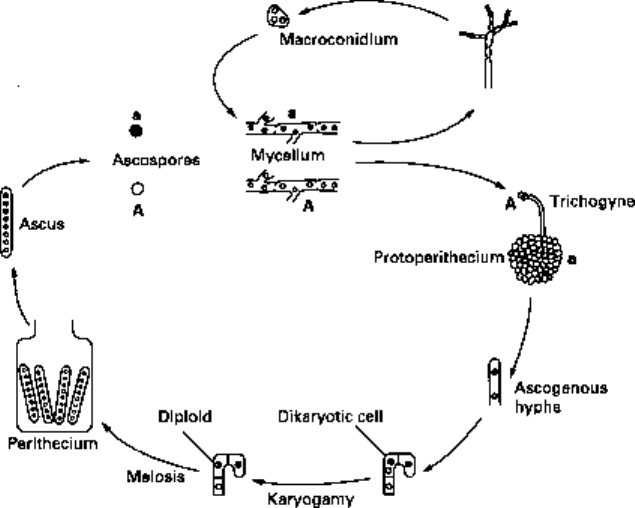
Fig. 2.9 The life cycle of Neurospora crassa. The figure illustrates both the asexual cycle via
macroconidia and the sexual cycle. In the case of the latter, the diagram represents the
interaction between a male of mating type A and a female of mating type a. The trichogyne
grows towards the male. There is fusion, one male nucleus enters and pairs with a female nucleus.
Rounds of synchronous nuclear divisions result in dikaryotic (i.e. containing two nuclei)
cells. Karyogamy produces a true diploid which immediately undergoes meiosis and
ascosporogenesis. The cycle is completed by germination of the individual ascospores to found fresh
mycelia.
from the heterokaryotic (i.e. containing many separate nuclei) vegetative mycelium
produce either macroconidia or microconidia. The macroconidia contain several nuclei
and re-establish vegetative mycelium when they germinate. Each microconidium is
uninucleate; their role in the life cycle is to fuse with the trichogyne (a specialized
hypha of opposite mating type.) The trichogyne is carried on the protoperithecium,
which, as its name suggests, is the precursor to the perithecium (fruiting body). Inside
the perithecium, nuclear fusion takes place, followed by meiosis and further differen-
tiation to produce an ascus containing eight ascospores. When the ascospores germinate,
they produce haploid mycelia which can form heterokaryons by means of hyphal fusions
with mycelia of the opposite mating type.
It is instructive to consider the similarities and differences in the life cycle of
S. cerevisiae and N. crassa. Disregarding the obvious difference that the former is a
yeast whilst the latter is a mould, it should be noted that both fungi have a vegetative
haplophase. The diplophase of S. cerevisiae can proliferate, whereas in N. crassa the
diploid rapidly undergoes meiosis and ascospore formation. Both organisms can exist
in one of two mating types, each of which is controlled by a single genetic locus.
Neurospora crassa has truly male and female thalli (singular, thallus, is the vegetative
body of a fungus) which are morphologically distinct. Neurospora crassa is an obligate
aerobe, hence, elimination of oxygen will prevent its growth.
6 Penicillium and Aspergillus
The scientifically informed layperson is aware that Penicillium species are associated
with a number of beneficial products. These include the important antibiotic penicillin
(originally from P. notatum, but which soon came to be prepared from P. chrysogenum
because this species produces more: see Chapters 5 and 7), and the ripening of Stilton,
Roquefort and Camembert cheeses with strains of P. roquefortii (blue vein cheeses)
and P. camembertii (surface-ripened cheeses). However, many more would be surprised
to learn that today's commercial penicillin-producing strains all derive from an organism
which was originally isolated from a rotting Canteloupe melon. This fact emphasizes
the real ecological place of such moulds where, of course, decomposition of fruits and
vegetables is an essential part of the recycling of materials in the biosphere. Life could
not continue on our planet in the absence of decomposer organisms.
A less desirable characteristic of many moulds is the production of mycotoxins.
One group of mycotoxins, the aflatoxins, which are derived from decaketides, are a
particular cause for concern. Aflatoxin Bj is carcinogenic in animals and mammalian
cell lines in tissue culture. It has been linked to specific mutations in the human
tumour suppressor gene p53 thus causing primary hepatocellular carcinoma. Hence,
contamination by aflatoxins of food, feed, and medical, veterinary, pharmaceutical and
laboratory preparations has serious health and economic consequences. Aspergillus
parasiticus and A. flavus are notorious as producers of aflatoxins. As with other species
of Aspergillus, they are very widespread in the environment and, aided by their rapid
growth on a variety of substrates, they are commonly found as contaminants of all
sorts of materials. Members of the genus can give rise to a group of diseases known
collectively as aspergilloses. These includes allergies, toxicoses, tissue invasion and
local colonization. Aspergillus fumigatus is the most common cause of aspergillosis.
Not all members of the genus are wholly bad. Aspergillus oryzae has been used for
centuries in the production of soy sauce. Although this is of little consequence in the
West, the underlying technology became the foundation of the Japanese amino acid
and nucleotide business which have worldwide economic importance.
It appears that the various strains of Penicillium used in cheese production do not
produce any such toxins. Presumably this is an example of selection acting on the early
human cheese-makers: the folk who produced cheese which contained mycotoxins ate
their own cheese and died. Hence, they did not hand on their skills and strains of mould
to subsequent generations! Microbiological contamination by wild moulds always carries
the possibility of chemical contamination with their toxins, so we should not think of
Penicillium species as being of universal benefit to humankind. Indeed, direct infections
by Penicillium species have been reported to various parts of the human body including
the cornea, ear, respiratory tract, urinary tract and heart (following the surgical insertion
of artificial valves: Kwon-Chung & Bennett 1992).
Penicillium has no sexual cycle. The organisms merely produce conidia (asexually
produced spores) which are readily dispersed by slight draughts (Fig. 2.10). New growth
can commence after landing on a suitable substrate. The brush-like appearance is typical
Yeasts and moulds 49

Fig. 2.10 A typical Penicillium.
(Fig. 2.10). The organism Penicillium marneffei is said to be thermally dimorphic: at
25-30°C it produces colonies like any other of the genus. At 35-37°C it is yeast-like
(Larone 1995). It is endemic in South-East Asia and is reported as causing deep-seated
infections in both immunocompromised and normal individuals who have visited those
parts. The wider significance of this to fungal biology and especially to the taxonomy
of Penicillium species remains to be established, but the importance to human health is
already clear.
Epidermophy ton, Microsporum and Trichophyton
All three of these are dermatophytes, i.e. filamentous fungi which can utilize keratin
for their nutrition. Keratin is the chief protein in skin, hair and nail. Hence, all of these
organisms are responsible for superficial mycoses in mammals. It is often stated that
dermatophytes are the only fungi to have evolved which rely upon infection for their
own survival. This mistaken belief results from a view which is too human-centred and
neglects, for example, the presence of symbiotic fungi in the stomachs of ruminants.
The genus Microsporum contains several interesting species including M. audouinii,
which, in years gone by, caused epidemics of 'ringworm' in children, but rarely in
adults: M.ferrugineum appears to fulfil this role today; M. canis which infects children,
cats and dogs, but not adults—it is said that the children acquire the infection from
the animals; M. gypseum affects mainly lower animals; M. gallinae infects poultry and
humans; M. nanum can be common in pigs, but is rare in humans. The appearance of a
tinea ('ringworm') is the host's reaction to the proteolytic (protein degrading) enzymes
secreted by the fungus. In highly sensitized or hyper-allergic individuals this can be
very pronounced.
Epidermophyton floccosum infects the skin and nails but not the hair, whereas
different species of the genus Trichophyton display both geographical and anatomical
variations. For example, T. rubrum is currently the most common dermatophyte of
humans: it infects skin and nails but almost never hairy parts of the body; T.
mentagrophytes, which is frequently the cause of athlete's foot, can infect all parts of
the human body; the aptly-named T. tonsurans is the major causative agent of scalp
ringworm in the USA whereas T. megninii is hardly ever found in the Western world.
These varied distributions presumably reflect a complex matrix of variables including
climate, nutrition, age, physiological status, the proximity of animals and other aspects
of human lifestyle.
Certain identification of each individual dermatophyte requires great skill especially
in the case of Microsporum where there is considerable morphological similarity between
the species: hyphae are septate with numerous macroconidia which are thick-walled
and rough in most cases. Microconidia are usually present. Epidermophyton is broadly
similar except that microconidia are not formed. Distinguishing individual species
of Trichophyton from each other is less problematical, although an unwary observer
might confuse T. mentagrophytes with T. rubrum. Generally, in Trichophyton species,
macroconidia are rare, thin-walled and smooth; there are numerous microconidia.
Clearly, although these organisms only cause superficial infections, a rapid, genetic-
based identification system would be a boon.
8 References
Andreasen A.A. & Stier J.B. (1953) Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol
requirement for growth in a defined medium. / Cell Comp Physiol, 41, 23-36.
Andreasen A.A. & Stier J.B. (1954) Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated
fatty acid requirement for growth in a defined medium. J Cell Comp Physiol, 43, 271-281.
Briza P., Ellinger A., Winkler G. & Breitenbach M. (1990) Characterization of a D, L-dityrosine-containing
macromolecule from yeast ascospore walls. J Biol Chem, 265, 15118-15123.
Cass A., Finklestein A. & Krespi V. (1970) The ion permeability induced in thin lipid membranes by
the polyene antibiotics nystatin and amphotericin B. J Gen Physiol, 56, 100-124.
Crowe J.H., Crowe L.M. & Chapman D. (1984) Preservation of membranes in anydrobiotic organisms:
the role of trehalose. Science, 223, 701-703.
Dickinson J.R. (1988) The metabolism of sporulation in yeast. Microbiol Sci, 5, 121-123.
Dickinson J.R. (1996) 'Fusel' alcohols induce hyphal-like extensions and pseudohyphal formation in
yeasts. Microbiology, 142, 1391-1397.
Dickinson J.R. & Hewlins M.J.E. (1991)
13
C NMR analysis of a developmental pathway mutation in
Saccharomyces cerevisiae reveals a cell derepressed for succinate dehydrogenase. J Gen Microbiol,
137, 1033-1037.
Dickinson J.R., Dawes I.W., Boyd A.S.F. & Baxter R.L. (1983)
13
C NMR studies of acetate metabolism
during sporulation of Saccharomyces cerevisiae. Proc Nat Acad Sci USA, 80, 5847-5851.
Emmons C.W. (1955) Saprophytic sources of Cryptococcus neoformans associated with the pigeon.
Am J Hygiene, 62, 227-232.
Fraenkel D.G. (1982) Carbohydrate metabolism. In: The Molecular Biology of the Yeast Saccharomyces,
vol. 2. Metabolism and Biosynthesis (eds J.N. Strathern, E.W. Jones & J.R. Broach), pp. 1-37. Cold
Spring Harbor, NY: Cold Spring Harbor Laboratory.
Friefelder D.M. (1960) Bud formation in Saccharomyces cerevisiae. J Bacteriol, 80, 567-568.
Gimeno C.J., Ljungdahl P.O., Styles C.A. & Fink G.R. (1992) Unipolar cell divisions in the yeast
S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell, 68, 1077-1090.
Gow N.A.R. (1994) Growth and guidance of the fungal hypha. Microbiology, 140, 3139-3205.
Gow N.A.R., Swoboda R.K., Bertram G., Gooday G.W. & Brown A.J.P. (1993) Key genes in the
regulation of dimorphism of Candida albicans. In: Dimorphic Fungi (eds H. Vanden Bossche, F.C.
Odds & D. Kerridge), pp. 61-71. New York: Plenum Press.
Hartwell L.H. (1974) Saccharomyces cerevisiae cell cycle. Bacteriol Rev, 38, 164-198.
Hartwell L.H. (1978) Cell division from a genetic perspective. J Cell Biol, 77, 627-637.
Yeasts and moulds 51
Henry S.A. (1982) Membrane lipids of yeast: biochemical and genetic studies. In: The Molecular
Biology of the Yeast Saccharomyces, vol. 2. Metabolism and Biosynthesis (eds J.N. Strathern, E.W.
Jones & J.R. Broach), pp. 101-158. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Herskowitz I., Rine J. & Strathern J.N. (1992) Mating-type determination and mating-type inter-
conversion in Saccharomyces cerevisiae. In: The Molecular and Cellular Biology of the Yeast
Saccharomyces cerevisiae. vol. 2, Gene Expression (eds E.W. Jones, J.R. Pringle & J.R. Broach),
pp. 583-656. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Kennedy B.K. & Guarente L. (1996) Genetic analysis of aging in Saccharomyces cerevisiae. Trends
Genet, 12, 355-359.
Kron S.J. & Gow N.A.R. (1995) Budding yeast morphogenesis: signalling, cytoskeleton and cell cycle.
Curr Opin Cell Biol, 7, 845-855.
Kron S.J., Styles C.A. & Fink G.R. (1994) Symmetric cell division in pseudohyphae of the yeast
Saccharomyces cerevisiae. MolBiol Cell, 5, 1003-1022.
Kuriyama H. & Slaughter J.C. (1995) Control of cell morphology of the yeast Saccharomyces cerevisiae
by nutrient limitation in continuous culture. LettAppl Microbiol, 20, 37-40.
Kwon-Chung K.J. & Bennett J.E. (1992) Medical Mycology. Philadelphia: Lea & Febiger.
Larone D.H. (1995) Medically Important Fungi: A Guide to Identification, 3rd edn. Washington:
American Society for Microbiology.
Lew D.J. & Reed S.l. (1995) Cell cycle control of morphogenesis in budding yeast. Curr Opin Genet
Dev, 5, 17-23.
Leopold A.C. (1986) Membranes, Metabolism and Dry Organisms. Ithaca: Cornell University Press.
Manners D.J., Masson A.J. & Patterson J.C. (1973a) The structure of a j8-(l-3)-D-glucan from yeast cell
walls. BiochemJ, 135, 19-30.
Manners D.J., Masson A.J., Patterson J.C, Bjorndal H. & Lindberg B. (1973b) The structure of a /J-(l-
6)-D-glucan from yeast cell walls. Biochem J, 135, 31-36.
Murphy J.W. (1996) Slick ways Cryptococcus neoformans foils host defences. Am Soc Microbiol News,
62, 77-80.
Necas O. (1971) Cell wall synthesis in yeast protoplasts. Bacteriol Rev, 35, 149-170.
Norman A.W, Demel R.A., DeKruyff B., Geurts Van Kessel W.S.M. & Van Deenen L.L.M. (1972)
Studies on the biological properties of polyene antibiotics: comparison of the other polyenes with
filipin in their ability to interact specifically with sterol. Biochim Biophys Acta, 290, 1-14.
Odds EC. (1988) Candida and Candidosis. London: Balliere Tindall.
Pringle J.R. (1978) The use of conditional lethal cell cycle mutants for temporal and functional sequence
mapping of cell cycle events. J Cell Physiol, 95, 393-406.
Pringle J.R. & Hartwell L.H. (1981) The Saccharomyces cerevisiae cell cycle. In: The Molecular Biology
of the Yeast Saccharomyces, vol. 1. Life Cycle and Inheritance (eds J.N. Strathern, E.W. Jones &
J.R. Broach), pp. 97-142. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Soil D.R. (1992) High frequency switching in Candida albicans. Clin Microbiol Rev, 5, 183-203.
Sanjuan R., Zueco J., Stock R., Font de Mora J. & Sentandreu R. (1995) Identification of glucan-
mannoprotein complexes in the cell wall of Candida albicans using a monoclonal antibody that
reacts with a (l,6)-/3-glucan epitope. Microbiology, 141, 1545-1551.
Soil D.R., Galask R., Isley S. et al. (1989) Switching of Candida albicans during recurrent episodes of
recurrent vaginitis. J Clin Microbiol, 27, 681-690.
Van Rinsum J., Klis EM. & van den Ende H. (1991) Cell wall glucomannoproteins of Saccharomyces
cerevisiae mnn9. Yeast, 7, 717-726.
Wright R.M., Repine T. & Repine J.E. (1993) Reversible pseudohyphal growth in haploid Saccharomyces
cerevisiae is an aerobic process. Curr Genet, 23, 388-391.
Viruses
1 Introduction
2 General properties of viruses
2.1 Size
2.2 Nucleic acid content
2.3 Metabolic capabilities
3 Structure of viruses
3.1 Helical symmetry
3.2 Icosahedral symmetry
4 The effect of chemical and physical
agents on viruses
5 Virus-host-cell interactions
6 Bacteriophages
6.1 The lytic growth cycle
6.2 Lysogeny
6.3 Epidemiological uses
7 Human viruses
7.1 Cultivation of human viruses
7.1.1 Cell culture
7.1.2 The chick embryo
7.1.3 Animal inoculation
8 Multiplication of human viruses
8.1 Attachment
8.2 Penetration and uncoating
8.3 Production of viral proteins and
replication of viral nucleic acid
8.4 Assembly and release of progeny
9 The problems of viral chemotherapy
9.1 Interferon
10 Tumour viruses
11 The human immunodeficiency virus
12 Prions
13 Further reading
Introduction
Following the demonstration by Koch and his colleagues that anthrax, tuberculosis
and diphtheria were caused by bacteria, it was thought that similar organisms
would, in time, be shown to be responsible for all infectious diseases. It gradually
became obvious, however, that for a number of important diseases no such bacterial
cause could be established. Infectious material from a case of rabies, for example,
could be passed through special filters which held back all particles of bacterial
size, and the resulting bacteria-free filtrate still proved to be capable of inducing
rabies when inoculated into a susceptible animal. The term virus had, up until
this time, been used quite indiscriminately to describe any agent capable of
producing disease, so these filter-passing agents were originally called filterable viruses.
With the passage of time the description 'filterable' has been dropped and the name
virus has come to refer specifically to what are now known to be a distinctive
group of microorganisms different in structure and method of replication from
all others.
General properties of viruses
All forms of life—animal, plant and even bacterial—are susceptible to infection by
Viruses 53
3
