Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

deterioration have quite unpleasant tastes and smell even at low levels, and would
deter most from using such a medicine.
1.2.2 Chemical and physico-chemical deterioration of pharmaceutical products
Microorganisms form a major part of the natural recycling processes for biological
matter in the environment. As such, they possess a wide variety of degradative
capabilities, which they are able to exert under relatively mild physico-chemical
conditions. Mixed natural communities are often far more effective co-operative
biodeteriogens than the individual species alone, and sequences of attack of complex
substrates occur where initial attack by one group of microorganisms renders them
susceptible to further deterioration by secondary, and subsequent, microorganisms.
Under suitable environmental selection pressures even novel degradative pathways
emerge, able to attack newly introduced synthetic chemicals (xenobiotics). However,
the rates of degradation of materials released into the environment can vary
greatly, from half lives of hours (phenol) to months ('hard' detergents) to years
(halogenated pesticides). The overall rate of deterioration of a chemical will depend
upon:
1 its chemical structure;
2 the physico-chemical properties of a particular environment;
3 the type and quantity of microbes present;
4 whether the metabolites produced can serve as sources of usable energy and
precursors for biosynthesis of cellular components, and hence the creation of more
microorganisms.
Pharmaceutical formulations may be considered as specialized micro-environments
and their susceptibility to microbial attack assessed using conventional ecological
criteria. Some naturally-occurring ingredients are particularly sensitive to attack, and
quite a few synthetic components, such as modern surfactants, have been deliberately
constructed to be readily degraded after disposal into the environment. Crude vegetable
and animal drug extracts often contain wide assortments of microbial nutrients besides
the therapeutic agents. This, combined with frequently conducive and unstable physico-
chemical characteristics, leaves many formulations with a high potential for microbial
attack, unless steps are taken to minimize it.
1.2.3 Pharmaceutical ingredients susceptible to microbial attack
Surface-active agents. Anionic surfactants such as the alkali metal and amine soaps of
fatty acids are generally stable due to the slightly alkaline pH of the formulations,
although readily degraded once diluted out into sewage. Alkyl and alkylbenzene
sulphonates and sulphate esters are metabolized by co-oxidation of their terminal methyl
groups followed by sequential /3-oxidation of the alkyl chains and fission of the aromatic
rings. The presence of chain branching involves additional a-oxidative processes.
Generally, ease of degradation decreases with increasing chain length and complexity
of branching of the alkyl chain. Sulphonate and sulphate ester residues are converted
to sulphate, although sulphonate residues are significantly more recalcitrant than the
esters.
Microbial spoilage and preservation of pharmaceutical products 357
Non-ionic surfactants such as alkylpolyoxyethylene alcohol emulsifiers are readily
metabolized by a wide variety of microorganisms. Increasing chain lengths and
branching again decreases ease of attack. Alkylphenol polyoxyethylene alcohols are
similarly attacked, but are significantly more resistant. Lipolytic cleavage of the fatty
acids from sorbitan esters, polysorbates and sucrose esters is often followed by
degradation of the cyclic nuclei, producing numerous small molecules readily utilizable
for microbial growth.
Ampholytic surfactants based on phosphatides, betaines and alkylamino-substituted
amino acids are an increasingly important group of surfactants and are generally reported
to be reasonably biodegradable.
The cationic surfactants used as antiseptics and preservatives in pharmacy are usually
only slowly degraded, at high dilution, in sewage. Pseudomonads have been found
growing readily in quaternary ammonium antiseptic solutions, largely at the expense
of other ingredients such as buffering materials, although some metabolism of the
surfactant has also been observed.
Organic polymers. Many of the thickening and suspending agents used in pharmacy
are subject to microbial depolymerization by specific classes of extracellular enzymes,
yielding nutritive fragments and monomers. Examples of such enzymes, with their
substrates in parentheses are: amylases (starches), pectinases (pectins), cellulases
(carboxymethylcelluloses, but not alkylcelluloses), uronidases (polyuronides such as
in tragacanth and acacia), dextranases (dextrans) and proteases (proteins). Agar (complex
polysaccharides) is an example of a relatively inert polymer and, as such, is used as a
support for solidifying microbiological culture media. The lower molecular weight
polyethylene glycols are readily degraded by sequential oxidation of the hydrocarbon
chain, but the larger congeners are rather more recalcitrant. Synthetic packaging
polymers such as nylon, polystyrene and polyester are extremely resistant to attack,
although cellophane (modified cellulose) is susceptible under some humid conditions.
Humectants. Low molecular weight materials such as glycerol and sorbitol are included
in some products to reduce water loss and are usually readily metabolized unless present
in high concentrations (see section 1.3.3).
Fats and oils. These hydrophobic materials are usually attacked extensively when
dispersed in aqueous formulations such as oil-in-water emulsions, although fungal attack
is reported in condensed moisture films on the surface of oils in bulk, or where water
droplets have contaminated the bulk oil phase. Oil-in-water emulsion-based medicines
are less commonly encountered than in food formulations where their microbial attack
can also occur, aided by the high solubility of oxygen in many oils. Lipolytic rupture of
triglycerides liberates glycerol and fatty acids, the latter often then undergoing fi-
oxidation of the alkyl chains and the production of odiferous ketones. While the microbial
metabolism of pharmaceutical hydrocarbon oils is rarely reported, this is a problem in
engineering and fuel technology when water droplets have accumulated in oil storage
tanks and subsequent fungal colonization has catalysed serious corrosion.
Sweetening, flavouring and colouring agents. Many of the sugars and other sweetening
agents used in pharmacy are ready substrates for microbial growth. However, some
are used in very high concentrations to reduce water activity in some aqueous products
and inhibit microbial attack (see section 1.3.3). At one time, a variety of colouring
agents (such as tartrazine and amaranth) and flavouring agents (such as peppermint
water) were kept as stock solutions for extemporaneous dispensing purposes but they
frequently supported the growth of Pseudomonas spp. including Ps. aeruginosa. It
is now recommended that such stock solutions contain preservatives or are made
freshly as required by dilution of alcoholic solutions which are much less susceptible
to microbial attack.
Therapeutic agents. It is possible to demonstrate that a variety of microorganisms under
laboratory conditions can metabolize a wide assortment of drugs, resulting in loss of
activity. Materials as diverse as alkaloids (morphine, strychnine, atropine), analgesics
(aspirin, paracetamol), thalidomide, barbiturates, steroid esters and mandelic acid can
be metabolized and serve as substrates for growth. Indeed the use of microorganisms
to carry out subtle transformations on steroid molecules forms the basis of the
commercial production of potent therapeutic steroidal agents (see Chapter 25). Reports
of drug destruction in actual medicines are less frequent. However, examples include
the metabolism of atropine in eye drops by contaminating fungi, inactivation of penicillin
injections by /3-lactamase-producing bacteria (see Chapter 5), steroid metabolism in
damp tablets and creams by fungi, the microbial hydrolysis of aspirin in suspension by
esterase-producing bacteria, and chloramphenicol deactivation in an oral medicine by
a chloramphenicol acetylase-producing contaminant.
Preservatives and disinfectants. Many preservatives and disinfectants can be metab-
olized by a wide variety of Gram-negative bacteria, although more commonly at
concentrations below their effective 'use' levels. However, quaternary ammonium
antimicrobial agents are only slowly attacked. Organomercurial preservatives discharged
into rivers from paper mills have been extensively converted to insidiously toxic
alkylmercury compounds which could reach humans via an ascending food chain.
Degradation of agents at 'use' concentrations in pharmacy and medicine is less
commonly reported, but there are incidents of the growth of pseudomonads in stock
solutions of quaternary ammonium antiseptics and chlorhexidine with resultant infection
of patients. Pseudomonas spp. have metabolized 4-hydroxybenzoate ester preservatives
contained in eye-drops and caused serious eye infections, and metabolized them in oral
suspensions and solutions. It is important to remember this possibility when selecting
preservatives for formulations.
1.2.4 Observable effects of microbial attack on pharmaceutical products
Microbial contaminants will usually need to be able to attack ingredients of a medicine
and create substrates necessary for biosynthesis and energy production before they can
replicate to levels where obvious spoilage becomes apparent since, for example, 10
6
microbes will have an overall degradative effect around 10
6
time faster than one cell.
However, growth and attack may well be localized in surface moisture films or very
unevenly distributed within the bulk of viscous formulations such as creams. Early
Microbial spoilage and preservation of pharmaceutical products 359
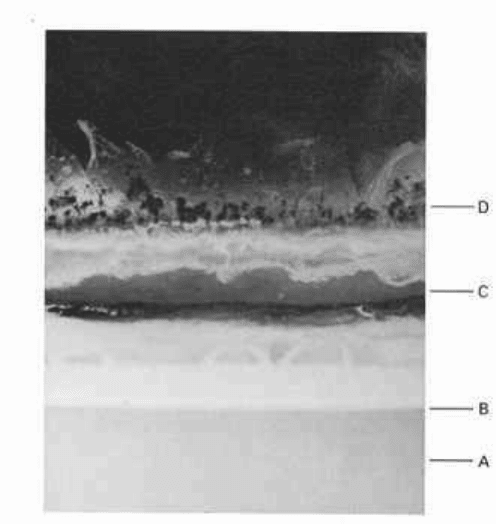
Fig. 18.1 Section (xl.5)
through an inadequately
preserved olive oil, oil-in-water,
emulsion in an advanced state of
microbial spoilage showing:
A, discoloured, oil-depleted,
aqueous phase; B, oil globule-
rich creamed layer; C, coalesced
oil layer from 'cracked'
emulsion; D, fungal mycelial
growth on surface. Also present
are a foul taste and evil smell!
indications of spoilage are often organoleptic, with the release of very unpleasant
smelling and tasting metabolites such as 'sour' fatty acids, 'fishy' amines, 'bad eggs',
bitter, 'earthy' or sickly tastes and smells. Products frequently become unappealingly
discoloured by microbial pigments of various shades. Thickening and suspending agents
such as tragacanth, acacia or carboxymethylcellulose can be depolymerized, resulting
in loss of viscosity, and sedimentation of suspended ingredients. Alternatively, microbial
polymerization of sugars and surfactant molecules can produce slimy, viscous, masses
in syrups, shampoos and creams, and fungal growth in creams has produced 'gritty'
textures. Changes in product pH can occur depending on whether acidic or basic
metabolites are released, and become so modified as to permit secondary attack by
microbes previously inhibited by the initial product pH. Gaseous metabolites may be
seen as trapped bubbles within viscous formulations.
When a complex formulation such as an oil-in-water emulsion is attacked, a gross
and progressive spoilage sequence may be observed. Metabolism of surfactants will
reduce stability and accelerate 'creaming' of the oil globules. Lipolytic release of fatty
acids from oils will lower pH and encourage coalescence of oil globules and 'cracking'
of the emulsion. Fatty acids and their ketonic oxidation products will provide a sour
taste and unpleasant smell, whilst bubbles of gaseous metabolites may be visible, trapped
in the product, and pigments may discolour the product (see Fig. 18.1).
1.3 Factors affecting microbial spoilage of pharmaceutical products
An understanding of the influence of the chemical and physico-chemical parameters of
an environment on microorganisms might allow for subtle manipulation of a formulation
to create conditions which are as unfavourable as possible for growth and spoilage,
360 Chapter 18
within the limitations of patient acceptability and therapeutic efficacy. Additionally,
the overall characteristics of a particular formulation will indicate its susceptibility to
attack by various classes of microorganisms.
1.3.1 Types, and size, of contaminant inoculum
Whilst there will be some chance that a particularly aggressive microbe may enter and
contaminate a medicine, some element of prediction is possible if one considers the
environment and usage to which the product is likely to be subjected during its life and
the history of similar medicines (see Chapters 17 and 19). A formulator can then build
in as much protection as possible against non-standard encounters, such as additional
preservation for a syrup if osmotolerant yeast contamination is particularly likely.
Should failure subsequently occur, a knowledge of microbial ecology and careful
identification of the contaminant(s) can be most useful in tracking down the defective
steps in the design or production process. Very low levels of contaminants which are
unable to replicate in a product might not cause appreciable spoilage but, should an
unexpected surge in the contaminant bioburden occur, the built-in protection could
become swamped and spoilage ensue. This might arise if:
1 raw materials were unusually contaminated;
2 a lapse of the plant-cleaning protocol occurred;
3 large microbial growths detached themselves from within supplying pipework;
4 a change in production procedures allowed unexpected growth of contaminants
during the modified operation;
5 there was demolition work in the vicinity of the manufacturing site or;
6 there had been gross misuse of the product during administration.
However, inoculum size alone is not always a reliable indicator of likely spoilage
potential. A very low level of, say, aggressive pseudomonads in a weakly preserved
solution may suggest a greater risk than tablets containing fairly high numbers of fungal
and bacterial spores.
When an aggressive contaminant enters a medicine, there may be an appreciable
lag period before significant spoilage begins, the duration of which decreases
disproportionately with increasing contaminant loading. It is possible to provide some
control over extemporaneously dispensed formulations by specifying short shelf-lives
of, say, 2 weeks. However, since there is usually a long delay between manufacture
and administration of factory-made medicines, growth and attack could ensue during
this period unless additional steps are taken to prevent it.
The isolation of a particular microorganism from a markedly spoiled product does
not necessarily mean that it was the initiator of the attack. It could be a secondary
opportunist contaminant which has overgrown the primary spoilage organism once the
physico-chemical properties had been favourably modified by the primary spoiler.
1.3.2 Nutritional factors
The simple nutritional requirements and metabolic adaptability of many common
saprophytic spoilage microorganisms enable them to utilize many of the components
of medicines as substrates for biosynthesis and growth, including not only the intended
Microbial spoilage and preservation of pharmaceutical products 361
ingredients but also the wide array of trace materials contained in them. The use of
crude vegetable or animal products in a formulation provides an additionally nutritious
environment. Even demineralized water prepared by good ion-exchange methods will
normally contain sufficient nutrients to allow significant growth of many water-borne
Gram-negative bacteria such as Pseudomonas spp. When such contaminants fail to
grow in a medicine it is unlikely to be as a result of nutrient limitation but due to other,
non-supportive, physico-chemical or toxic properties.
Most acute pathogens require specific growth factors normally associated with the
tissues they infect but which are often normally absent in pharmaceutical formulations.
They are thus unlikely to multiply in them, although they may remain viable and infective
for an appreciable time in some dry products where the conditions are suitably protective.
1.3.3 Moisture content: water activity (A )
Microorganisms require ready access to water in appreciable quantities for growth.
Although some solute-rich medicines such as syrups may appear to be 'wet', microbial
growth in them may be difficult since the microbes have to compete for water molecules
with the vast numbers of sugar and other molecules of the formulation which also
readily interact with water via hydrogen bonding. An estimate of the proportion of the
uncomplexed water in a formulation available to equilibrate with any microbial
contaminants and facilitate growth can be obtained by measuring its water activity
(A
w
). (This can be calculated from: A
w
= vapour pressure of formulation -*- vapour
pressure of water under similar conditions). The greater the solute concentration,
the lower is the water activity. With the exception of halophilic bacteria, most
microorganisms grow best in dilute solutions (high A
w
) and, as solute concentration
rises (lowering A
w
), growth rates decline until a minimal, growth-inhibitory A
w
is
reached. Limiting A
w
values are of the order of: Gram-negative rods, 0.95; staphylococci,
micrococci and lactobacilli, 0.9; and most yeasts, 0.88. Syrup-fermenting osmotolerant
yeasts have been found spoiling products with A
w
levels as low as 0.73, whilst some
filamentous fungi can grow at even lower values, with Aspergillus glaucus as low as
0.61.
The A
w
of aqueous formulations can be lowered to increase resistance to microbial
attack by the addition of high concentrations of sugars or polyethylene glycols. However,
even Syrup BP (66% sucrose; A
w
= 0.86) has been reported to fail occasionally to inhibit
osmotolerant yeasts and additional preservation may be necessary. With a trend towards
the elimination of sucrose from medicines continuing, alternative solutes are being
investigated, such as sorbitol and fructose, which are not thought to encourage dental
caries. The use of brine to preserve some meats would be organoleptically unacceptable
for medicines. A
w
can also be reduced by drying, although the dry, often hygroscopic
medicines (tablets, capsules, powders, vitreous 'glasses') will require suitable packaging
to prevent resorption of water and consequent microbial growth (Fig. 18.2). Tablet
film coatings are now available which greatly reduce water vapour uptake during storage
whilst allowing ready dissolution in bulk water. These might contribute to increased
microbial stability during storage in particularly humid climates, although suitable foil
strip packing may be more effective, if also more expensive.
362 Chapter 18
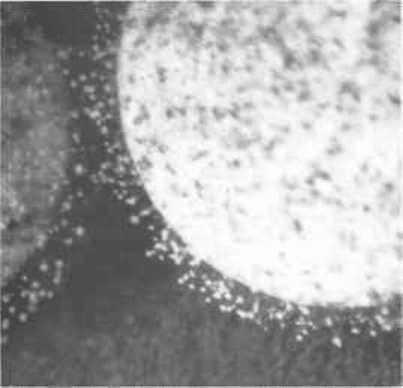
Fig. 18.2 Fungal growth on a
tablet which has become damp
(raised A
w
) during storage under
humid conditions. Note the
sparseness of mycelium, and
conidiophores. The contaminant
is thought to be a Penicillium sp
Condensed water films can accumulate on the surface of otherwise 'dry
5
products
such as tablets or bulk oils following storage in damp atmospheres with fluctuating
temperatures, resulting in sufficiently high localized A
w
to initiate fungal growth. More
water, produced from respiration, may then raise A
w
even further, encouraging growth.
Dilute aqueous films similarly formed on the surface of viscous products such as syrups
and creams, or exuded by syneresis from hydrogels, can and do reach sufficiently high
A
w
to permit surface yeast and fungal spoilage.
Inhibition of microbial growth by reduction of A
w
is more complex than by a simple
binding of water molecules alone as some solutes are more effective than others at
inhibiting microbial attack by particular types of microorganisms even when used to
generate similar A
w
levels. Mechanisms are thought to involve interference with cellular
osmoregulation and energy production.
1.3.4 Redox potential
The ability of microbes to grow in an environment is influenced by its oxidation-
reduction balance (redox potential) since they will require compatible terminal electron
acceptors to permit function of their respiratory pathways. Vacuum packing of foodstuffs,
or the inclusion of oxygen absorbers in the package to minimize oxygen levels, reduces
attack by some of the obligate aerobic spoilage bacteria, but does not eliminate all spoilage.
Oxygen removal to control spoilage in medicines is not a practical proposition although
it is used to control non-biological oxidation. The use of pressurized carbon dioxide for
soft drinks preservation relies more on the specific antimicrobial action of carbonic
acid than to removal of oxygen. Some viscous foodstuffs, particularly those containing
meat, have sufficiently low redox potentials to permit growth of dangerous anaerobic
Clostridia, but this is unlikely in most pharmaceuticals. The redox potential even in
fairly viscous emulsions may be quite high due the appreciable solubility of oxygen in
most fats and oils.
Microbial spoilage and preservation of pharmaceutical products 363
1.3.5 Storage temperature
Spoilage of pharmaceuticals could occur over the range of about -20° to 60°C, although
much less likely at the extremes. The actual storage temperature may selectively
determine spoilage by particular types of microorganisms. Storage in a deep freeze at
-20°C or lower is used for long-term storage of foodstuffs and some pharmaceutical
raw materials, and dispensed total parenteral nutrition (TPN) feeds have been stored in
hospitals for short periods at -20°C to even further minimize the risk of growth of any
contaminants which might have been introduced during their aseptic compounding.
Reconstituted syrups and multi-dose eyedrop packs are sometimes dispensed with the
instruction to 'store in a cool place' such as a domestic fridge (8°-12°C), partly to
reduce the risk of in-use contamination growing before the expiry date. Conversely,
pharmacopoeial Water for Injections is recommended to be held at 80°C or above after
distillation and prior to packing and sterilization to prevent possible regrowth of Gram-
negative bacteria, and the release of endotoxins.
1.3.6 pH
Extremes of pH prevent microbial attack, although feeble mould growth even in
dilute hydrochloric acid necessitates the preservation of analytical acid standards.
Around neutrality bacterial spoilage is more likely, with reports of pseudomonads and
related Gram-negative bacteria growing in antacid mixtures, flavoured mouth washes
and in distilled or demineralized water. Above pH 8, for instance with soap-based
emulsions, spoilage is rare. For products with low pH levels such as the fruit juice-
flavoured syrups (ca. pH 3-4) mould or yeast attack is more likely. Yeasts can metabolize
organic acids and raise the pH to levels where secondary bacterial growth can occur.
Although the use of low pH adjustment to preserve foodstuffs is well established
(pickling, coleslaw, yoghurt etc.) it is not practicable to make deliberate use of this for
medicines.
1.3.7 Packaging design
Packaging can have a major influence on microbial stability of some formulations, to
control the entry of contaminants during both storage and use. Enormous efforts have
gone into the design of containers to prevent the ingress of contaminants into medicines
for parenteral administration because of the high risks of infection by this route. Self-
sealing rubber wads must be used to prevent microbial entry into multi-dose injection
containers (Chapter 21) following withdrawals with a hypodermic needle. Wide-
mouthed cream jars will allow the entry of fingers with their concomitant high bioburden
of contamination, and this can be reduced by replacement with narrow nozzle and
flexible screw capped tubes. Where medicines rely on their low A
w
to prevent spoilage,
packaging such as strip foils must be of water vapour-proof materials with fully efficient
seals. Cardboard outer packaging and labels themselves can become substrates for
microbial attack under humid conditions, and preservatives are often included to reduce
their risk of damage.
364 Chapter 18
1.3.8 Protection of microorganisms within pharmaceutical products
The survival of microorganisms in particular environments is influenced by the presence
of various relatively inert materials. Thus, microbes can be more resistant to heat or
desiccation in the presence of some polymers such as starch, acacia or gelatin. Adsorption
onto naturally occurring particulate material may aid establishment and survival in
some environments. There is a belief, but limited hard evidence, that the presence of
suspended particles such as kaolin, magnesium trisilicate or aluminium hydroxide gel
may influence contaminant longevity in medicines containing them, and that the presence
of some surfactants, suspending agents and proteins can increase the resistance of
microorganisms to preservatives, over and above their direct inactivating effect on the
agents.
2 Preservation of medicines using antimicrobial agents:
basic principles
2.1 Introduction
An antimicrobial 'preservative' may be included in a formulation to further reduce the
risk of spoilage and, preferably, kill any anticipated low levels of contaminants remaining
in a non-sterile medicine after manufacture or which might enter while stored, or during
the repeated withdrawal of doses from a multi-dose container. If a medicine is unlikely
to encourage growth or survival of contaminants and the infective risk is low, such as
with tablets, capsules and dry powders, then a preservative might be pointless.
Preservatives should not be added to deal with erratic failures in poorly controlled
manufacturing processes, due to the uncertainty of success, and their possible depletion
before fulfilling their intended role (see section 2.2). The bad practice of including
preservatives in medicines sterilized by filtration to guard against undetected failure of
the filter did not tackle the real problem of the need for properly validated filtration
systems.
Ideally, such preservatives should:
1 be able to kill rapidly all microbial contaminants as they enter the medicine;
2 not be irritant or toxic to the patient;
3 be stable and effective throughout the life of the medicine; and,
4 be selective in reacting with the contaminants and not the ingredients of the medicine.
Unfortunately, the most active antimicrobial agents are often generally non-selective
in action, inter-reacting significantly with formulation ingredients and patients as well
as microorganisms. Once the more toxic, irritant and reactive agents are excluded,
those remaining generally have only modest antimicrobial efficacy, and there are now
no preservatives considered sufficiently non-toxic for use in highly sensitive areas,
e.g. for injection into central nervous system tissues or for use within the eye. A number
of microbiologically effective preservatives used in cosmetics are reported to cause
significant incidences of contact dermatitis, and are thus precluded from use in
pharmaceutical creams. Although it may be preferable to rapidly kill all contaminants
as they enter a medicine, this may only be possible for relatively simple aqueous solutions
such as eye-drops or injections. For physico-chemically complex systems such as
Microbial spoilage and preservation of pharmaceutical products 365
emulsions and creams, only inhibition of growth and rather slow, or no, rates of killing
may be realistically achieved.
In order to maximize what preservative efficiency is possible, an appreciation of
those parameters which influence antimicrobial activity within medicines is essential.
2.2 Effect of preservative concentration, temperature and size of inoculum
Changes in the efficacy of preservatives vary exponentially with changes in
concentration (see concentration exponent, 77, Chapter 11), the extent of variation
depending upon the type of agent. For example, halving the concentration of phenol
(rj = 6) gives a 64-fold (2
6
) reduction in activity, whilst a similar dilution for
chlorhexidine (77 = 2) reduces killing power by only fourfold (2
2
). Changes in product
temperature will alter efficacy in proportions, related to different types of preservative
and certain groups of microorganisms (see temperature coefficient, Q
10
, Chapter 11).
Thus, a drop in temperature from 30 to 20°C could result in fivefold and 45-fold
losses of killing power towards Escherichia coli by phenol (Q
10
= 5) or ethanol
(Q = 45), respectively. If both temperature and concentration vary concurrently, the
situation is more complex, but it has been suggested, for example, that if a 0.1%
chlorocresol (77 = 6, Q
10
= 5) solution completely killed a suspension of E. coli at
30°C in 10 minutes, it would require around 90 minutes to achieve a similar effect if
the temperature was lowered to 20°C and slight overheating during production had
resulted in a 10% loss by vaporization in the chlorocresol concentration (other
factors remaining constant).
Preservative molecules are used up as they inactivate microorganisms and as they
interact non-specifically with the significant quantities of contaminant 'dirt' also
introduced during use. This will result in a progressive and exponential decline in the
efficiency of the remaining preservative. Preservative 'capacity' is a term used to describe
the cumulative level of contamination that a preserved formulation is likely to cope
with before becoming so depleted as to become ineffective. This will vary with
preservative type and complexity of the formulation.
2.3 Factors affecting the 'availability' of preservatives
Most preservatives interact in solution with many of the commonly used ingredients of
pharmaceutical formulations to varying extents via a number of weak bonding attractions
as well as with any microorganisms present. This can result in unstable equilibria in
which only a small proportion of the total preservative present is 'available' to inactivate
the relatively small microbial mass, and the resultant rate of killing may be far lower
than might be anticipated from the performance of simple aqueous solutions. The
'unavailable' preservative may still, however, contribute to the general irritancy of the
product. It is commonly believed that where the solute concentrations are very high,
and A
w
is appreciably reduced, the efficiency of preservatives is often appreciably
reduced and may be virtually inactive at very low A
w
. A practice of including pre-
servatives in very low A
w
products such as tablets and capsules misses the point.
This would only offer minimal protection for the dry tablets, and if they should be-
come damp they will be spoiled for other, non-microbial, reasons.
366 Chapter 18
