Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

cells with abnormal characteristics. Monkey kidney cell cultures are tested for simian
herpes B virus, simian virus 40, mycoplasma and tubercle bacilli. Cultures of human
diploid cells and continuous line cells are subjected to detailed karyological examination
(examination of chromosomes by microscopy) to ensure that the cells have not
undergone any changes likely to impair the quality of a vaccine or lead to undesirable
side-effects.
2.6.2 Final-product control
Vaccines containing killed microbes or their products are generally tested for potency
in assays in which the amount of the vaccine that is required to protect animals from a
defined challenge dose of the appropriate pathogen, or its product, is compared with
the amount of a standard vaccine that is required to provide the same protection. The
usual format of the test is the 3 + 3 dose quantal assay that is used to estimate the
potency of whooping-cough vaccine {British Pharmacopoeia 1993). Three logarithmic
serial doses of the test vaccine and three logarithmic serial doses of the standard vaccine
are made and each is used to inoculate a group of 16 mice. In the case of both the
vaccine and the standard the middle dose is chosen, on the basis of experience, so that
it is sufficient to induce a protective response in about 50% of the animals to which it
is given. Each lower dose may then be expected to protect fewer than 50% of the mice
to which it is given and each higher dose to protect more than 50% of the animals to
which it is given. Fourteen days later all of the mice are infected ('challenged') with
Bordetella pertussis and, after a further 14 days, the number of mice surviving in each
of the six groups is counted. The number of survivors in each group is then used to
calculate the potency of the test vaccine relative to the potency of the standard vaccine
by the statistical method of probit analysis (Finney 1971). The potency of the test
vaccine may be expressed either as a percentage of the potency of the standard vaccine
but, as the standard vaccine will have an assigned potency in International Units (IU),
the potency of the test vaccine may be expressed in similar units. Tests similar to that
used to estimate the potency of whooping-cough vaccine are prescribed for the estimation
of the potencies of diphtheria vaccine and of tetanus vaccine. In the cases of these two
vaccines the bacterial toxins are used as the challenge material {British Pharmacopoeia
1993).
Vaccines containing live microorganisms are generally tested for potency by counts
of their viable particles. In the case of the only live bacterial vaccine in common use,
BCG vaccine, dilutions of vaccine are made and dropped in fixed volumes on to solid
media capable of supporting the microorganisms' growth. After a fortnight the colonies
generated by the drops are counted and the live count of the undiluted vaccine is
calculated. The potency of live viral vaccines is estimated in much the same way except
that a substrate of living cells is used. Dilutions of vaccine are inoculated on to tissue
culture monolayers in Petri dishes or in plastic trays, and the live count of the vaccine
is calculated from the infectivity of the dilutions and dilution factor involved.
Safety tests. Because many vaccines are derived from basic materials of intense
pathogenicity—the lethal dose of a tetanus toxin for a mouse is estimated to be 3 x
10
_5
(ig—safety testing is of paramount importance. Effective testing provides a
Manufacture and quality control of immunological products 315
guarantee of the safety of each batch of every product and most vaccines in the final
container must pass one or more safety tests as prescribed in a pharmacopoeial
monograph. This generality does not absolve a manufacturer from the need to perform
'in-process' tests as required, but it is relaxed for those preparations which have a final
formulation that makes safety tests on the final product either impractical or meaningless.
Bacterial vaccines are regulated by relatively simple safety tests. Those vaccines
composed of killed bacteria or bacterial products must be shown to be completely free
from the living microbes used in the production process and inoculation of appropriate
bacteriological media with the final product provides an assurance that all organisms
have been killed. Those containing diphtheria and tetanus toxoids require in addition,
a test system capable of revealing inadequately detoxified toxins; inoculation of guinea-
pigs, which are exquisitely sensitive to both diphtheria and tetanus toxins, is always
used for this purpose. Inoculation of guinea-pigs is also used to exclude the presence of
abnormally virulent organisms in BCG vaccine.
Viral vaccines present problems of safety testing far more complex than those
experienced with bacterial vaccines. With killed viral vaccines the potential hazards
are those due to incomplete virus inactivation and the consequent presence of residual
live virus in the preparation. The tests used to detect such live virus consist of the
inoculation of susceptible tissue cultures and of susceptible animals. The cultures are
examined for cytopathic effects and the animals for symptoms of disease and histological
evidence of infection at autopsy. This test is of particular importance in inactivated
poliomyelitis vaccine, the vaccine being injected intraspinally into monkeys. At autopsy,
sections of brain and spinal cord are examined microscopically for the histological
lesions indicative of proliferating poliovirus.
With attenuated viral vaccines the potential hazards are those associated with
reversion of the virus during production to a degree of virulence capable of causing
disease in vaccinees. To a large extent this possibility is controlled by very careful
selection of a stable seed but, especially with live attenuated poliomyelitis vaccine, it is
usual to compare the neurovirulence of the vaccine with that of a vaccine known to be
safe in field use. The technique involves the intraspinal inoculation of monkeys with a
reference vaccine and with the test vaccine and a comparison of the neurological lesions
and symptoms, if any, that are caused. If the vaccine causes abnormalities in excess of
those caused by the reference it fails the test.
Tests of general application. In addition to the tests designed to estimate the potency
and to exclude the hazards peculiar to each vaccine there are a number of tests of more
general application. These relatively simple tests are as follows.
1 Sterility. In general, vaccines are required to be sterile. The exceptions to this
requirement are smallpox vaccine made from the dermis of animals and bacterial
vaccines such as BCG, Ty21A and tularaemia vaccine which consist of living but
attenuated microbes. WHO requirements and pharmacopoeial standards stipulate, for
vaccine batches of different size, the numbers of containers that must be tested and
found to be sterile. The preferred method of sterility testing is membrane filtration as
this technique permits the testing of large volumes without dilution of the test media.
The test system must be capable of detecting aerobic and anaerobic organisms and
fungi (see Chapter 23).
2 Freedom from abnormal toxicity. The purpose of this simple test is to exclude the
presence in a final container of a highly toxic contaminant. Five mice of 17-22g and
two guinea-pigs of 250-350 g are inoculated with one human dose or 1.0ml, whichever
is less, of the test preparation. All must survive for 7 days without signs of illness.
3 Presence of aluminium and calcium. The quantity of aluminium in vaccines
containing aluminium hydroxide or aluminium phosphate as an adjuvant is limited to
1.25 mg per dose and it is usually estimated compleximetrically. The quantity of calcium
is limited to 1.3 mg per dose and is usually estimated by flame photometry.
4 Free formalin. Inactivation of bacterial toxins with formalin may lead to the presence
of small amounts of free formalin in the final product. The concentration, as estimated
by colour development with acetylacetone, must not exceed 0.02%.
5 Phenol concentration. When phenol is used to preserve a vaccine its concentration
must not exceed 0.25% w/v or, in the case of some vaccines, 0.5% w/v. Phenol is
estimated by the colour reaction with amino-phenazone and hexacyanoferrate.
3 Immunosera
Immunosera are preparations derived from the blood of animals, usually from the blood
of horses. To prepare an immunoserum a horse is injected with a sequence of spaced
doses of an antigen until a trial blood sample shows that the injections have induced a
high titre of antibody to the injected antigen. A large volume of blood is then removed
by venepuncture and collected into a vessel containing sufficient citrate solution to
prevent clotting. The blood cells are allowed to settle and the supernatant plasma is
drawn off. The plasma is then fractionated by the addition of ammonium sulphate
and the globulin fraction is recovered and treated with pepsin to yield a refined
immunoserum, Harms (1948). This refined immunoserum contains no more than a
trace of the albumin that was present in the plasma. The refined immunoserum is
titrated for the potency of its antibody content, diluted to the required concentration
and transferred into ampoules. Two or more monovalent immunosera may be blended
together to provide a multivalent immunoserum.
The quality of immunosera is controlled by potency tests and by conventional tests
for safety and sterility. The potency tests have a common design in that, in the case of
all immunosera, the potency is estimated by comparing the amount of an immunoserum
that is required to neutralize an effect of an homologous antigen with the amount of a
standard preparation that is required to achieve the same effect. Serial dilutions of the
immunoserum and of a standard preparation are made and to each is added a constant
amount of the homologous antigen. Each mixture is then inoculated into a group of
animals, usually guinea-pigs or mice, and the dilutions of the immunoserum and of the
standard which neutralize the effects of the antigen are noted. As the potencies of the
standard preparations are expressed in IU the potencies of the immunosera are determined
in corresponding units per millilitre {British Pharmacopoeia 1993).
Table 15.3 lists the immunosera for which there is a need, or a potential need, today
and indicates the required potencies of these immunosera and the salient features of the
potency assay methods.
Manufacture and quality control of immunological products 317
In each of the assays of potency the amount of the immunoserum and the amount of a corresponding
standard antitoxin that are required to neutralize the effects of a defined dose of the corresponding
toxin are determined. The two determined amounts and the assigned unitage of the standard antitoxin
are then used to calculate the potency of the immunoserum in International Units (IU).
Human immunoglobulins
Human immunoglobulins are preparations of the immunoglobulins, principally
immunoglobulin G (IgG), that are present in human blood. They are derived from the
plasma of donated blood and from plasma obtained by plasmapheresis. Normal
immunoglobulin, that is immunoglobulin that has relatively low titres of antibodies, is
prepared from pools of plasma obtained from not fewer than a thousand individuals;
specific immunoglobulins, that is immunoglobulins with a high titre of a particular
antibody, are usually prepared from smaller pools of plasma obtained from individuals
who have suffered recent infections or who have undergone recent immunization and
who thus have a high titre of a particular antibody. Each contribution of plasma to a
pool is tested for the presence of hepatitis B surface antigen (HBsAg), for antibodies to
human immunodeficiency viruses I and II (HIV I and II) and for antibodies to hepatitis
C virus in order to identify, and to exclude from a pool, any plasmas capable of
transmitting infection from donor to recipient.
The immunoglobulins are obtained from the plasma pools by fractionation methods
that are based on ethanol precipitation in the cold with rigorous control of protein
concentration, pH and ionic strength (Cohn et al. 1946; Kistler & Nitschmann 1962).
Some of the fractionation steps may contribute to the safety of immunoglobulins by
inactivating or removing contaminating viruses that have not been recognized by testing
of the blood donations. The immunoglobulin may be presented either as a freeze-dried
or a liquid preparation at a concentration that is 10 to 20 times that in plasma. Glycine
may be added as a stabilizer and thiomersal as a preservative.
The quality control of immunoglobulins includes potency tests and conventional
tests of safety and sterility. The potency tests consist of neutralization tests that parallel
those used for the potency assay of immunosera, except that in the cases of some
immunoglobulins the assays are made in vitro. In addition to the safety and sterility
tests, total protein is determined by nitrogen estimations, the protein composition by
318 Chapter 15
Table 15.3 Immunosera used in the prevention of infections in
Immunoserum
Botulinum antitoxin
Diphtheria antitoxin
Tetanus antitoxin
Potency assay method
Neutralization of the lethal
effects of botulinum
toxins A, B and E in mice
Neutralization of the erythrogenic
effect of diphtheria toxin
in the skin of guinea-pigs
Neutralization of the
paralytic effect of tetanus
toxin in mice
humans
Potency requirement
500IUm|-
1
of type A
500IU mM of Type B
50IU ml-
1
of Type E
1000 IU ml"
1
if prepared
in horses
500IU mM if prepared in
other species
1000 IU ml"
1
for prophylaxis
3000 IU ml"
1
for treatment

In each of the assays of potency the amount of the immunoglobulin and the amount of a
corresponding standard preparation that are required to neutralize the infectivity or other biological
activity of a defined amount of virus or to neutralize a defined amount of a bacterial toxin are
determined. The two determined amounts and the assigned unitage of the standard preparation are
then used to calculate the potency of the immunoglobulin in International Units (IU). ELISA,
enzyme-linked immunosorbent assay.
cellulose acetate electrophoresis and molecular size by liquid chromatography. The
presence of immunoglobulins derived from species other than humans is excluded by
precipitin tests. Table 15.4 lists six human immunoglobulins and their requisite potencies
and indicates the methods in which the potencies are determined.
Tailpiece
Immunological products, notably vaccines, provide very secure protection from diseases
caused by small pathogenic entities such as bacterial toxins and many viruses. They
provide somewhat less secure protection from larger pathogens such as bacteria and
little protection, if any, from much larger pathogens such as malaria parasites. There
thus appears to be a rough inverse correlation between the efficacies of vaccines and
the sizes of the pathogens from which each vaccine is intended to provide protection.
This relationship may reflect the way in which vaccine-induced antibodies react with a
toxin or pathogen. Small homogeneous tetanus toxin molecules may be completely
invested by tetanus antitoxin molecules and thus wholly neutralized. In contradistinction
malarial parasites may be unaffected by antibodies that attach only to a cell component
that is not essential for the parasite's survival. It has recently been suggested (Beverly
1996) that in order to make effective vaccines against larger pathogens it may first be
necessary to identify those molecules in the pathogens that are essential for each
Manufacture and quality control of immunological products 319
Table 15.4 Immuno;
Immunoglobulin
Hepatitis B
Measles
Normal
Rabies
Tetanus
Varicella/zoster
globulins used in the prevention and treatment of infections in humans
Potency assay method
Radioimmunoassay or
enzymoimmunoassay
Neutralization of the
infectivity of measles virus
for cell cultures
Neutralization tests in cell
cultures or in animals
Neutralization of the
infectivity of rabies virus for mice
Neutralization of the
paralytic effect of tetanus toxin
in mice
ELISA in paralled with
a standard varicella-zoster
immunoglobulin
Potency requirement
Not less than lOOIUmh
1
Not less than 50IU ml"
1
Measurable amounts of one
bacterial antibody and of one
viral antibody for which there
are international standards
Not less than 150111ml-
1
Not less than SOIUml"
1
Not less than lOOIUmh
1
pathogen's survival. A vaccine containing such molecules might induce antibodies to a
pathogen's essential molecules and thus provide immunity against larger pathogens
comparable with that provided by the vaccines against toxins and small pathogens.
The cost of the vaccines used in the routine immunization of infants, children and
adolescents is roughly equivalent to the cost of 100 loaves of bread. In the industrialized
countries that is a small price to pay for what is virtually life-long protection from
diphtheria, tetanus, whooping-cough, H. Influenzae type B infection, poliomyelitis,
measles, mumps and rubella. In many developing countries it is a price far beyond the
reach of either individuals or health authorities but a price that is in large part borne by
the World Health Organization's Expanded Programme of Immunization.
Further reading
Beverley P.C.L. (1996) A job in time. MRC News Winter, 1996.
British Medical Association and Pharmaceutical Press (1997) The British National Formulary. London:
BMA. (This publication contains a useful section on immunological products. New editions appear
at intervals.)
British Pharmacopoeia (1993) London: Her Majesty's Stationery Office. (The British Pharmacopoeia
contains edited versions of the monographs for immunological products that appear in the European
Pharmacopoeia.)
Cohn E.J., Strong L.E., Hughes W.L., Hulford D.J., Ashworth J.N., Melin M. & Taylor H.I. (1946)
Preparation and properties of serum proteins IV. J Am Chem Soc, 68, 459-475.
Datapharm Publications Ltd (1996) The Data Sheet Compendium. London. (This publication contains
reproductions of the Data Sheets of immunological products that are licensed by the Medicines
Control Authority.)
Finney D.J. (1971) Probit Analysis. London: Cambridge University Press.
Harms A.J. (1948) The purification of antitoxic plasmas by enzyme treatment and heat denaturation.
Biochem J, 42, 340-347.
Kistler P. & Nitschmann HS. (1962) Large scale production of human plasma fractions. Vox Sang, 7,
414-424.
Sheffield F. (1990) The measurement of immunity. In: Topley and Wilson's Principles of Bacteriology,
Virology and Immunity (eds M.T. Parker & L.H. Collier), 8th edn. pp. 437-448. London: Edward
Arnold.
Vaccination and immunization
1 Introduction 6.1
6.2
2 Spread of infection
2.1 Common source infections 6.2.1
2.2 Propagated source infections 6.2.2
6.2.3
3 Objectives of a vaccine/immunization 6.2.4
programme 6.3
3.1 Severity of the disease 6.4
3.2 Effectiveness of the vaccine/
immunogen 6.4.1
3.3 Safety 6.4.2
3.4 Cost 6.4.3
3.5 Longevity of the immunity 6.4.4
4 Classes of immunity 6.5
4.1 Passive acquired immunity
4.2 Active acquired immunity
7
5 Classes of vaccine
5.1 Live vaccines 8
5.2 Killed and component vaccines
9
6 Routine immunization against
infectious disease
Introduction
People rarely suffer from the same infectious disease twice. When such re-infection
does occur it is usually either with an antigenically modified strain (common cold,
influenza), the patient is immunocompromised (immunosuppressive drugs, immu-
nological disorders) or a long time has elapsed since the first infection. Alternatively
the patient may have failed to eliminate the primary infection which has then remained
latent and emerges in a modified or similar form (herpes simplex, cold sores; herpes
zoster, chickenpox). Immunity towards re-infection was recognized long before
the discovery of the causal agents of infectious disease. Efforts were therefore made
towards developing treatment strategies that might generate immunity to infection.
An early development was the attempted control of smallpox (Variola major) through
the deliberate introduction, into the skin of healthy individuals, of material taken
from active smallpox lesions. Such treatments produced single localized lesions and
commonly, but not always, protected the recipient from contracting full-blown smallpox.
The process became known as variolation, and, unknown to its practitioners, attenuated
the disease through changing the route of infection of the causal organism. Unfortunately,
occasional cases of smallpox resulted from such treatment. Further developments
Poliomyelitis vaccination
Measles, mumps and rubella
vaccination (MMR)
Measles
Mumps
Rubella
MMR vaccine
Tuberculosis
Diphtheria, tetanus and pertussis (DTP)
immunization
Diphtheria
Tetanus
Pertussis (whooping-cough)
DTP vaccine combinations and
administration
Haemophilus influenza Type B (HiB)
immunization
Juvenile immunization schedule
Immunization of special risk groups
Further reading
Vaccination and immunization 321
16
recognized that immunity developed towards one disease often brings with it cross-
immunity towards another related condition. Cowpox is a disease of cattle that can be
transmitted to man. Symptoms are similar but less severe than those of smallpox.
Material taken from active cowpox (Vaccinia) lesions was therefore substituted into
the variolation procedures. This conferred much of the protection against smallpox
that had become associated with variolation but without the associated risk. Edward
Jenner's discovery, made over two centuries ago, became known as vaccination
and heralded a new era in disease control. The term vaccination is now widely used
to describe prophylactic measures that use live microorganisms or their products
to induce immunity. The more general term immunization describes procedures that
induce immunity in the recipient but which do not necessarily involve the use of
microorganisms. Nowadays vaccination and immunization procedures are used not
only to protect the individual against infection but also to protect communities
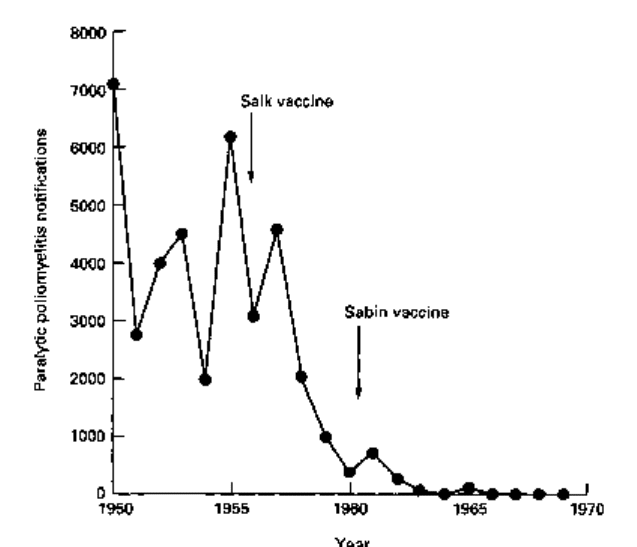
against epidemic disease. Such public health measures have met with spectacular
success as illustrated in Fig. 16.1 for the incidence of paralytic poliomyelitis. In
instances, where there is no reservoir of the pathogen other than in infected
individuals, and survival outside the host is limited (i.e. smallpox, poliomyelitis and
measles) then such programmes, worldwide, have the potential to eradicate the disease
permanently.
Fig. 16.1 Reported incidence of paralytic poliomyelitis in England and Wales during the 1950s and
1960s. After introduction of vaccination programmes the incidence of disease dropped from an
endemic incidence of ca. 5000 cases per year to fewer than 10.
322 Chapter 16
2
Spread of infection
Infectious diseases may either be spread from a common reservoir of the infectious
agent that is distinct from diseased individuals (common source) or they might transfer
directly from a diseased individual to a healthy one (propagated source).
2.1 Common source infections
In common source infections, the reservoir of infection might be animate (i.e. insect
vectors of malaria and yellow fever) or they might be inanimate (infected drinking
water, cooling towers, contaminated food supply). In the simplest of cases the source
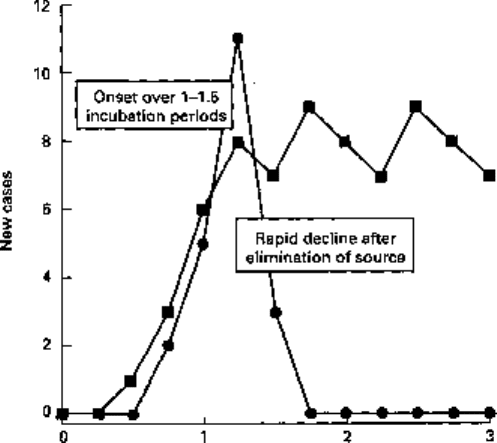
of infection is transient (i.e. food sourced to a single retail outlet or to an isolated event
such as a wedding reception). In such instances the onset of new cases is rapid,
phased over 1-1.5 incubation periods, and the decline in new cases closely follows the
elimination of the source (Fig. 16.2). This leads to an acute outbreak of infection limited
socially and geographically to those linked with the source. Such an incident was
epitomized by the outbreak of Escherichia coli 0157 infections, in Lanarkshire, in the
winter of 1996.
If the source of the infection persists, after onset, then the incidence of new cases is
maintained at a level which is commensurate with the infectivity of the pathogen and
the frequency of exposure of individuals. In this manner, if cases of the variant
Creutzfeldt-Jacob disease (vCJD), first recognized in the mid-1990s, relates to human
exposure to bovine spongiform encephalopathy-infected beef in the early 1980s, then
Incubation periods (time)
Fig. 16.2 Incidence pattern for common-source outbreaks of infection where the source persists (•)
and where it is short-lived (•).
Vaccination and immunization 323
2.2
the incidence of vCJD will increase over 1-1.5 incubation periods (i.e. 10-15 years)
and be sustained for many years before a decline. In such a scenario vCJD, related to a
single common-source outbreak, would persist well into the next millenium.
For those infectious diseases that are transmitted to humans via insect vectors the
onset and decline phases of epidemics are rarely observed other than as a reflections of
the seasonal variation in the prevalence of the insect. Rather, the disease is endemic
within the population group and has a steady incidence of new cases. Diseases such as
these are generally controlled by public health measures and environmental control of
the vector with vaccination and immunization being deployed to protect individuals
(e.g. yellow fever vaccination).
Propagated source infections
Propagated outbreaks of infection relate to the direct transmission of an infective
agent from a diseased individual to a healthy, susceptible one. Mechanisms of such
transmission were described in Chapter 4 and include inhalation of infective aerosols
(measles, mumps, diphtheria), direct physical contact (syphilis, herpes virus) and, where
sanitation standards are poor, through the introduction of infected faecal material into
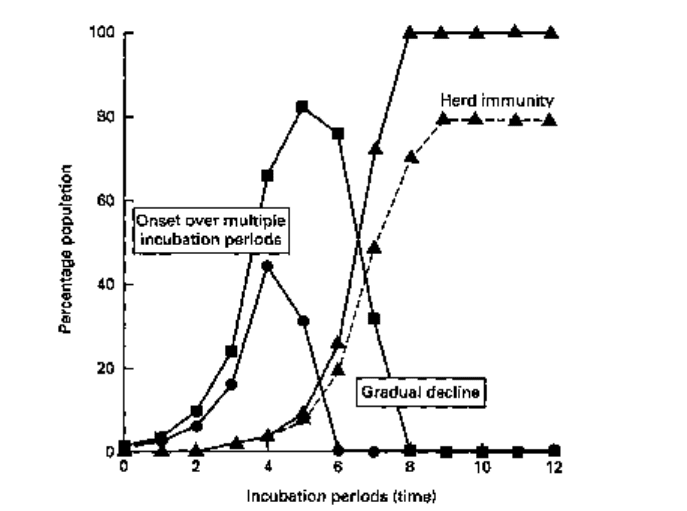
drinking water (cholera, typhoid). The ease of transmission, and hence the rate of onset
of an epidemic (Fig. 16.3) relates not only to the susceptibility status, and general state
of health of the individuals but also to the virulence properties of the organism, the
route of transmission, the duration of the infective period associated with the disease,
Fig. 16.3 Propagated outbreaks of infection showing the incidence of new cases (•), diseased
individuals (•), and recovered immune (A). The dotted line indicates the incidence pattern for an
incompletely mixed population group.
324 Chapter 16
