Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

7.1
Fungicidal activity
Fungal spores or mycelium may be added to the solution under test. At selected time
intervals, samples can be subcultured into suitable media and the presence or absence
of growth noted after incubation. A quantitative assessment similar to that described
for bactericidal activity (section 3.2, Table 11.3) can also be undertaken.
7.2 Fungistatic activity
Both the liquid and the solid dilution tests described above for bacteria (sections 3.6.1
and 3.6.4) may be used; suitable media must, of course, be employed.
7.3 Choice of test organism
For the evaluation of preparations to be used against pathogenic fungi, suitable cultures
of these pathogens should be used. To test substances intended to inhibit general
contaminants, cultures of common fungi obtained conveniently by exposing Petri dishes
of solid media to the atmosphere may be used, or alternatively dust or soil may be used
as a source of a mixed inoculum.
8 Virucidal activity
The testing of disinfectants for virucidal activity is not an easy matter. As pointed out
earlier (Chapter 3), viruses are unable to grow in artificial culture media and thus some
other system, usually employing living cells, must be considered. One such example is
tissue culture, but not all virus types can propagate under such circumstances and so an
alternative approach has to be adopted in specific instances. The principles of such
methods are given below.
8.1 Tissue culture or egg inoculation
A standardized viral suspension is exposed, in the presence of yeast suspension, to
appropriate dilutions of disinfectant in WHO hard water. At appropriate times, dilutions
are made in inactivated horse serum and each dilution is inoculated into tissue cell
culture or embryonated eggs (as appropriate for the test virus). The drop in infectivity
of the treated virus is compared with that of the control (untreated) virus.
Since disinfectant itself might be toxic to the tissue culture or eggs, a toxicity test
must also be carried out. Here, appropriate dilutions of disinfectant are mixed with
inactivated horse serum and inoculated into tissue cells or eggs (as appropriate). These
are examined daily for damage.
8.2 Plaque assays
Plaque assays, at present, apply to only a very limited number of viruses, e.g. poliovirus,
herpes virus, human rotavirus. The principle of these assays is as follows: test virus is
dried on to coverslips which are immersed in various concentrations of test disinfectant
Evaluation of non-antibiotic antimicrobial agents 245
for various time intervals and a plaque-counting method used to determine surviving
viral particles. The plaques are similar to those described in Chapter 3, except that a
host cell other than bacteria (Chapter 3) has to be employed.
For assaying herpes virus, monolayers of baby hamster kidney (BHK) cells are
used. Virus titre is expressed as the number of plaque-forming units (pfu) per millilitre
before and after exposure to a disinfectant, so that the virucidal efficacy of the test
agent can be determined. A diagrammatic representation is given in Fig. 11.7.
3.8.3 'Acceptable' animal model
The hepatitis B virus (HB V) does not grow in tissue culture and an 'acceptable' animal
model has been found to be the chimpanzee. This is observed for clinical infection
after inoculation with treated and untreated virus, care being taken in the test series that
residual disinfectant is removed by adequate means before inoculation into the animal.
This procedure is limited by the number of animals that can be used and by the
strictures imposed by a humane approach. HBV has not yet been transmitted to non-
primate animals.
3.8.4 Duck hepatitis B virus: a possible model of infectivity of human hepatitis B virus
Duck hepatitis B virus (DHBV) has been proposed as a possible model for the
inactivation of human HBV by chemical disinfectants. The principle of the test method
uses viral DNA polymerase (DNAP) as a target, total inhibition in vitro of DNAP by
chemical disinfectants being predictive of inactivation of infectivity in vivo.
3.8.5 Immune reaction
Three types of particles are associated with HBV: small spherical particles, 22 nm in
diameter; tubular particles, also having a diameter of 22 nm; and larger spherical particles
(42 nm diameter) known as the Dane particles. The Dane particle alone has a typical
virus structure and appears to be infectious but is the least common form. It consists of
246 Chapter 11
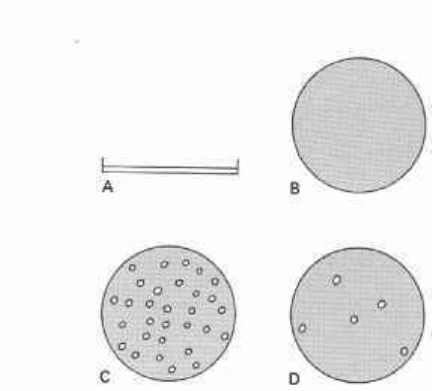
Fig. 11.7 A, diagrammatic representation of
plaque assay for evaluating virucidal activity
and B, monolayers of baby hamster kidney
(BHK) cells; C, virus titre: untreated virus
(o represents a plaque-forming unit, pfu, in
BHK cells): D, virus titre: disinfectant-
treated virus (before plating onto BHK, the
disinfectant must be neutralized in an
appropriate manner). Note the greatly
reduced number of pfu in D, indicative of
fewer uninactivated virus particles than in C.
a complex, double-layered sphere with an electron-dense core. It contains partially
double-stranded circular DNA and is regarded as the putative virion (for further
information on virions see Chapter 3). The Dane particles contain three antigens:
hepatitis B surface antigen (HBsAg) which is also present on 22-nm particles, hepatitis
B core antigen (HBcAg) found in the inner core and hepatitis B e antigen (HBeAg)
found in the core and responsible for infectivity.
The specific immunological detection of the HBV surface antigen (HBsAg) is
considered as being presumptive evidence for the presence of viable HBV. The
hypothesis, then, on which this method is based is that if the disinfectant can destroy
the reactivity of the HBsAg, it can also destroy the infectivity of HBV. A problem with
some disinfectants, e.g. formaldehyde and glutaraldehyde, is that their actions are
essentially fixative in nature. The HBsAg immunological reaction is thus not destroyed
at concentrations known to be high enough to kill the most resistant forms (bacterial
spores) of microorganisms. Furthermore, concentrations of disinfectants necessary to
inactivate HBsAg within a reasonable period of time are often comparatively high.
This type of procedure may thus suggest that an unnecessarily high disinfectant
concentration (so-called overkill) may be employed in practice to achieve a virucidal
effect.
3.8.6 Virus morphology
The serum from patients with clinical symptoms of hepatitis B commonly contain
three distinct structures that possess HBsAg (section 3.8.5 above). The effects of different
concentrations of various disinfectants on the structure of Dane particles have been
studied, but it is unlikely that morphological changes can be related to virucidal activity.
3.8.7 Endogenous reverse transcriptase
The human immunodeficiency virus (HIV; lymphadenopathy-associated virus, LAV;
human T-cell lymphotrophic virus type 3, HTLV III) is responsible for acquired immune
deficiency syndrome (AIDS; see Chapter 3). Because of the hazard and difficulties of
growing the virus outside humans, a different approach has to be examined for
determining viral sensitivity to disinfectants.
Studies have demonstrated that one such method is to examine the effects of dis-
infectants on endogenous RNA-dependent DNA polymerase (i.e. reverse transcriptase)
activity. In essence, HIV is an RNA virus; after it enters a cell the RNA is converted to
DNA under the influence of reverse transcriptase. The virus induces a cytopathic effect
on T lymphocytes, and in the assay reverse transcriptase activity is determined after
exposure to different concentrations of various disinfectants. However, it has been
suggested that monitoring residual viral reverse transcriptase activity is not a satisfactory
alternative to tests whereby infectious HIV can be detected in systems employing fresh
human peripheral blood mononuclear cells.
3.8.8 Bacteriophage
A model for evaluating virucidal agents has been described which employs
Evaluation of non-antibiotic antimicrobial agents 247
bacteriophages as indicator organisms. Bacteriophages used include those infecting
Escherichia coli, Bactewides fragHis and Pseudomonas aeruginosa.
4 Semi-solid antibacterial preparations
The use of the term 'semi-solid' has been coined to embrace a group of pharmaceutical
preparations known as pastes, ointments, creams and gels. The chief feature which
distinguishes the first three is their viscosity or, to use a more descriptive word, their
stiffness, which decreases in the order: paste, ointment, cream. They may consist of an
intimate mixture of the active agent with either an oleaginous base or, alternatively, an
emulsion with either water or an oleaginous substance as a continuous phase. Gels are
preparations in which the base is usually a carbohydrate polymer (starch, pectin,
methylcellulose, tragacanth, sterculia gum) and water, or more rarely having a base
of protein origin, such as gelatin, with a suitable quantity of water. More recently
polyethylene glycols and other organic polymers have been used.
When formulating antibacterial preparations it is imperative to realize that the
properties of the base may seriously modify the antibacterial activity of the medicament.
It is quite useless to formulate a well-proven antiseptic into an otherwise elegant
pharmaceutical preparation without determining if the final formulation is, itself, an
effective antibacterial agent.
4.1 Tests for bacteriostatic activity
The first official test was published by the Food, Drug and Insecticide Administration
of the US Department of Agriculture, in which portions of the preparation were placed
on the surface of nutrient agar inoculated with Staph, aureus. After incubation the
zones of inhibition, if any, around the preparation were measured. This test was modified
later by incorporating 10% of horse serum in the agar 'to simulate conditions in a
wound' and a control consisting of unmedicated base was also used in each experiment.
This test is known as the cup-plate test (see also section 3.6.3 and Fig. 11.5).
In addition to placing the test preparation onto sectors of seeded agar, it may be
placed in a trough cut in uninoculated agar and test organisms streaked in parallel
lines up to the edge of the trough. Failure to grow up to the edge is indicative of
inhibition.
Thus, the cup-plate method is useful to test several preparations or varying
formulations of the same preparation against one organism under identical conditions,
and the ditch-plate method enables one preparation to be tested against several organisms
(see Fig. 11.5A,B).
4.2 Tests for bactericidal activity
A number of tests have been described which imitate, at least in part, the principle of
the phenol coefficient test for liquid disinfectants. A culture of the test organism is
mixed intimately with the semi-solid preparation, and the mixture subcultured by means
of a loop into a suitable broth designed to disperse the base and neutralize the antibacterial
activity of the medicament.
248 Chapter 11
Thus, the culture may be mixed and transferred to a hypodermic syringe sur-
rounded by a constant-temperature jacket; at desired intervals, the mixture is
subcultured by ejecting small volumes from the syringe nozzle into subculture
medium.
A technique, devised by one of the authors (W.B.H.), was designed to test the
preparation when spread on to an infected surface. The surface of a nutrient agar plate
was inoculated evenly with the test organism and incubated to produce an even surface
growth. The preparation under test was spread evenly upon this, and at selected time
intervals a core of agar, cells and preparation were removed with a sterile cork-borer
and the disc of agar and cell removed by means of a sterile needle and inoculated into
recovery medium, which was then incubated. As much of the preparation is removed
as is possible and care taken to ensure its dispersal in the medium. The organism should,
if still viable, grow through the back of the agar disc to give growth in the subculture
tube also.
Tests on skin
It is possible to also test semi-solid antibacterial preparations on the skin itself, as
described for liquid disinfectants (section 3.5.1). A portion of the skin—the backs of
the fingers between the joints is a useful spot—is treated with the test organism, the
preparation is then applied and after a suitable interval the area is swabbed and the
swab incubated in a suitable medium. Alternatively, the method employing pig skin,
described in section 3.5.1, may well be adapted to the problem of testing semi-solid
skin disinfectants.
General conclusions
It is suggested that, as a minimum routine for the final test of an alleged antibacterial
semi-solid formulation, the following be used.
1 The cup-plate technique for bacteriostatic activity (section 3.6.3).
2 A test for bactericidal activity.
3 A skin test.
For routine assessment of test formulations during development work the cup-plate
and ditch-plate methods are adequate.
Solid disinfectants
Solid disinfectants (disinfectant powders) usually consist of a disinfectant substance
diluted by an inert powder. For example phenolic substances adsorbed onto kieselguhr
form the basis of many disinfectant powders, while another widely used powder of
respectable antiquity is hypochlorite powder. Disinfectant or antiseptic powders for
use in medicine include substances such as acriflavine, or antifungal compounds such
as zinc undecenoate or salicylic acid mixed with talc.
Solid disinfectants may be evaluated in vitro by applying them to suitable test
organisms growing on solid medium. Discs may be cut from the agar and subcultured,
observing the usual precautions.
Evaluation of non-antibiotic antimicrobial agents 249
To test their inhibitory power, the powders may be dusted onto the surface of
seeded agar plates, using the inert diluent as a control and noting the extent of
growth.
Disinfectant and sanitary powders are the subject of a British Standard (BS
1013:1946), now withdrawn, which describes a method of determining the RW
coefficient of such powders. A weighed quantity was shaken with distilled water at
18°C for 30 minutes and this suspension was used in the test already described (section
3.1.1).
6 Evaluation of air disinfectants
One of the most potent routes for transmission of bacterial disease is via the air. Cross-
infection in hospital wards, infection in operating theatres, the transmission of disease
in closed spaces such as cinemas and other places of assembly, in the ward rooms and
crew's quarters of ships and in submarines are all well known. Of equal importance is
the provision of a bacteria-free environment for aseptic manipulations generally. Clearly,
the disinfection of atmospheres is a worthwhile field of study and to this end much
research has been done. It is equally clearly important to be able to evaluate preparations
claimed to be air disinfectants.
Heretofore the milieu on or in which the disinfectant has been required to act has
been either solid or liquid; now antibacterial action in the gas or vapour phase or in the
form of aerosol (colloidal) interaction must be considered, and this presents the problem
of determining the viable airborne population.
6.1 Determination of viable airborne microorganisms
The simplest way of assessing the viable microbial population of the air is to expose
Petri dishes containing a solid nutrient medium to the air, followed by incubation;
indeed this method was used in 1881 by Koch. Although this method does depend on
the organisms or organism-bearing particles actually falling on the plate by gravity it is
a method which is still used to assess the general cleanliness of air in pharmaceutical
factories where aseptic operations are taking place, in food processing areas or in hospital
wards. More positive data may be obtained, however, if a force other than gravity is
used to collect airborne particles.
An early attempt at quantification consisted of placing a Petri dish containing a
nutrient agar in a box beneath an inverted funnel, the stem of which passed out of the
box into the atmosphere. By applying a partial vacuum to the box, air was drawn in
through the stem of the funnel and impinged on the agar. The plate could be incubated
directly and developing colonies counted. Provided the air drawn in was metered, a
direct quantitative assessment of the viable airborne population could be made. This
idea led logically to the development of the slit sampler illustrated in Fig. 11.8. The
principle is similar to that described immediately above, but the Petri dish is placed on
a turntable which can be revolved at varying speeds and the funnel is replaced by a
cylinder in which the end nearest the nutrient medium is furnished with a slit ca. 2.5 mm
wide. The arrangement is set so that the slit runs parallel to a radius of the dish but
leaves a clear space around the circumference and at the centre of the plate. In operation,
250 Chapter 11
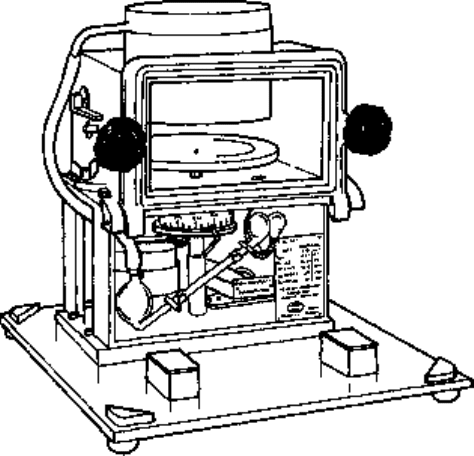
Fig. 11.8 Slit sampler (C.F. Casella & Co., Ltd).
a vacuum is applied to the chamber containing the turntable, air passes in through the
slit and the nutrient medium revolves so that the airborne particles, if any, are trapped
on the medium and spread in a sector over the medium.
Experimental evaluation
In brief, the experimental technique is to create a bacterial population in a close chamber,
obtain a quantitative assessment of the viable airborne bacterial population by means
of a suitable sampling device, submit the population to the disinfectant action, whether
ultraviolet light, chemical vapour or aerosol, and then determine the airborne population
at suitable intervals.
Preservatives
Preservatives may include disinfectant and antiseptic chemicals together with certain
compounds used almost exclusively as preservatives. They are added to many industrial,
including pharmaceutical, products which may, by their nature, support the growth of
bacteria and moulds causing spoilage of the product and possibly infection of the user.
In the field of pharmaceutical preservation, addition of an inhibitory substance to a
multidose injection (Chapter 21) or the prevention of growth in aqueous suspensions
of drugs intended for oral administration (Chapter 18) are prime examples.
Preservatives are widely employed in cosmetic preservation for lotions, creams
and shampoos. Preservation is also an important aspect of formulation in emulsion
paints and cutting fluids, i.e. fluids used to cool and lubricate lathe and drilling
tools.
Evaluation of non-antibiotic antimicrobial agents 251
7.1 Evaluation of preservatives
Potential chemical preservatives may be evaluated in the first place by the methods
outlined above, especially by determining MIC values (section 3.6) or by viable counts
(section 3.2). The RW, CM and KS tests (sections 3.1.1 and 3.1.2) have no relevance in
preservative evaluation. It will be recalled (section 2.5) that formula ingredients may
reduce the efficiency of a preservative which has shown up well in conventional tests
using culture media as the suspending fluid.
Emulsions, especially oil-in-water emulsions which, incidentally, figure widely
in cosmetic products, are especially prone to failure because the preservative may
partition into the oily phase of the emulsion while contaminants will flourish in the
aqueous phase now deprived of preservative by partitioning (see Chapter 18 for further
details).
The cardinal requirement, therefore, for preservative efficacy is the evaluation of
the finally preserved preparation and this may be performed by means of a challenge
test. In essence, the (hopefully) preserved product is deliberately inoculated (challenged)
with suitable test organisms and incubated and examined to see if the inoculum has
been able to grow or if its growth has been successfully suppressed. There has been
extensive debate on challenge testing and the subject has been reviewed by Cowen and
Steiger(1976).
The British Pharmacopoeia (1993) contains a test for efficacy of preservatives. In
essence, the product is deliberately challenged separately by the fungus Aspergillus
niger, the yeast Candida albicans and the bacteria Ps. aeruginosa and Staph, aureus.
These organisms represent potential contaminants in the environment in which products
are prepared, stored or used. Other organisms may be used in specified circumstances,
e.g. the osmophilic yeast, Zygosaccharomyces rouxii for preparations with a high sucrose
content, and E. coli for oral liquid preparations.
Different performance criteria are laid down for injectable and ophthalmic
preparations, topical preparations and oral liquid preparations. Inhibition of the challenge
organism is determined by viable counting techniques. The British Pharmacopoeia
(1993) should be consulted for full details of the experimental procedures to be used.
The United States Pharmacopeia (1995, 23rd edn) also gives procedures for
evaluating the efficacy of antimicrobial preservatives in pharmaceutical products.
7.2 Preservative combinations
The use of preservative combinations may be used to extend the range and spectrum of
preservation. Thus, in the series of alkyl esters of 4-hydroxybenzoic (/?-hydroxybenzoic)
acid (parabens), water solubility decreases in the order: methyl, ethyl, propyl and butyl
ester. By combining these products it is possible to achieve a situation where both the
aqueous and oil phase of an emulsion are protected.
Combinations may also be used to extend the spectrum of a preservative system.
Thus, the preservative Germall 115 has an essentially antibacterial activity and very
low, if not zero, antifungal activity. By combining Germall 115 with parabens, which
possess antifungal activity, a broader spectrum (antibacterial/antifungal) preservative
system is obtained.
252 Chapter 11
7.2.1
Synergy in preservative combinations
7.2.2
Very occasionally a combination of antimicrobial agents exhibits synergy. Synergy is
measured against a single microorganism and is exhibited when a combination of two
compounds exerts a greater inhibitory effect than could be expected from a simple
additive effect of the two compounds in the mixture.
Evaluation of synergy
Synergy may be evaluated and displayed by preparing mixtures of the two compounds
being investigated and determining their growth inhibitory power by means of an MIC
determination (section 3.6.1).
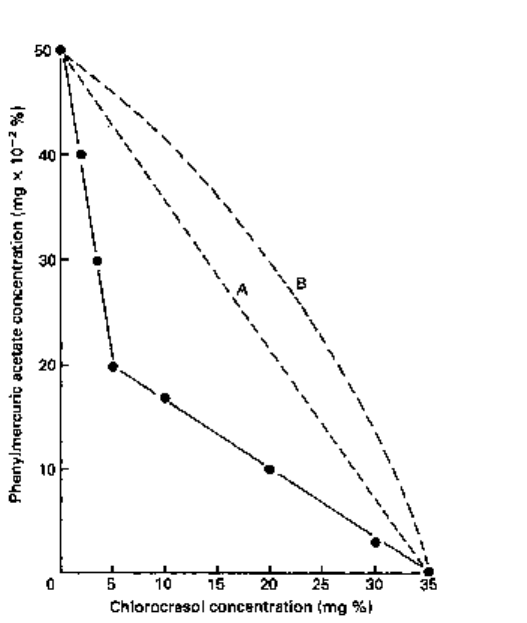
The results may be plotted in the form of a graph (called an isobologram) and an
example is given in Fig. 11.9. This graph may be interpreted as follows: 50 x 10
-2
mg%
and 35 mg% of phenylmercuric acetate and chlorocresol, respectively, used alone,
inhibits the growth of Staph, aureus. In combination, 20 x 10"
2
mg% of phenylmercuric
acetate and 5 mg% of chlorocresol inhibit the growth of this organism. Thus, growth
Fig. 11.9 Isobologram (•-•) drawn from minimum growth inhibitory concentrations (MIC values)
of chlorocresol and phenylmercuric acetate used alone and in combination against Staph, aureus,
showing synergy. A, result if combination was merely additive; B, result if combination was
antagonistic.
Evaluation of non-antibiotic antimicrobial agents 253
inhibition is obtained with a lower total quantity of preservative. If the combinations
were merely additive, the isobologram plot would follow the course of the dashed line
(A), and if antagonistic the dashed curve (B).
Synergy has been discussed in depth by Denyer et al. (1985) and Hodges & Hanlon
(1991).
7.2.3 Rapid methods
In many cases, especially in the food industry, it would be very useful if the performance
of a biocide or the extent of contamination of product, apparatus and working surfaces
could be deduced sooner than that provided by a method which depends on visible
microbial growth (12-24 hours). Many methods have been devised to secure a more
rapid result and have been designated rapid tests. They have recently been reviewed by
Denyer (1990).
Two such methods will be mentioned here.
1
Epifluorescence depends on the fact that certain dyes, acridine orange being widely
used, will stain cellular material. When examined in fluorescent light any living cells
present will fluoresce green or greenish yellow, whereas dead cells will appear orange
to red. The methods will be found in the literature under direct epifluorescent microscopy
(DEM) and direct epifluorescent filter technique (DEFT). In DEM, material suspected
of being contaminated or a sample in which living bacteria are sought are examined
directly; in DEFT the sample being examined is filtered and the residue on the filter
examined as above.
2 Bioluminescence. In another method, luminous bacteria, or bacteria not normally
luminous but which have been manipulated genetically to become luminous, are used.
Their death under chemical stress or presence in hygiene studies are assessed in a
sensitive light meter. A variant of this method depends on the fact that bacterial adenosine
triphosphatase (ATPase), present in bacteria, will catalyse the normal biological light-
producing reaction to give detectable light in a sensitive meter.
This is a very brief summary but is included as readers may come across these
methods or a reference to rapid methods in their general reading or work experience.
8 Appendix: British Standards
British Standards relating to disinfectants (date in brackets at end of an entry means that the Standard
was confirmed on that date without further revision).
(1986) 'Specification for black and white disinfectants'. BS 2462: 1986 [1991].
(1986) 'Glossary of terms relating to disinfectants'. BS 5283: 1986 [1991].
(1976) 'Aromatic disinfectant fluids'. BS 5197: 1976 [1991].
(1984) 'Method for determination of the antimicrobial activity ofQAC disinfectant formulations'. BS
6471: 1984 [1994].
(1990) 'Specification for QAC based aromatic disinfectant fluids'. BS 6424: 1984 [1990].
(1985) 'Method for determination of the Rideal-Walker coefficient of disinfectants'. BS 541: 1985
[1991].
(1986) 'Method for assessing the efficacy of disinfectants by the modified Chick-Martin test: BS 808:
1986 [1991].
254 Chapter 11
