Hjorth-Jensen M. Computational Physics
Подождите немного. Документ загружается.

12.3. SIMULATION OF MOLECULAR SYSTEMS 229
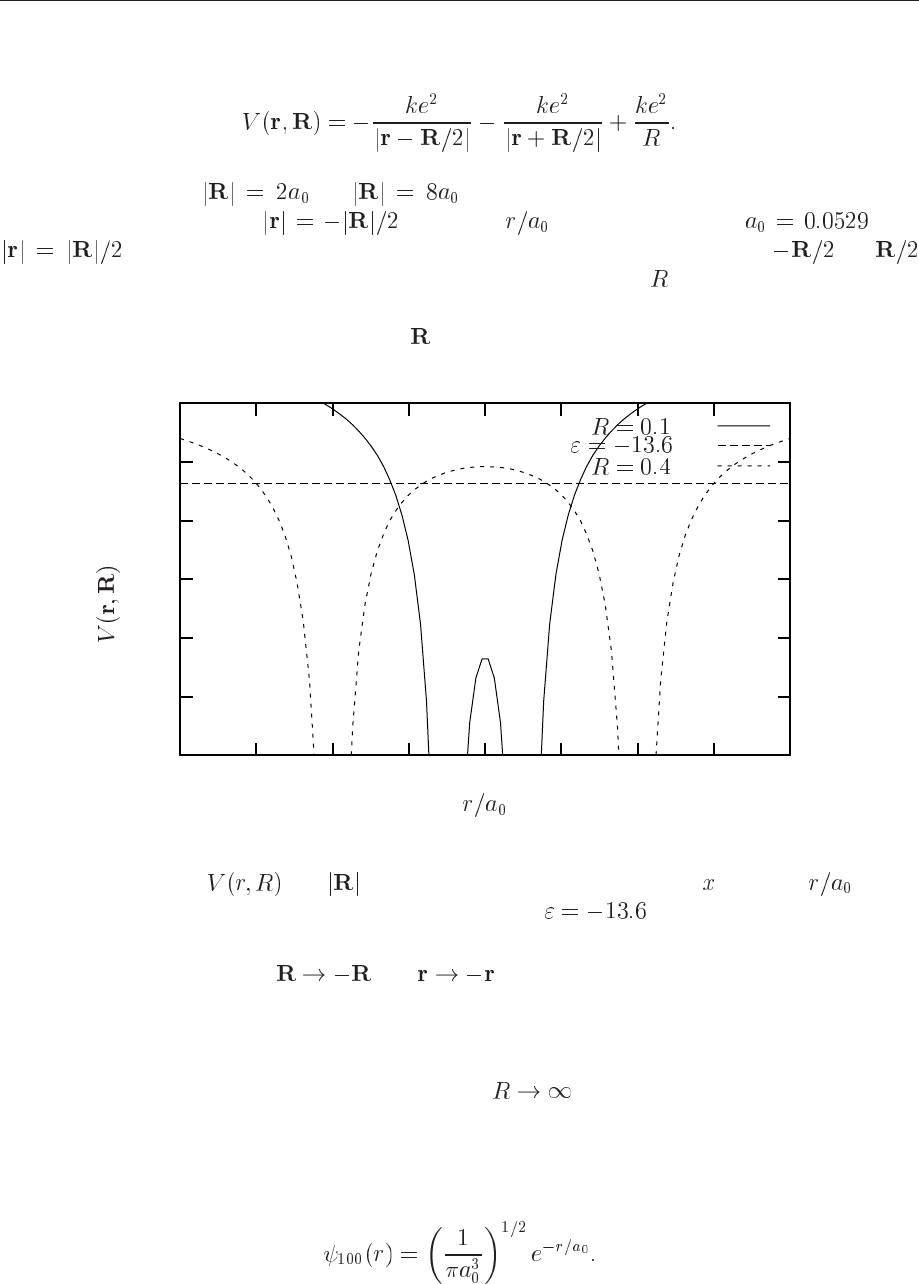
protons. In Fig. 12.5 we show a plot of the potential energy
(12.77)
Here we have fixed
og , being 2 and 8 Bohr radii, respectively. Note
that in the region between (units are in this figure, with ) and
the electron can tunnel through the potential barrier. Recall that og
correspond to the positions of the two protons. We note also that if is increased, the potential
becomes less attractive. This has consequences for the binding energy of the molecule. The
binding energy decreases as the distance
increases. Since the potential is symmetric with
nm
eV
nm
[eV]
86
42
0
-2-4
-6-8
0
-10
-20
-30
-40
-50
-60
Figure 12.5: Plot of
for =0.1 and 0.4 nm. Units along the -axis are . The
straight line is the binding energy of the hydrogen atom, eV.
respect to the interchange of
and it means that the probability for the electron
to move from one proton to the other must be equal in both directions. We can say that the
electron shares it’s time between both protons.
With this caveat, we can now construct a model for simulating this molecule. Since we have
only one elctron, we could assume that in the limit
, i.e., when the distance between the
two protons is large, the electron is essentially bound to only one of the protons. This should
correspond to a hydrogen atom. As a trial wave function, we could therefore use the electronic
wave function for the ground state of hydrogen, namely
(12.78)

230 CHAPTER 12. QUANTUM MONTE CARLO METHODS
Since we do not know exactly where the electron is, we have to allow for the possibility that
the electron can be coupled to one of the two protons. This form includes the ’cusp’-condition
discussed in the previous section. We define thence two hydrogen wave functions
(12.79)
and
(12.80)
Based on these two wave functions, which represent where the electron can be, we attempt at the
following linear combination
(12.81)
with
a constant.
12.3.2 Physics project: the H
molecule
in preparation
12.4 Many-body systems
12.4.1 Liquid
He
Liquid
He is an example of a so-called extended system, with an infinite number of particles.
The density of the system varies from dilute to extremely dense. It is fairly obvious that we
cannot attempt a simulation with such degrees of freedom. There are however ways to circum-
vent this problem. The usual way of dealing with such systems, using concepts from statistical
Physics, consists in representing the system in a simulation cell with e.g., periodic boundary
conditions, as we did for the Ising model. If the cell has length
, the density of the system is
determined by putting a given number of particles
in a simulation cell with volume . The
density becomes then .
In general, when dealing with such systems of many interacting particles, the interaction it-
self is not known analytically. Rather, we will have to rely on parametrizations based on e.g.,
scattering experiments in order to determine a parametrization of the potential energy. The in-
teraction between atoms and/or molecules can be either repulsive or attractive, depending on the
distance
between two atoms or molecules. One can approximate this interaction as
(12.82)
where
are some integers and constans with dimension energy and length, and with
units in e.g., eVnm. The constants and the integers are determined by the constraints
12.4. MANY-BODY SYSTEMS 231
[nm]
0.50.450.40.350.30.25
0.007
0.006
0.005
0.004
0.003
0.002
0.001
0
-0.001
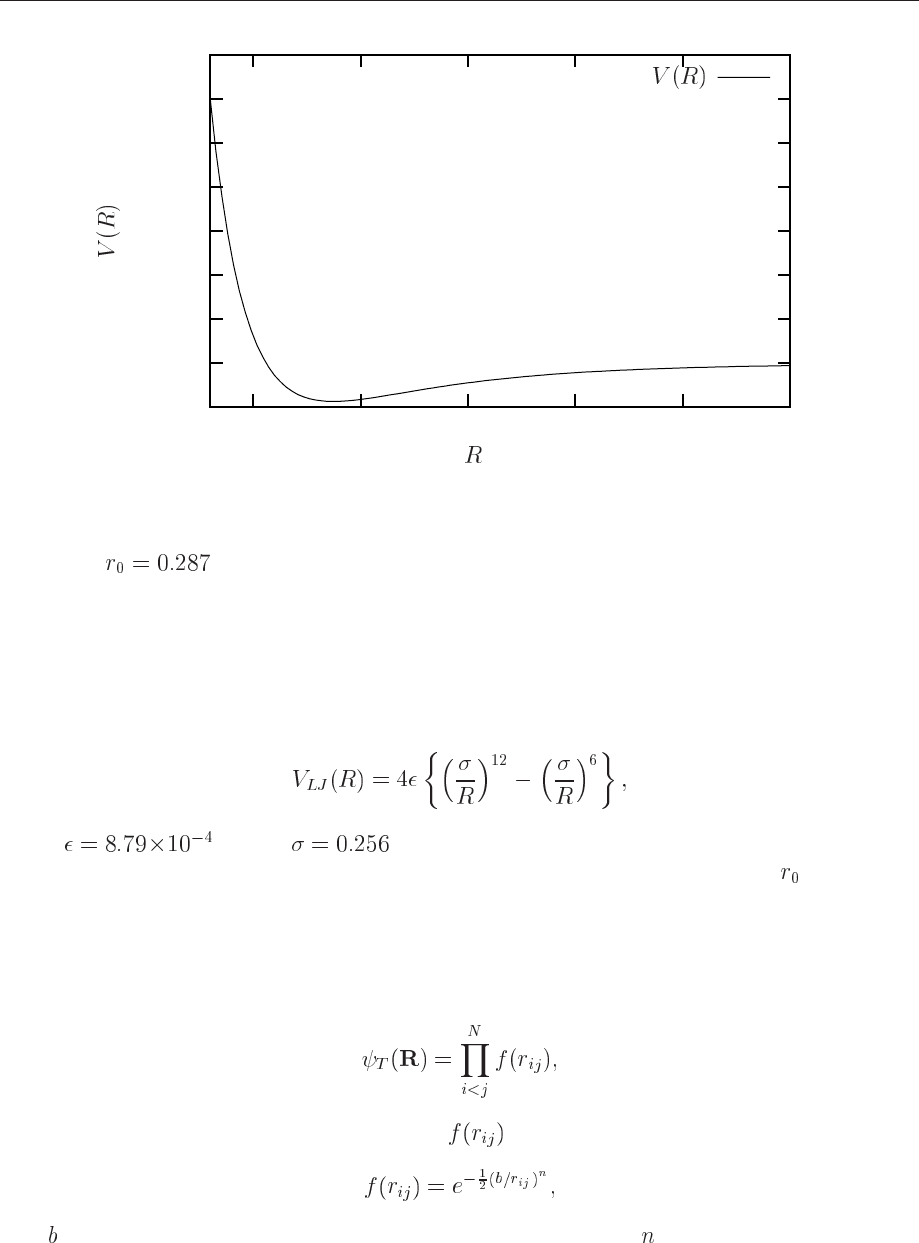
Figure 12.6: Plot for the Van der Waals interaction between helium atoms. The equilibrium
position is
nm.
that we wish to reproduce both scattering data and the binding energy of say a given molecule.
It is thus an example of a parametrized interaction, and does not enjoy the status of being a
fundamental interaction such as the Coulomb interaction does.
A well-known parametrization is the so-called Lennard-Jones potential
(12.83)
where
eV and nm for helium atoms. Fig. 12.6 displays this interaction
model. The interaction is both attractive and repulsive and exhibits a minimum at . The reason
why we have repulsion at small distances is that the electrons in two different helium atoms start
repelling each other. In addition, the Pauli exclusion principle forbids two electrons to have the
same set of quantum numbers.
Let us now assume that we have a simple trial wave function of the form
(12.84)
where we assume that the correlation function
can be written as
(12.85)
with
being the only variational parameter. Can we fix the value of using the ’cusp’-conditions
discussed in connection with the helium atom? We see from the form of the potential, that it
232 CHAPTER 12. QUANTUM MONTE CARLO METHODS
diverges at small interparticle distances. Since the energy is finite, it means that the kinetic
energy term has to cancel this divergence at small
. Let us assume that electrons and are very
close to each other. For the sake of convenience, we replace . At small we require then
that
(12.86)
In the limit
we have
(12.87)
resulting in
and thus
(12.88)
with
(12.89)
as trial wave function. We can rewrite the above equation as
(12.90)
with
For this variational wave function, the analytical expression for the local energy is rather simple.
The tricky part comes again from the kinetic energy given by
(12.91)
It is possible to show, after some tedious algebra, that
(12.92)
In actual calculations employing e.g., the Metropolis algorithm, all moves are recast into
the chosen simulation cell with periodic boundary conditions. To carry out consistently the
Metropolis moves, it has to be assumed that the correlation function has a range shorter than
. Then, to decide if a move of a single particle is accepted or not, only the set of particles
contained in a sphere of radius
centered at the referred particle have to be considered.
12.4.2 Bose-Einstein condensation
in preparation

12.4. MANY-BODY SYSTEMS 233
12.4.3 Quantum dots
in preparation
12.4.4 Multi-electron atoms
in preparation
Chapter 13
Eigensystems
13.1 Introduction
In this chapter we discuss methods which are useful in solving eigenvalue problems in physics.
13.2 Eigenvalue problems
Let us consider the matrix A of dimension n. The eigenvalues of A is defined through the matrix
equation
(13.1)
where
are the eigenvalues and the corresponding eigenvectors. This is equivalent to a
set of
equations with unknowns
W can rewrite eq (13.1) as
with being the unity matrix. This equation provides a solution to the problem if and only if the
determinant is zero, namely
which in turn means that the determinant is a polynomial of degree in and in general we will
have distinct zeros, viz.,
235
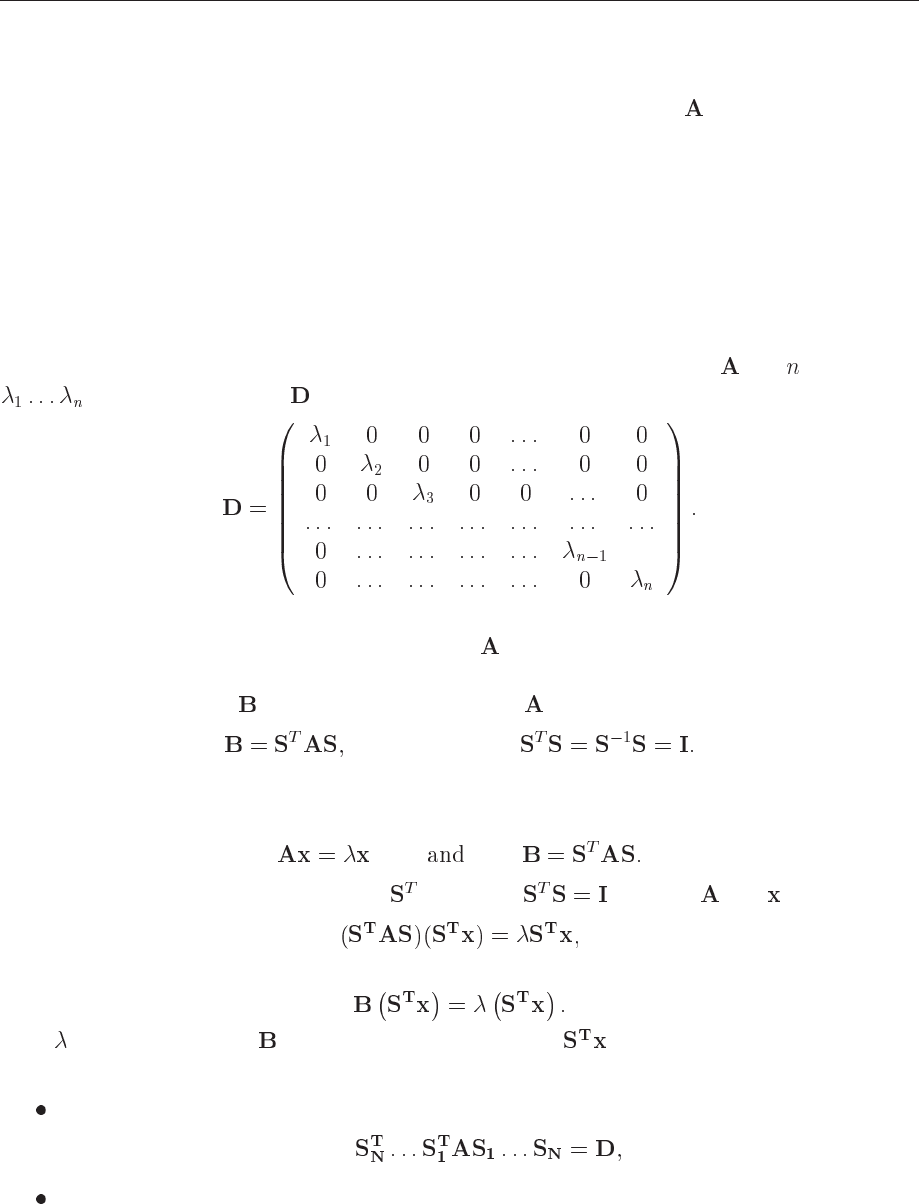
236 CHAPTER 13. EIGENSYSTEMS
Procedures based on these ideas con be used if only a small fraction of all eigenvalues and
eigenvectors are required, but the standard approach to solve eq. (13.1) is to perform a given
number of similarity transformations so as to render the original matrix
in: 1) a diagonal form
or: 2) as a tri-diagonal matrix which then can be be diagonalized by computational very effective
procedures.
The first method leads us to e.g., Jacobi’s method whereas the second one is e.g., given by
Householder’s algorithm for tri-diagonal transformations. We will discuss both methods below.
13.2.1 Similarity transformations
In the present discussion we assume that our matrix is real and symmetric, although it is rather
straightforward to extend it to the case of a hermitian matrix. The matrix
has eigenvalues
(distinct or not). Let be the diagonal matrix with the eigenvalues on the diagonal
(13.2)
The algorithm behind all current methods for obtaning eigenvalues is to perform a series of
similarity transformations on the original matrix to reduce it either into a diagonal form as
above or into a tri-diagonal form.
We say that a matrix
is a similarity transform of if
where (13.3)
The importance of a similarity transformation lies in the fact that the resulting matrix has the
same eigenvalues, but the eigenvectors are in general different. To prove this, suppose that
(13.4)
Multiply the first equation on the left by
and insert between and . Then we get
(13.5)
which is the same as
(13.6)
Thus
is an eigenvalue of as well, but with eigenvector .
Now the basic philosophy is to
either apply subsequent similarity transformations so that
(13.7)
or apply subsequent similarity transformations so that A becomes tri-diagonal. Thereafter,
techniques for obtaining eigenvalues from tri-diagonal matrices can be used.
Let us look at the first method, better known as Jacobi’s method.
13.2. EIGENVALUE PROBLEMS 237
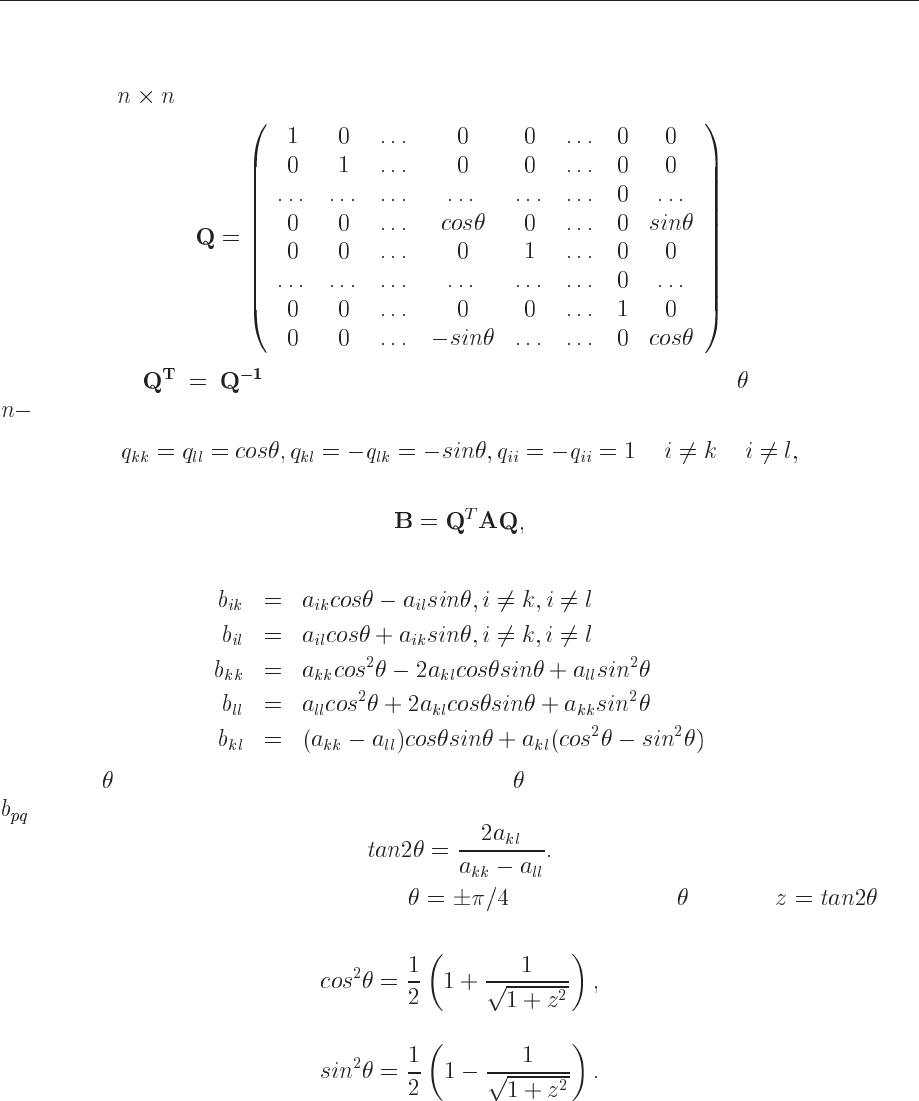
13.2.2 Jacobi’s method
Consider a (
) orthogonal transformation matrix
(13.8)
with property
. It performs a plane rotation around an angle in the Euclidean
dimensional space. It means that its matrix elements different from zero are given by
(13.9)
A similarity transformation
(13.10)
results in
(13.11)
The angle is arbitrary. Now the recipe is to choose so that all non-diagonal matrix elements
become zero which gives
(13.12)
If the denominator is zero, we can choose . Having defined through , we
do not need to evaluate the other trigonometric functions, we can simply use relations like e.g.,
(13.13)
and
(13.14)
The algorithm is then quite simple. We perform a number of iterations untill the sum over the
squared non-diagonal matrix elements are less than a prefixed test (ideally equal zero). The
algorithm is more or less foolproof for all real symmetric matrices, but becomes much slower
than methods based on tri-diagonalization for large matrices. We do therefore not recommend
the use of this method for large scale problems. The philosophy however, performing a series of
similarity transformations pertains to all current models for matrix diagonalization.

238 CHAPTER 13. EIGENSYSTEMS
13.2.3 Diagonalizationthroughthe Householder’s methodfortri-diagonalization
In this case the energy diagonalization is performed in two steps: First, the matrix is transformed
into a tri-diagonal form by the Householder similarity transformation and second, the tri-diagonal
matrix is then diagonalized. The reason for this two-step process is that diagonalising a tri-
diagonal matrix is computational much faster then the corresponding diagonalization of a general
symmetric matrix. Let us discuss the two steps in more detail.
The Householder’s method for tri-diagonalization
The first step consists in finding an orthogonal matrix
which is the product of orthog-
onal matrices
(13.15)
each of which successively transforms one row and one column of into the required tri-
diagonal form. Only transformations are required, since the last two elements are al-
ready in tri-diagonal form. In order to determine each
let us see what happens after the first
multiplication, namely,
(13.16)
where the primed quantities represent a matrix of dimension which will subsequentely be
transformed by
. The factor is a possibly non-vanishing element. The next transformation
produced by has the same effect as but now on the submatirx only
(13.17)
Note that the effective size of the matrix on which we apply the transformation reduces for every
new step. In the previous Jacobi method each similarity transformation is performed on the full
size of the original matrix.
After a series of such transformations, we end with a set of diagonal matrix elements
(13.18)
and off-diagonal matrix elements
(13.19)
