Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.

19.7 Random mixture of atoms 435
G
m
/RT
(µ
B
–
o
µ
B
)/RT
0
0
0 1.0
−0.8
−0.5
x
B
0 1.0
x
B
(a) (b)
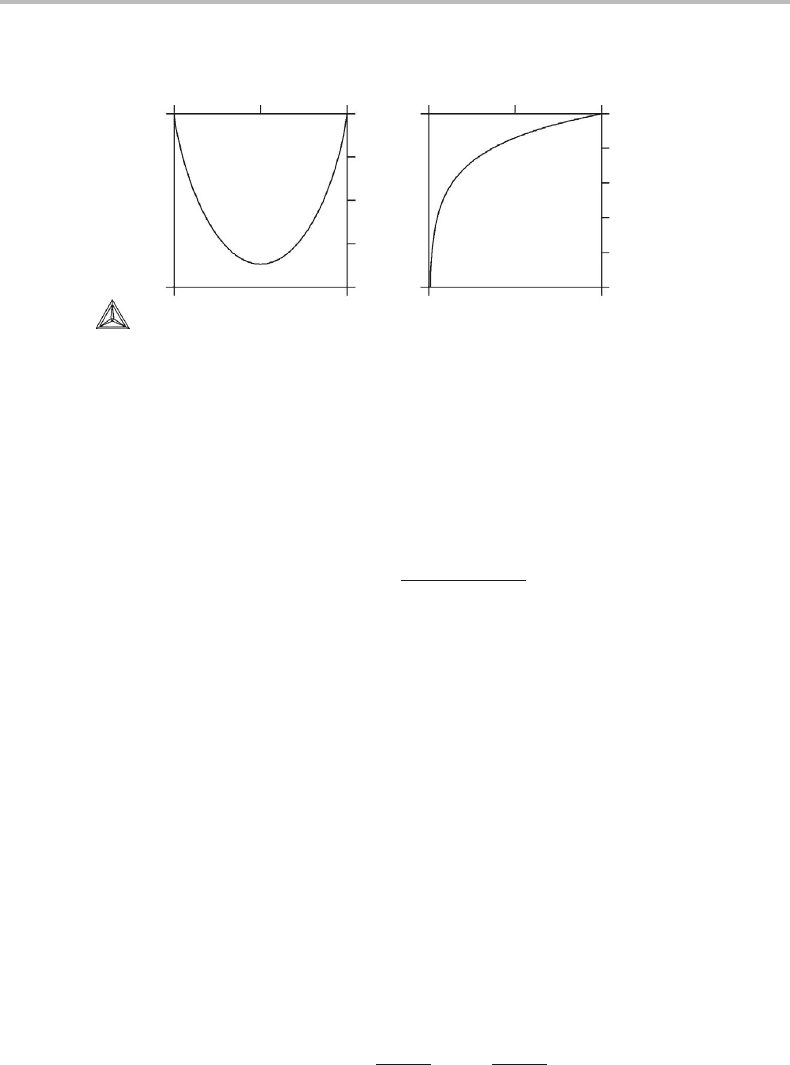
Figure 19.7 (a) Gibbs energy of mixing for an ideal, binary solution. (b) Variation of a chemical
potential in the same solution. In both cases the values have been normalized by dividing
with RT.
Let us consider a substance where all the sites are equivalent. Since all the various
atoms dissolved in the substance can substitute for each other, such a substance is called
a substitutional solution. The number of different ways in which N
A
atoms of A, N
B
atoms of B, N
C
atoms of C, etc., can be arranged is
W =
N !
N
A
!N
B
!N
C
! ...
. (19.40)
This randomness will give the following contribution to the entropy of the system accord-
ing to Boltzmann’s relation,
S/k = ln W = ln N! − ln N
i
!
∼
=
N ln N − N
i
ln N
i
=−N (N
i
/N) ln(N
i
/N) =−N x
i
ln x
i
,
(19.41)
since N is equal to N
i
and x
i
= N
i
/N.
We may construct a model for an ideal substitutional solution by requiring that there
is no energy change on mixing the atoms. The only effect on the Gibbs energy would
come from the configurational disorder,
G =−T S = NkTx
i
ln x
i
, (19.42)
where N is the number of moles of atoms. Let us consider an ideal system with one mole
of atoms. Using Eq. (4.6)weobtain
G
m
=−T S = N
A
kTx
i
ln x
i
= RTx
i
ln x
i
(19.43)
µ
j
= G
m
+
∂G
m
∂x
j
− x
i
∂G
m
∂x
i
= RT ln x
j
. (19.44)
Figure 19.7 gives G
m
and µ
B
for an ideal, binary A–B system. It is worth emphasizing
that µ
B
goes to −∞as x
B
goes to 0. As a consequence the G
m
curve should be vertical
at its end-points. However, this tendency starts to develop so close to the end-points that
it is hardly discernible.

436 Modelling of disorder
In the model for an ideal solution there is no internal variable because the situation
has been completely fixed by the assumption of random mixing. The situation will be
different if all the arrangements do not have the same energy. The disorder will then be
incomplete and one can introduce internal variables describing short- and long-range
order. This phenomenon will be described in Section 22.6.However, there is a class of
solution phases where all the sites are not equivalent and they may be, at the same time,
close to random in one sense and well ordered in another. They will be discussed in the
next sections.
Exercise 19.6
An A–B–C alloy is prepared by mixing x
C
moles of pure C with 1 – x
C
moles of an
A–B alloy. Calculate (a) the entropy of mixing in 1 – x
C
moles of the initial A–B alloy,
(b) the entropy due to the mixing of C into the A–B alloy and (c) the sum of the two
contributions.
Hint
The A content of the initial alloy can be expressed as x
A
/(x
A
+ x
B
)ifx
A
and x
B
refer to
the final, ternary alloy. The effect of mixing C with a mixture of A + Bisthe same as the
effect of mixing C with a single element which could very well consist of two isotopes.
Remember that x
A
+ x
B
= 1 − x
C
.
Solution
S(a) = (1 − x
C
) ·{x
A
/(x
A
+ x
B
) · ln[x
A
/(x
A
+ x
B
)] + x
B
/(x
A
+ x
B
) · ln[x
B
/(x
A
+
x
B
)]}=x
A
ln x
A
+ x
B
ln x
B
− (x
A
+ x
B
) ln(x
A
+ x
B
); S(b) = x
C
ln x
C
+ (1 − x
C
)
ln(1 − x
C
) = x
C
ln x
C
+ (x
A
+ x
B
) ln(x
A
+ x
B
); S(c) = x
A
ln x
A
+ x
B
ln x
B
+
x
C
ln x
C
.
19.8 Restricted random mixture
Many crystalline phases have more than one family of sites. It is convenient to describe
such phases with the use of sublattices and the state may be defined by giving the site
fractions (see Section 4.8).
y
t
j
= N
t
j
/N
t
. (19.45)
If the atoms in one sublattice are mixed with each other at random, they give the following
contribution to the entropy
S
t
=−kN
i
N
t
i
N
t
ln
N
t
i
N
t
=−R
i
y
t
i
ln y
t
i
. (19.46)

19.9 Crystals with stoichiometric vacancies 437
Since entropy is an extensive property which obeys the law of additivity, one could add
the contributions from the individual sublattices,
S
m
= S
t
=−R
s
a
s
i
y
s
i
ln y
s
i
. (19.47)
This expression holds for one mole of atoms if there are no vacant sites. a
s
is then defined
as N
s
/N, the fraction of all sites belonging to the s sublattice, and a
s
is unity. One
may instead like to consider one mole of formula units where the formula is written with
integers for all a
s
. Then a
s
is the number of atoms per formula unit.
This may be regarded as an ideal solution model for the particular type of crystalline
structure. If all the elements can go into all the sublattices with the same probability, this
model reduces to the previous ideal solution model because y
s
i
is then identical to x
i
.
Exercise 19.7
Consider a so-called Laves phase with two components each on two sublattices, (A, B)
1
(C, D)
2
. Calculate the entropy of mixing assuming random mixing within each sublattice
for equal amounts of A and B and also for C and D. Compare with the ideal entropy of
mixing when all four components are mixed randomly with each other.
Hint
Consider 3 moles of atoms, 0.5 of A, 0.5 of B, 1 of C and 1 of D.
Solution
S/R =−1 · (0.5ln0.5 + 0.5ln0.5) − 2 · (0.5ln0.5 + 0.5ln0.5) =+3ln2 = 2.08.
Foranideal solution we get
S/R = 3[(1/6) ln(1/6) + (1/6) ln(1/6) + (2/6) ln(2/6) +(2/6) ln(2/6)] = 3.99.
19.9 Crystals with stoichiometric vacancies
A binary crystal can vary in composition even if each component is restricted to its own
sublattice. An example is w¨ustite which has separate sublattices for Fe and O. The Fe
sublattice can have vacant sites and we should thus write the formula as (Fe,Va)
1
O
1
.We
shall call such vacancies stoichiometric vacancies because in the simplest case, their
number is fixed by the stoichiometric imbalance between the amounts of the elements.
The entropy of disorder in a crystal with any kind of vacancies is given by the expression
already discussed, if the vacancies are distributed at random on a sublattice. The vacancies
are then treated as the atoms of any element and one must define their site fraction, y
s
Va
.
On the other hand, the vacancies are not included in the mole fractions x
i
which give

438 Modelling of disorder
the composition of the crystal. Consequently, when evaluating x from y, one should not
include the vacancies in the summation. We can give the equation as
x
j
=
s
a
s
y
s
j
s
a
s
−
s
a
s
y
s
Va
. (19.48)
On the other hand, it is not always possible to calculate the y values from the overall
composition given by the set of x values. If each element is dissolved in one sublattice
only, then we can still evaluate the site fractions from the composition of the crystal
provided that there is at least one sublattice without vacancies. First we can identify that
sublattice as the one having the largest value of x
s
i
/a
s
. Let us denote that sublattice by
r. The site fractions in any sublattice t are then given by
y
t
j
= x
j
a
r
/a
t
i
x
r
i
. (19.49)
If y
t
j
in any sublattice is less than unity then the difference from unity gives the site
fraction of vacancies
y
t
Va
= 1 −
i
y
t
i
. (19.50)
This relation illustrates why they are called stoichiometric vacancies. The amount of
stoichiometric vacancies does not change directly with the temperature but there may
be an indirect effect if the phase is in equilibrium with another phase. The composition
may then vary by an exchange of atoms between the phases. The amount of vacancies
can also vary if the composition of the other phase varies by an action from the outside.
A typical example is an oxide in equilibrium with an atmosphere of variable P
O
2
.
Exercise 19.8
We know the composition of an oxide by chemical analysis as x
U
= 0.252, x
Pu
= 0.094
and x
O
= 0.654. Calculate the site fractions under the assumption that the oxide is a
so-called stoichiometric phase with only a small deviation from stoichiometry.
Hint
Suppose that U and Pu occupy one sublattice and O another. The number of sites can
then be estimated from the number of atoms supposing there are no vacancies. Vacancies
can then be assumed on one sublattice for stoichiometric reasons.
Solution
x
U
+ x
Pu
= 0.346; x
O
/(x
U
+ x
Pu
) = 0.654/0.346 = 1.89. This is close to 2. We may
thus assume that the formula is (U,Pu)
1
(O,Va)
2
and we find y
O
= 0.654 · 1/2 · 0.346 =
0.945; y
Va
= 0.055.

19.10 Interstitial solutions 439
19.10 Interstitial solutions
Phases with two or more sublattices are often called compounds or intermediary phases,
or intermetallic phases when appropriate. Such compounds may be strictly stoichiometric
or may show a deviation from stoichiometry, caused by defects. One such defect is the
vacancy. A related case is the interstitial solution where some solute atoms dissolve in
acrystalline solvent by going into interstitial sites that are initially empty. These sites
form a sublattice. An example is the solution of carbon in the bcc modification of iron,
so-called ferrite or α-Fe. The formula can be written as Fe
1
(Va,C)
3
. The entropy of
interstitial solutions can be treated with the method discussed in the preceding sections.
A more complicated case occurs when an element goes mainly into ordinary lattice sites
but some of its atoms go into interstitial sites.
In Section 4.8 we found that it was possible to derive an expression for the chemical
potential of a compound in a stoichiometric phase but not for the chemical potential of an
element. The situation is different for a phase with vacancies. For the interstitial solution
of C in α–Fe we can by standard methods derive expressions for µ
FeC
3
and µ
FeVa
3
.We
can thus evaluate µ
C
from
1
3
(µ
FeC
3
− µ
Fe
VA
3
) =
1
3
µ
Fe
+ µ
C
−
1
3
µ
Fe
− µ
Va
= µ
C
− µ
Va
= µ
C
. (19.51)
because we can usually assume that the chemical potential of vacancies is zero. Assuming
random mixing of carbon atoms and vacancies we obtain the following contributions to
the entropy of mixing, the Gibbs energy of mixing and the chemical potential of carbon
S
m
=−3R(y
Va
ln y
Va
+ y
C
ln y
C
) (19.52)
G
m
= 3RT(y
Va
ln y
Va
+ y
C
ln y
C
) (19.53)
µ
C
= µ
C
− µ
Va
=
1
3
G
m
+
∂G
m
∂y
C
−
y
i
∂G
m
∂y
i
− G
m
−
∂G
m
∂y
Va
+
y
i
∂G
m
∂y
i
=
1
3
∂G
m
∂y
C
−
∂G
m
∂y
Va
=
1
3
· 3RT ln
y
C
y
Va
= RT ln
y
C
1 − y
C
. (19.54)
Interstitial solutions will be further discussed in Section 21.2.
It is important to notice that the deviation from stoichiometry of a compound may also
be caused by some atoms going into sites of the ‘wrong’ sublattice (in that connection
called anti-sites) or into interstitial sites.
Exercise 19.9
The interstitial solution of carbon in fcc- and bcc-iron can be represented with the formula
(Fe)
1
(Va,C)
1
and (Fe)
1
(Va,C)
3
, respectively. The martensitic transformation from fcc to
bcc is very rapid and carbon is not able to take advantage of the additional interstitial
sites. Estimate how much larger the driving force for the martensitic transformation

440 Modelling of disorder
would have been if carbon could be distributed among all the sites available in bcc-Fe.
Make a numerical calculation for an Fe-C alloy with a molar content of 0.02 C.
Hint
For simplicity, suppose that the redistribution of carbon atoms has an effect on the Gibbs
energy through the ideal entropy term only. Consider a system with one mole of Fe atoms
and thus z
C
= 0.02/0.98 = 0.0204 moles of C atoms.
Solution
G = 3RT[(z
C
/3) ln(z
C
/3) + (1 − z
C
/3) ln(1 − z
C
/3)] − RT[z
C
ln z
C
+ (1 − z
C
)
ln(1 − z
C
)] = RT(−0.1219 + 0.0996) =−0.223RT. The driving force would inc-
rease with 0.223RT.

20
Mathematical modelling of
solution phases
20.1 Ideal solution
The thermodynamic properties of some solutions were illustrated graphically in
Section 7.1 and some mathematical expressions were also given. We shall now give
a more thorough discussion. In Section 18.7 we mentioned the possibility of modelling
a special physical effect, p, in a substance and defining the remaining part of the Gibbs
energy as the property of a hypothetical state, h, which does not have that physical effect,
G
m
= G
h
m
+ G
p
m
. (20.1)
This approach can also be applied to solution phases. The most important application
is the treatment of the thermodynamic effects of mixing the atoms in a solution. The
hypothetical state would then be a so-called mechanical mixture of the pure components
at the same temperature and pressure,
G
h
m
= x
i
o
G
i
. (20.2)
The physical effect, G
p
m
would here be the contribution due to the intimate mixing of the
atoms in a solution. It is usually denoted by
M
G
m
,which is thus defined by
G
m
= x
i
o
G
i
+
M
G
m
. (20.3)
The mechanical mixture may be regarded as a reference for the properties of a solution
and
M
G
m
gives the solution behaviour. It is called Gibbs energy of mixing but a better
name would have been ‘Gibbs energy of solution’ because it represents the effect of
forming a solution from a mechanical mixture. For a binary system the reference is a
straight line in the molar Gibbs energy diagram, in a ternary system it is a plane, etc.
Earlier, in Fig. 7.1,itwas demonstrated that one can use this straight line in a binary
system as the line of reference in a molar Gibbs energy diagram and the concept of Gibbs
energy of mixing was introduced.
The simplest model for the intimate mixing of atoms in a solution is based on the
assumption of random mixing and no particular interactions between atoms of different
kinds. For that case we have
G
m
= x
i
o
G
i
− T ·
M
S
ideal
m
, (20.4)

442 Mathematical modelling of solution phases
A
B
B
A
C
G
m
(a) (b)
Figure 20.1 Characteristic shapes of the molar Gibbs energy curves in (a) binary and (b) ternary
systems, caused by the entropy of mixing.
and expressions for the ideal entropy of mixing in phases with different structures were
derived in Sections 19.7 to 19.10.Asolution obeying such an equation may be called an
ideal solution. For an ideal substitutional solution we have
M
S
ideal
m
=−Rx
i
ln x
i
(20.5)
G
m
= x
i
o
G
i
+ RTx
i
ln x
i
, (20.6)
and we may also define a partial ideal entropy of mixing for component I,
M
S
ideal
i
=−R ln x
i
(20.7)
Sometimes one explicitly requires that the molar volume of an ideal solution should be
equal to a weighted average of the values for the pure components. It is easy to see that
Eq. (20.4) satisfies this requirement because the derivative with respect to P yields
V
m
= ∂G
m
/∂ P = x
i
∂
o
G
m
/∂ P = x
o
i
V
i
. (20.8)
The term −T ·
M
S
ideal
m
is important even in real, non-ideal solutions and it gives the Gibbs
energy its characteristic shape of a hanging rope for a binary system and a canopy for a
ternary. This has already been illustrated in a number of diagrams in Sections 7.1 and
7.10 and is again shown in Fig. 20.1.
Exercise 20.1
Suppose one knows that a binary solution is not ideal because the molar volume obeys
the relation: V
m
= x
A
o
V
A
+ x
B
o
V
B
+ x
A
x
B
·|
o
V
A
−
o
V
B
|. Examine the effect on G
m
if
o
V
A
−
o
V
B
= 2 · 10
−6
m
3
mol.
Hint
Represent G
m
with x
A
o
G
A
+ x
B
o
G
B
− T ·
M
S
ideal
m
+ x
A
x
B
L and evaluate the effect
on L.

20.2 Mixing quantities 443
Solution
Compare with V
m
= ∂G
m
∂ P = x
A
o
V
A
+ x
B
o
V
B
+ x
A
x
B
∂ L/∂ P. Thus, ∂ L/∂ P =
(
o
V
A
−
o
V
B
) and, neglecting the pressure dependence of
o
V
A
−
o
V
B
,wefindL = L
o
+
P ·
(
o
V
A
−
o
V
B
). At 1 bar (10
5
Pa) this effect would increase L by 10
5
· 2 ·10
−6
=
0.2Jmol. This is negligible in most cases.
20.2 Mixing quantities
It is convenient to compare the value of any molar quantity in a solution with the weighted
average of the values for the pure components and define a molar quantity of mixing,
M
A
m
,
A
m
= x
o
i
A
i
+
M
A
m
. (20.9)
The additional requirement for an ideal solution, mentioned in the preceding sec-
tion, may thus be formulated by stating that the volume of mixing,
M
V
m
, must be
zero.
Partial quantities of mixing can be defined relative to the value for the pure
component,
M
A
i
= A
i
−
o
A
i
. (20.10)
It is evident that all relations derived for A
m
and A
i
in Section 4.2 also apply to
M
A
m
and
M
A
i
because the references
o
A
i
will drop out from all such relations. We get, for
instance,
M
A
m
= A
m
− x
i
o
A
i
= x
i
A
i
− x
i
o
A
i
= x
i
(A
i
−
o
A
i
) = x
M
i
A
i
. (20.11)
We find, as for A
m
in Section 4.1,
M
A
j
=
M
A
m
+
∂
M
A
m
∂x
j
−
x
i
∂
M
A
m
∂x
i
. (20.12)
Exercise 20.2
Show that the partial enthalpy of mixing can be calculated from
M
H
j
= ∂(
M
G
j
/T )/
∂(1/T ).
Hint
We know H
j
= ∂(G
j
/T )/∂(1/T ). Use
M
H
j
= H
j
−
o
H
j
and
M
G
j
= G
j
−
o
G
j
.
Solution
∂(
M
G
j
/T )∂(1/T ) = ∂(G
j
/T )/∂(1/T ) − ∂(
o
G
j
/T )/∂(1/T ) = H
j
−
o
H
j
=
M
H
j
.

444 Mathematical modelling of solution phases
20.3 Excess quantities
The various mixing quantities were defined relative to the mechanical mixture of the
components. In the same way one may define excess quantities relative to an ideal
solution, e.g.
G
m
=
x
i
o
G
i
− T ·
M
S
ideal
m
+
E
G
m
. (20.13)
The excess quantities represent the deviation from ideal behaviour and are thus subject
to direct study and modelling. In this chapter we shall examine mathematical models
of the excess Gibbs energy and in the next chapter we shall discuss some very simple
physical models.
From the excess Gibbs energy one may define partial excess Gibbs energies and obtain,
by standard procedures,
E
G
j
=
E
G
m
+ ∂
E
G
m
/∂x
j
− x
i
∂
E
G
m
/∂x
i
(20.14)
E
G
i
= G
i
−
o
G
i
+ T ·
M
S
ideal
i
(20.15)
E
G
m
= x
i
E
G
i
(20.16)
E
S
i
= S
i
−
o
S
i
−
M
S
ideal
i
(20.17)
E
H
i
= H
i
−
o
H
i
=
M
H
i
. (20.18)
We note that it is not necessary to introduce the concept of excess enthalpy because
all enthalpy of mixing is in excess of the ideal solution behaviour. For a substitutional
solution we get
E
G
j
= G
i
−
o
G
i
− RT ln x
i
. (20.19)
E
S
i
= S
i
−
o
S
i
− R ln x
i
(20.20)
Exercise 20.3
Using a Maxwell relation at constant T and P we get ∂ G
i
/∂ N
j
= ∂
2
G/∂ N
i
∂ N
j
=
∂
2
G/∂ N
j
∂ N
i
= ∂G/∂ N
i
. Using this relation, show that ∂
E
G
i
/∂ N
j
= ∂
E
G
j
/∂ N
i
.
Hint
G
i
=
o
G
i
+ RT ln x
i
+
E
G
i
; ∂ x
i
/∂ N
j
=−x
i
/N; ∂x
j
/∂ N
i
=−x
j
/N.
Solution
∂G
i
/∂ N
j
=RT(1/x
i
)·(−x
i
/N) + ∂
E
G
i
/∂ N
j
=−RT/N +∂
E
G
i
/∂ N
j
; ∂G
j
/∂ N
i
=
−RT/N + ∂
E
G
j
/∂ N
i
.Ifthe two left-hand sides are equal, we obtain ∂
E
G
i
/∂ N
j
=
∂
E
G
j
/∂ N
i
.
