Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.


1.6 Second law of thermodynamics 15
this was done it was decided to express the difference between the boiling and freezing
point of water at 1 atm as 100 units, in agreement with the Celsius scale. This unit is
now called kelvin (K).
Let us now return to the irreversible process of heat conduction from a warm reservoir
to a cold one. By transferring an amount dQ one would decrease the entropy of the warm
reservoir by dQ/T
b
and increase the entropy of the cold one by dQ/T
a
. The net change
of the entropy would thus be
dS =−dQ/T
b
+ dQ/ T
a
= dQ · (T
b
− T
a
)T
b
T
a
. (1.36)
This irreversible process thus produces entropy. One talks about internal entropy
production
d
ip
S > 0. (1.37)
The subscript ‘ip’ indicates that this change of the entropy of the system is due to an
internal process. This is the second law of thermodynamics and it should be noted that it
concerns what happens inside a system, whereas the first law concerns interactions with
the surroundings. As we have seen, the transfer of heat to the system, dQ, will increase
the entropy by dQ/T and, by also considering the effect of additional matter, dN,inan
open system we can write the second law as
dS = dQ/ T + S
m
dN + d
ip
S > dQ/T + S
m
dN. (1.38)
S
m
is the molar entropy of the added material and can be derived exactly as H
m
in the
first law was derived in Section 1.3.With the alternative definition of heat in Eq. (1.12)
we would obtain
dS = dQ
∗
/T − (H
m
/T − S
m
)dN + d
ip
S = dQ
∗
/T − ((H
m
− TS
m
)/T )dN
+d
ip
S > dQ
∗
/T − ((H
m
− TS
m
)/T )dN. (1.39)
When a spontaneous process proceeds, it is in a direction that can be predicted from
the above criterion. A spontaneous process is always an irreversible process, other-
wise it would have no preferred direction and it would be reversible. A reversible pro-
cess is a hypothetical construction and can be defined by either one of the following
criteria,
d
ip
S = 0 (1.40)
dS = dQ/ T + S
m
dN. (1.41)
Exercise 1.6
Suppose a simple model for an internal reaction yields the following expression for
the internal production of entropy under conditions of constant T, V and N,
ip
S =
−ξ K /T − R[ξ ln ξ − (1 + ξ ) ln(1 + ξ )], where ξ is a measure of the progress of the
reaction going from 0 to 1. Find the equilibrium value of ξ, i.e. the value of ξ for which
the reaction cannot proceed spontaneously.

16 Basic concepts of thermodynamics
Hint
The spontaneous reaction will stop whend
ip
S is no longer positive, i.e. whend
ip
S/dξ = 0.
Solution
d
ip
S /dξ = d(
ip
S)/dξ =−K /T − R[1 + ln ξ − 1 − ln(1 + ξ )] = 0; ξ/(1 + ξ ) =
exp(−K /RT); ξ = 1/[exp(K/RT) − 1].
Exercise 1.7
Find a state function from which one could evaluate the heat flow out of the system when
a homogeneous material is compressed isothermally.
Hint
Heat is not a state function of a system. In order to solve the problem we must know how
the change was made. Let us first assume that it was reversible.
Solution
Forreversible conditions Q =
T dS = T
1
dS = T
1
(S
2
− S
1
) and the heat extraction
−Q = T
1
(S
1
− S
2
). For irreversible conditions dQ < T dS; Q < T
1
(S
2
− S
1
) and the
extracted heat is −Q > T
1
(S
1
− S
2
), i.e. larger than before. However, if the final state
is the same, U must be the same because it is a state function and the higher value of
−Q must be compensated by a higher value of the work of compression W than during
reversible compression. How much higher −Q and W will be cannot be calculated without
detailed information on the factor making the compression irreversible.
Exercise 1.8
Consider a Carnot cycle with a non-ideal gas and suppose that the process is somewhat
irreversible. Use the second law to derive an expression for the efficiency.
Hint
For each complete cycle we have U = 0 and S = 0 because U and S are both
state functions.
Solution
U = W + Q
1
+ Q
3
= 0; S = Q
1
/T
a
+ Q
3
/T
b
+
ip
S = 0where W and
ip
S are the sums over the cycle. We seek η =−W/Q
3
and should thus eliminate
Q
1
by combining these equations: −Q
1
= Q
3
T
a
/T
b
+
ip
S · T
a
; −W = Q
1
+ Q
3
=
−Q
3
T
a
/T
b
−
ip
S · T
a
+ Q
3
= Q
3
(T
b
− T
a
)/T
b
−
ip
S · T
a
and thus η =−W/Q
3
=
(T
b
− T
a
)/T
b
−
ip
S · T
a
/Q
3
< (T
b
− T
a
)/T
b
because
ip
S, T
a
and Q
3
are all positive.

1.7 Condition of internal equilibrium 17
1.7 Condition of internal equilibrium
The second law states that an internal process may continue spontaneously as long as
d
ip
S is positive. It must stop when for a continued process one would have
d
ip
S ≤ 0. (1.42)
This is the condition for equilibrium in a system. By integrating d
ip
S we may obtain a
measure of the total production of entropy by the process,
ip
S.Ithas its maximum
value at equilibrium. The maximum may be smooth, d
ip
S = 0, or sharp, d
ip
S < 0, but
the possibility of that alternative will usually be neglected.
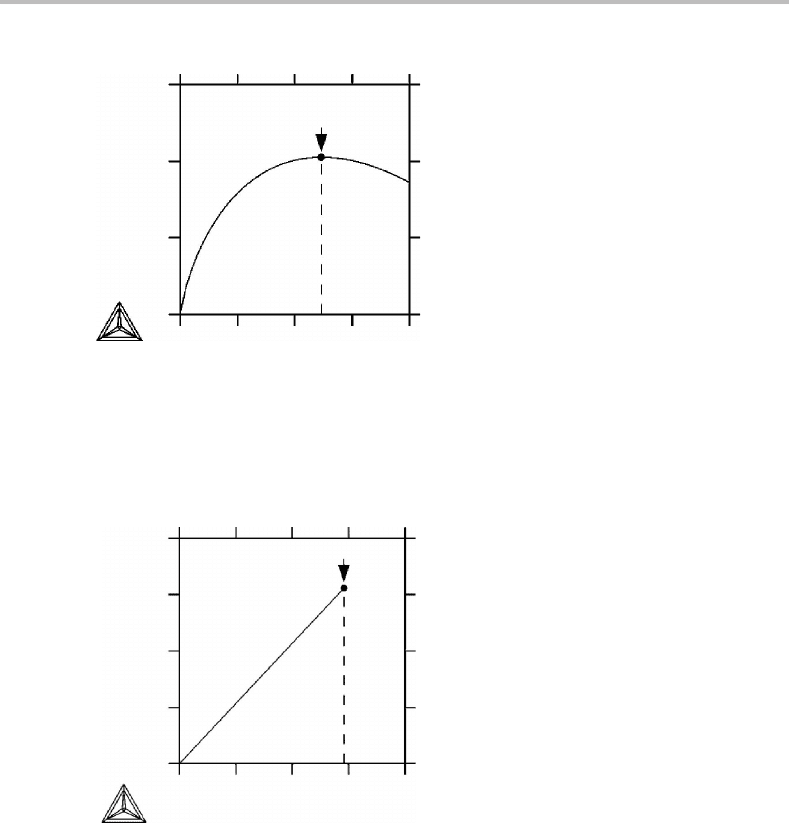
As an example of the first case, Fig. 1.5 shows a diagram for the formation of vacancies
in a pure metal. The internal variable, generally denoted by ξ ,ishere the number of
vacancies per mole of the metal.
As an example of the second case, Fig. 1.6 shows a diagram for the solid state reaction
between two phases, graphite and Cr
0.7
C
0.3
,bywhich a new phase Cr
0.6
C
0.4
is formed.
The internal variable here represents the amount of Cr
0.6
C
0.4
. The curve only exists up
to a point of maximum where one or both of the reactants have been consumed (in
this case Cr
0.7
C
0.3
). From the point of maximum the reaction can only go in the reverse
direction and that would give d
ip
S < 0which is not permitted for a spontaneous reaction.
The sharp point of maximum thus represents a state of equilibrium. This case is often
neglected and one usually treats equilibrium with the equality sign only, d
ip
S = 0.
If d
ip
S = 0itispossible that the system is in a state of minimum
ip
S instead of
maximum. By a small, finite change the system could then be brought into a state where
d
ip
S > 0 for a continued change. Such a system is thus at an unstable equilibrium.As
a consequence, for a stable equilibrium we require that either d
ip
S < 0, or d
ip
S = 0but
then its second derivative must be negative.
It should be mentioned that instead of introducing the internal entropy production,
d
ip
S, one has sometimes introduced dQ
/T where dQ
is called ‘uncompensated heat’.
It represents the extra heat, which must be added to the system if the same change of
the system were accomplished by a reversible process. Under the actual, irreversible
conditions one has dS = dQ/ T + d
ip
S. Under the hypothetical, reversible conditions
one has dS = (dQ + dQ
)/T . Thus, dQ
= T d
ip
S.Inthe actual process d
ip
S is produced
without the system being compensated by such a heat flow from the surroundings.
If the reversible process could be carried out and the system thus received the extra
heat dQ
,ascompared to the actual process, then the system must also have delivered
the corresponding amount of work to the surroundings in view of the first law. Because
of the irreversible nature of the process, this work will not be delivered and that is why
one sometimes talks about the ‘loss of work’inthe actual process which is irreversible
and produces some entropy instead of work, dW = dQ
= T d
ip
S.
Exercise 1.9
Check the loss of work in a cyclic process working with a high-temperature heat source
of T
b
and a low-temperature heat sink of T
a
and having some internal entropy production.
18 Basic concepts of thermodynamics
1.5
1.0
0
0
0.5
5 10 15 20
ξ (fraction of vacancies, u
Va
⋅10
5
)
on of vacancies, u
Va
⋅10
5
)
(∆
ip
S/R)⋅10
4
equilibrium
value of ξ
s,
Figure 1.5 The internal entropy production due to the formation of thermal vacancies in 1 mole
of a pure element at a temperature where the energy of formation of a vacancy is 9kT, k being
the Boltzmann constant. The initial state is a pure element without any vacancies. The internal
variable is here the number of vacancies expressed as moles of vacancies per mole of metal, u
Va
.
0.4
0.3
0.2
0.2 0.4 0.6 0.8
0.1
0
0
∆
ip
S/R
equilibrium
value of ξ
ξ (moles of Cr
0.6
C
0.4
)
Figure 1.6 The internal entropy production due to the solid state phase transformation
C +Cr
0.7
C
0.3
→ Cr
0.6
C
0.4
at 1500 K and 1 bar. The initial state is 0.5 mole each of C(graphite)
and Cr
0.7
C
0.3
. The internal variable here represents the amount of Cr
0.6
C
0.4
.
Hint
In Exercise 1.8 we found −W = Q
3
(T
b
− T
a
)/T
b
−
ip
S · T
a·
From this result we can
calculate the ‘loss of work’, e.g. if the amount of heat extracted from the heat source is
the same in the irreversible case as in the reversible one. Give this loss per heat extracted
from the heat source, and give it per heat given to the colder heat sink, Q
1
.
Solution
Forareversible cycle one would have −W = Q
3
(T
b
− T
a
)/T
b
. The ‘loss of work’ per
extracted heat is thus
ip
S · T
a
/Q
3
.

1.8 Driving force 19
For the second case we should eliminate Q
3
from the two equations in the solu-
tion of Exercise 1.8: −Q
3
=
ip
S · T
b
+ Q
1
T
b
/T
a
; −W = Q
1
+ Q
3
=−Q
1
(T
b
− T
a
)/
T
a
−
ip
S · T
b
.
The ‘loss of work’ per received heat is thus
ip
S · T
b
/(−Q
1
). The two results are
equal in the reversible limit where Q
3
/T
b
=−Q
1
/T
a
according to Eq. (1.35).
1.8 Driving force
Let the internal variable ξ represent the extent of a certain internal process. The internal
entropy production can then be regarded as a function of this variable and we may define
its derivative d
ip
S/dξ as a new state variable. It may also be regarded as a state function
because it may be expressed as a function of a set of state variables, including ξ,which
define the state. For convenience, we shall multiply by T under isothermal conditions to
obtain a new state variable,
D ≡ T
d
ip
S
dξ
. (1.43)
One may use D = 0asthe condition of equilibrium. This quantity was introduced by
De Donder [2]when considering chemical reactions between molecules and it was thus
called affinity.However, it has a much wider applicability and will here be regarded as
the driving force for any internal process. The symbol D, chosen here, may either be
regarded as an abbreviation of driving force or as an honour to De Donder. It is usually
convenient to define ξ byavariable that is an extensive property, subject to the law of
additivity. The driving force D will then be an intensive variable.
If a system is not in a state of equilibrium, there may be a spontaneous internal process
for which the second law gives d
ip
S > 0 and thus
T d
ip
S = Ddξ>0. (1.44)
It is evident that dξ and D must have the same sign in order for the process to proceed. By
convention, dξ is given a positive value in the direction one wants to examine and D must
then be positive for a spontaneous process in that direction. In many applications one
even attempts to predict the rate of a process from the magnitude of D. Simple models
often predict proportionality. This will be further discussed in Chapter 5.
If D > 0 for some internal process, then the system is not in a state of equilibrium. The
process may proceed and it will eventually approach a state of equilibrium where D = 0.
The equilibrium value of the variable ξ can, in principle, be evaluated from the condition
D = 0, which is usually more directly applicable than the basic condition d
ip
S = 0.
In the preceding section we connected an internal entropy production with the progress
of an internal process. However, we can now see that it is possible, in principle, to change
an internal variable without any entropy production. This can be done by changing the
external variables in such a way that the driving force D is always zero. Since D is zero at
equilibrium only, it is necessary to change the external variables so slowly that ξ can all
the time adjust itself to the new value required by equilibrium. In practice, this cannot be

20 Basic concepts of thermodynamics
completely achieved because the rate of the process should be zero if its driving force is
zero. An infinitely slow change is thus necessary. Such an idealized change is identical to
the reversible process mentioned in the preceding section and it is sometimes described
as an ‘equilibrium reaction’. It would take the system through a series of equilibrium
states.
It may be convenient to consider a reversible process if one knows a state of equilibrium
for a system and wants to find other states of equilibrium under some different conditions.
This is the reason why one often applies ‘reversible conditions’. As an example we may
consider the heating of a system under constant volume, discussed in Section 1.4. The
heat capacity under such conditions, C
V
,was found to be different under slow and rapid
changes. Both of these cases may be regarded as reversible because the internal entropy
production is negligible when D is small for a very slow change and also when dξ is
small for a frozen-in internal process. For both cases we may thus use dS = dQ/ T and
we obtain two different quantities,
C
V
≡
∂ Q
∂T
V
= T
∂ S
∂T
V
(1.45)
C
V,ξ
≡
∂ Q
∂T
V,ξ
= T
∂ S
∂T
V,ξ
. (1.46)
These expressions are equivalent to those given in Section 1.4 in terms of U.For inter-
mediate cases, which are not reversible, one should consider U and not S, i.e. use the
first law and not the second law.
Exercise 1.10
Consider an internal reaction which gives an entropy production under isothermal condi-
tions,
ip
S =−ξ K /T − R[ξ ln ξ − (1 + ξ ) ln(1 + ξ )]. Derive the stability at equilib-
rium, defined as B =−T ·d
2
ip
S/dξ
2
=−T · dD/dξ. (See Section 6.1.)
Hint
In Exercise 1.6 we have already calculated d
ip
S/dξ and ξ at equilibrium.
Solution
d
ip
S/dξ =−K /T − R[ln ξ − ln(1 + ξ )]; d
2
ip
S/dξ
2
=−R
[
1/ξ − 1/(1 +ξ )
]
.
However, at equilibrium 1/ξ = exp(K/RT) − 1; 1/(1 + ξ ) = [exp(K /RT) − 1]/
exp(K /RT).
Thus, B =+RT[1/ξ − 1/(1 + ξ )] = RT[1 − exp(K /RT)]
2
/ exp(K /RT).
This is always positive. The state of equilibrium must be stable.

1.9 Combined first and second law 21
1.9 Combined first and second law
Combination of the first and second laws, Eqs (1.11) and (1.38) yield by elimination of
dQ,
dS = dQ/T + S
m
dN + d
ip
S + (dU − dQ − dW − H
m
dN)/ T
= dU/T + (S
m
− H
m
/T )dN − dW/T + d
ip
S. (1.47)
Denoting H
m
− TS
m
by G
m
,asymbol that will be explained in Section 3.2, and intro-
ducing Ddξ/T for d
ip
S from Eq. (1.43) and only considering compression work, we
obtain
dS = (1/T )dU + (P/T )dV − (G
m
/T )dN + Ddξ/T. (1.48)
It should be noted that the alternative definition of heat, Eq. (1.12), would yield the same
result by eliminating dQ
∗
between Eqs (1.13) and (1.39). The combination of the two
laws is due to Gibbs [3] and Eq. (1.48), without the last term is often called Gibbs’
equation or relation. We shall simply refer to Eq. (1.48)asthecombined law and it
can be written in many different forms, expressing one state variable as a function of
the others. Such a function, based on the combined law, is regarded as a characteristic
state function for the set of variables occurring on the right-hand side. The variables in
that set are regarded as the natural variables for the quantity appearing on the left-hand
side.
It is more common to write the combined law in the following form
dU = T dS − PdV + G
m
dN − Ddξ. (1.49)
Here, U is the characteristic state function and its natural variables are S, V and N. One
usually regards S as an external variable although its value is also influenced by internal
processes and it is not possible to control its value by actions from the outside without
an intimate knowledge of the properties of the system.
When there are i internal processes, one should replace Ddξ by D
i
dξ
i
.Forthe sake
of simplicity this will be done only when we actually consider more than one process.
By grouping together the products of the external variables in Eq. (1.49)wewrite
dU = Y
a
dX
a
− Ddξ, (1.50)
where Y
a
represents potentials like T.Itisevident that the pressure should be expressed
as −P in order to be comparable with other potentials. As a consequence, we shall plot
P in the negative direction in many diagrams (see, for instance, Fig. 1.1). X
a
represents
extensive quantities like S and V. The pair of one potential and one extensive quantity,
Y
a
and X
a
,iscalled a pair of conjugate variables, for instance T, S or − P, V . Other
pairs of conjugate variables may be included through the first law by considering other
types of work, for instance gravitational work. It is important to notice that the change
in U is given in terms of the changes in variables all of which are extensive like S and V
and all of them are subject to the law of additivity.

22 Basic concepts of thermodynamics
Since U is a state variable which is a function of all the external variables, X
a
, X
b
,
etc., and the internal ξ variables, we have
Y
b
=
∂U
∂ X
b
X
c
,ξ
, (1.51)
where X
c
represents all the X variables except for X
b
.Itisinteresting to note that all the
Y variables are obtained as partial derivatives of an energy with respect to an extensive
variable. That is why they are regarded as potentials. One may also regard − D and ξ as
a pair of conjugate variables where − D is the potential and is obtained as
− D =
∂U
∂ξ
X
a
, (1.52)
where X
a
represents all the X variables. It should be emphasized that the Y potentials
have here been defined for a frozen-in state because ξ was treated as an independent
variable that is kept constant. Under conditions of maintained equilibrium one should
treat ξ as a dependent variable and the potentials are defined as
Y
b
=
∂U
∂ X
b
X
c
. (1.53)
We will soon see that for equilibrium states the two definitions of Y
b
give the same result.
In the following discussions we do not want to be limited to frozen-in states (dξ = 0),
nor to equilibrium states or reversible changes (D = 0) and we will thus retain the Ddξ
term in the combined law. It should again be emphasized that there are those two different
cases for which the term Ddξ is zero and can be omitted.
The combined law can be expressed in several alternative forms depending upon the
choice of independent external variables. These forms make use of new state functions
which will be discussed soon.
Exercise 1.11
Trytoinclude the effect of electrical work in the combined law.
Hint
There are two cases. First, consider the addition of an extra charge to the system. Second,
consider the case where the system is made part of an electrical circuit.
Solution
In the first case, the first law gives dU = dQ + dW + dW
el
where we may write dW
el
=
E · d(charge) =−EF dn
e
,where F is the Faraday constant (the negative of the charge
of one mole of electrons) and n
e
is the number of extra electrons (in mole). E is the
electrical potential. The combined law becomes dU = T dS − PdV − EFdn
e
− Ddξ.
However, E increases very rapidly with n
e
and reaches extremely high values before n
e
is large enough to have a chemical effect. This form is thus of little practical interest.

1.10 General conditions of equilibrium 23
Let us now consider a system that is part of an electrical circuit. It is evident that
the charge entering a system through one lead must be practically equal to the charge
leaving the system from the other lead, i.e. dn
e1
=−dn
e2
. The first law becomes
dU = dQ + dW + dW
e1
= dQ + dW − E
1
F dn
e1
− E
2
F dn
e2
= dQ + dW − (E
1
−
E
2
)F dn
e1
, and the combined law becomes dU =T dS − PdV −(E
1
− E
2
)
F dn
e1
− Ddξ. E
1
and E
2
are the electrical potentials on the two sides of the
system. At this time we do not need to speculate on what happens inside the system.
1.10 General conditions of equilibrium
A system is in a state of equilibrium if the driving forces for all possible internal processes
are zero. Many kinds of internal processes can be imagined in various types of systems
but there is one class of internal process that should always be considered, the transfer of a
quantity of an extensive variable from one part of the system, i.e. a subsystem, to another
subsystem. In this section we shall examine the equilibrium condition for such a process.
Let us first examine an internal process taking place in a system under constant values
of the external extensive variables S and V, here collectively denoted by X
a
, and let us
not be concerned about the experimental difficulties encountered in performing such an
experiment. We could then turn to the combined first and second law in terms of dU,
which is reduced as follows
dU = Y
a
dX
a
− Ddξ =−Ddξ. (1.54)
The driving force for the internal process will be
D =−(∂U/∂ξ)X
a
. (1.55)
The process can occur spontaneously and proceed until U has reached a minimum. The
state of minimum in U at constant S and V is thus a state of equilibrium.
The internal process we shall now consider is the transfer of dX
b
from one subsystem
(
)tothe other (
), keeping the remaining Xs constant at different values in the two
subsystems. It is convenient to measure the extent of this internal process by identifying
dξ with −dX
b
for the first subsystem and +dX
b
for the second. We thus obtain, by
applying the law of additivity to D,
− D =
∂U
∂ξ
X
a
=
∂U
∂(−X
b
)
X
c
+
∂U
∂ X
b
X
c
=−
∂U
∂ X
b
X
c
+
∂U
∂ X
b
X
c
.
(1.56)
The derivative ∂U/∂ X
b
is identical to the conjugate potential Y
b
and we thus find
D = Y
b
− Y
b
. (1.57)
The driving force for this process will be zero and the system will be in equilibrium with
respect to the process if the potential Y
b
has the same value in the two subsystems. We
have thus proved that each potential must have the same value in the whole system at

24 Basic concepts of thermodynamics
equilibrium. This applies to T, and to P with an exception to be treated in Chapter 16.It
also applies to chemical potentials µ
i
,which have not yet been introduced.
Exercise 1.12
One may derive a term −EF dn
e
for the electrical contribution to dU. Here E is the
electrical potential and −F dn
e
the electrical charge because dn
e
is the number of moles
of extra electrons and −F is the charge of one mole of electrons. Evaluate the driving
force for the transfer of electrons from one half of the system to the other if their electrical
potentials are E
and E
and can be kept constant. Define dξ as dn
e
.
Solution
−D = (∂U/∂ξ) =−(∂U/∂n
e
)
+ (∂U/∂n
e
)
= E
F − E
F ; D = (E
− E
)F .In
practice, the big question is whether the charge transfer will change the potential differ-
ence or whether there is a device for keeping it constant.
1.11 Characteristic state functions
Under experimental conditions of constant S, V and N it is most convenient to use the
combined law in the form given by Eq. (1.49) because then it yields simply
dU =−Ddξ. (1.58)
At equilibrium, D = 0, we obtain
D =−(∂U/∂ξ)
S,V ,N
= 0 (1.59)
for the internal process. If instead D > 0, then the internal process may proceed sponta-
neously and the internal energy will decrease and eventually approach a minimum under
constant S, V and N.
From an experimental point of view it is not very easy to control S but relatively
easy to control T.Achange of independent variable may thus be desirable and it can be
performed by subtracting d(TS)which is equal to T dS + SdT . The combined law in
Eq. (1.49)isthus modified to
d(U − TS) =−SdT − PdV + G
m
dN − Ddξ. (1.60)
We may regard this as the combined law for the variables T, V and N and the combination
U − TSis regarded as the characteristic state function for these variables, whereas U is
regarded as the characteristic state function for the variables S and V. The new function
