Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

220 3. Zero-Length Crosslinkers
intermediate. However, the fi nal product of this two-step reaction is identical to that obtained
using EDC alone: the activated carboxylate reacts with an amine to give a stable amide linkage
(Figure 3.2 ).
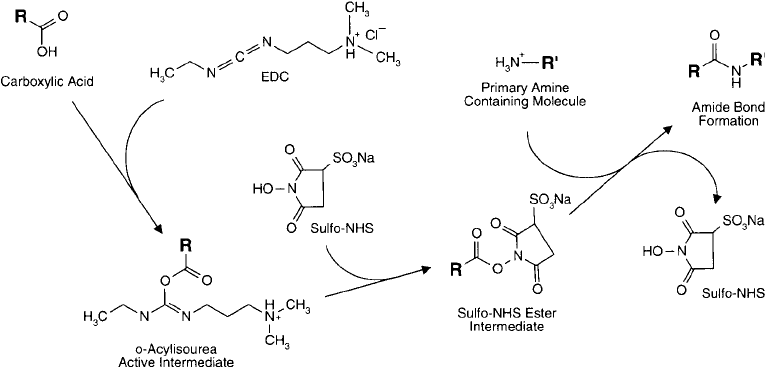
EDC/sulfo-NHS coupled reactions are highly effi cient and usually increase the yield of con-
jugation signifi cantly over that obtainable solely with EDC. Staros et al. (1986) shows that the
addition of just 5 mM sulfo-NHS to the EDC coupling of glycine to keyhole limpet hemocy-
anin increased the yield of derivatization about 20-fold as compared to using EDC alone. This
technique also can be used to create activated proteins containing sulfo-NHS esters (Grabarek
and Gergely, 1990). A protein can be incubated in the presence of EDC/sulfo-NHS, the active
ester form isolated and then mixed with a second protein or other amine-containing molecule
for conjugation. This two-step process allows the active species to form only on one protein,
thus gaining greater control over the conjugation ( Figure 3.3 ).
In addition to the potential side reactions of EDC as mentioned previously (Section 1.1, this
chapter), the additional effi ciency obtained by the use of a sulfo-NHS intermediate in the pro-
cess may cause other problems. In some cases, the conjugation actually may be too effi cient
to result in a soluble or active complex. Particularly when coupling some peptides to carrier
proteins, the use of EDC/sulfo-NHS often causes severe precipitation of the conjugate. Scaling
back the amount of EDC/sulfo-NHS added to the reaction may be done to solve this prob-
lem. However, eliminating the addition of sulfo-NHS altogether may have to be done in some
instances to preserve the solubility of the fi nal product.
The following protocol is a generalized description of how to incorporate sulfo-NHS ester
intermediates in EDC conjugation procedures. For specifi c applications of this technology, the
amount of each reagent and unconjugated species may have to be adjusted to obtain an optimal
conjugate. See also Chapter 14 and Chapter 9, Section 10 for protocols using EDC/sulfo-NHS
in the coupling of proteins to particles and quantum dots, respectively.
Figure 3.2 The effi ciency of an EDC-mediated reaction may be increased through the formation of a sulfo-
NHS ester intermediate. The sulfo-NHS ester is more effective at reacting with amine-containing molecules.
Thus, higher yields of amide bond formation may be realized using this two-step process as opposed to using a
single-step EDC reaction.

Protocol
1. Dissolve the protein to be modifi ed at a concentration of 1–10 mg/ml in 0.1 M sodium
phosphate, pH 7.4. NaCl may be added to this buffer if desired. For the modifi cation of
keyhole limpet hemocyanin (KLH; Thermo Fisher) as described by Staros et al., 1986,
include 0.9 M NaCl to maintain the solubility of this high-molecular-weight protein.
If lower or higher concentrations of the protein are used, adjust the amounts of the other
reactants as necessary to maintain the correct molar ratios.
2. Dissolve the molecule to be coupled in the same buffer used in step 1. For small mol-
ecules, add them to the reaction in at least a 10-fold molar excess over the amount of
protein present. If possible, the molecule may be added directly to the protein solution in
the appropriate excess. Alternatively, dissolve the molecule in the buffer at a higher con-
centration, and then add an aliquot of this stock solution to the protein solution.
3. Add the solution prepared in step 2 to the protein solution to obtain at least a 10-fold
molar excess of small molecule to protein.
4. Add EDC (Thermo Fisher) to the above solution to obtain at least a 10-fold molar excess of
EDC over the amount of protein present. Alternatively, a 0.05–0.1 M EDC concentration
Figure 3.3 EDC may be used in tandem with sulfo-NHS to create an amine-reactive protein derivative con-
taining active ester groups. The activated protein can couple with amine-containing compounds to form amide
bond linkages.
1. Carbodiimides 221
222 3. Zero-Length Crosslinkers
in the reaction usually works well. Also, add sulfo-NHS (Thermo Fisher) to the reaction
to bring its fi nal concentration to 5 mM. To make it easier to add the correct quantity of
EDC or sulfo-NHS, higher concentration stock solutions may be prepared if they are dis-
solved and used immediately. Mix to dissolve. If this ratio of EDC/sulfo-NHS to peptide
or protein results in precipitation, scale back the amount of addition until a soluble conju-
gate is obtained.
5. React for 2 hours at room temperature.
6. Purify the conjugate by gel fi ltration or dialysis using the buffer of choice (for many con-
jugates 0.01 M sodium phosphate, 0.15 M NaCl, pH 7.4 is appropriate). If some turbid-
ity has formed during the conjugation procedure, it may be removed by centrifugation or
fi ltration.
A modifi cation of a two-step protocol (Grabarek and Gergely, 1990) for the activation of
proteins with EDC/sulfo-NHS and subsequent conjugation with amine-containing molecules if
given below. The variation in the pH of activation from that described above provides greater
stability for the active ester intermediate. At pH 6.0, the amines on the protein will be pro-
tonated and therefore be less reactive toward the sulfo-NHS esters that form. In addition, the
hydrolysis rate of the esters is dramatically slower at slightly acid pH. Thus, the active species
may be isolated in a reasonable time frame without signifi cant loss in conjugation potential.
To quench the unreacted EDC, 2-mercaptoethanol is added to form a stable complex with the
remaining carbodiimide, according to Carraway and Triplett (1970). In the following protocol,
sulfo-NHS is used instead of NHS so that active ester is more water-soluble and ester hydroly-
sis is slowed (Anjaneyulu and Staros, 1987; Thelen and Deuticke, 1988).
Protocol
1. Dissolve the protein to be activated in 0.05 M MES, 0.5 M NaCl, pH 6.0 (reaction buffer),
at a concentration of 1 mg/ml.
2. Add to the solution in step 1 a quantity of EDC and sulfo-NHS (Thermo Fisher) to obtain
a concentration of 2 mM EDC and 5 mM sulfo-NHS. To aid in aliquoting the correct
amount of these reagents, they may be quickly dissolved in the reaction buffer at a higher
concentration, and then a volume immediately pipetted into the protein solution to obtain
the proper molar quantities.
3. Mix and react for 15 minutes at room temperature.
4. Add 2-mercaptoethanol to the reaction solution to obtain a fi nal concentration of 20 mM.
Mix and incubate for 10 minutes at room temperature. Note: If the protein being acti-
vated is sensitive to this level of 2-mercaptoethanol, instead of quenching the reaction
chemically, the activation may be terminated by desalting (step 5).
5. If the reaction was quenched by the addition of 2-mercaptoethanol, the activated pro-
tein may be added directly to a second protein or other amine-containing molecule for
conjugation. Alternatively, or if no 2-mercaptoethanol was added, the activated protein
may be purifi ed from reaction by-products by gel fi ltration using a desalting resin. The
desalting operation should be done rapidly to minimize hydrolysis and recover as much
active ester functionality as possible. The use of centrifugal spin columns of some sort
may afford the greatest speed in purifi cation (Thermo Fisher). After purifi cation, add the
activated protein to the second molecule for conjugation. The second protein or other

amine-containing molecule should be dissolved in 0.1 M sodium phosphate, pH 7.5.
This will bring the pH of the coupling medium above pH 7.0 to initiate the active ester
reaction.
6. React for at least 2 hours at room temperature.
7. Remove excess reactants by gel fi ltration or dialysis.
1.3. CMC
CMC, or 1-cyclohexyl-3-(2-morpholinoethyl)carbodiimide (usually synthesized as the metho
p-toluene sulfonate salt) (Aldrich), is a water-soluble reagent used to form amide bonds
between one molecule containing a carboxylate and a second molecule containing an amine.
The presence of the positively charged morpholino group creates its water solubility. Along
with EDC (Section 1.1, this chapter), CMC is the only other soluble carbodiimide commonly
available for biological conjugations. It was fi rst utilized in peptide synthesis (Sheehan and
Hlavka, 1956) and found to be superior to other coupling agents used at the time (Ondetti and
Thomas, 1965). It also has been used for the quantitative modifi cation and estimation of total
carboxyl groups in protein molecules (Hoare and Koshland, 1967) and for investigating the
secondary structure of nucleic acids (Metz and Brown, 1969). Another early application area
of CMC, relates not to solution phase crosslinking of two molecules, but to coupling of lig-
ands to insoluble support materials for use in affi nity chromatography (Lowe and Dean, 1971;
Marcus and Balbinder, 1972; Schmer, 1972).
CMC reacts with carboxylate groups by addition of the carboxyl across one of its diimide
bonds, resulting in the characteristic active ester, o-acylisourea intermediate common to
all carbodiimide mechanisms. Nucleophilic attack on this intermediate yields the acylated
product—usually an amide bond, resulting from the reaction with a primary amine ( Figure 3.4 ).
However, carbodiimide chemistry does create several potential side reactions. Sulfhydryl groups
may react with CMC to form a stable covalent complex unreactive toward further conjuga-
tion. The reagent also may react with phenols, alcohols, and other nucleophiles to quench the
crosslinking reaction. In aqueous solutions, hydrolysis of the carbodiimide and the active ester
are by far the most frequent side reactions. Reaction of the ester with water molecules regener-
ates the carboxylate and releases a soluble isourea by-product.
CMC should be able to participate in the two-step reaction using a sulfo-NHS ester intermedi-
ate similar to EDC, however there are no reports in the literature to this effect. Protocols for the
use of this reagent in biological crosslinking applications should be essentially the same as those
given previously for EDC, except substituting a molar equivalent quantity of CMC. See Sections
1.1 and 1.2 in this chapter for additional information concerning carbodiimide reactions.
1. Carbodiimides 223

224 3. Zero-Length Crosslinkers
1.4. DCC
DCC (dicyclohexyl carbodiimide) is one of the most frequently used coupling agents, especially
in organic synthesis applications. It has been used for peptide synthesis since 1955 (Sheehan
and Hess, 1955) and continues to be a popular choice for creating peptide bonds (Barany and
Merrifi eld, 1980). DCC is water-insoluble, but it has been used in 80 percent DMF for the
immobilization of small molecules onto carboxylate-containing chromatography supports for
use in affi nity separations (Larsson and Mosbach, 1971; Lowe et al., 1973). In addition to
forming amide linkages, DCC has been used to prepare active esters of carboxylate-containing
compounds using NHS or sulfo-NHS (Staros, 1982). Unlike the EDC/sulfo-NHS reaction
described in Section 1.2 (this chapter), active ester synthesis done with DCC is in organic sol-
vent, and therefore doesn ’t have the hydrolysis problems of water-soluble EDC-formed esters.
Thus, DCC is most often used to synthesize active ester containing crosslinking and modifying
reagents and not to perform biomolecular conjugations.
Figure 3.4 The water-soluble carbodiimide CMC reacts with carboxylates to form an active-ester intermediate.
In the presence of amine-containing molecules, amide bond formation can take place with release of an isourea
by-product.

DCC is a waxy solid that is often diffi cult to remove from a bottle. Its vapors are extremely
hazardous to inhalation and to the eyes. It should always be handled in a fume hood. The
isourea by-product of a DCC-initiated reaction, dicyclohexyl urea (DCU) ( Figure 3.5 ), is also
water-insoluble and must be removed by organic solvent washing. For synthesis of peptides or
affi nity supports on insoluble matrices this is not a problem, because washing of the support
material can be done without disturbing the conjugate coupled to the support. For solution
phase chemistry, however, reaction products must be removed by solvent washings, precipita-
tions, or recrystallizations.
A potential undesirable effect of DCC coupling reactions is the spontaneous rearrangement
of the o-acylisourea to an inactive N-acylurea (Stewart and Young, 1984) ( Figure 3.6 ). The rate
of this rearrangement is dramatically increased in aprotic organic solvents, such as DMF.
The activation effi ciency of DCC is extraordinarily high, especially in anhydrous solutions
that don ’t have competing hydrolysis problems. o-Acylisourea-activated carboxylates may
undergo two-side reactions that form other active groups. If DCC is added to an excess of a
carboxylate-containing molecule without the presence of an amine-containing target, then the
activated carboxylate may react with another carboxylic acid to form a symmetrical anhydride
(Figure 3.7 ). The formation of an anhydride intermediate may be a frequent mechanism in
route to the creation of an amide bond with an amine, especially under anhydrous conditions
(Rebek and Feitler, 1974; Nakajima and Ikada, 1995). In addition, a DCC-activated carbox-
ylate may react with an amino acid to form an azlactone ( Figure 3.8 ) (Coleman et al., 1990).
Both the anhydride and the azlactone will react with amines to form covalent amide linkages.
However, the ring-opening reaction of an azlactone will form a different product than the zero-
length crosslinking result of coupling directly to an amine-containing molecule ( Figure 3.9 ).
Figure 3.5 The organic-soluble carbodiimide DCC is often used to create amide bonds, especially between
water-insoluble compounds.
1. Carbodiimides 225

226 3. Zero-Length Crosslinkers
1.5. DIC
DIC (or diisopropyl carbodiimide) is another water-insoluble amide bond-forming agent that
has advantages over DCC (Section 1.4, this chapter). It is a liquid at room temperature and
Figure 3.6 The active-ester intermediate formed from the reaction of DCC with a carboxylate group may
undergo rearrangement to an inactive N -acylisourea product.
Figure 3.7 The reaction of DCC with a carboxylate compound in excess may create anhydride products in the
absence of nucleophiles.
Figure 3.8 A DCC-mediated reaction with a carboxylate group in the presence of a small amino acid may form
azlactone rings.

is therefore much easier to dispense than DCC. Its by-products, diisopropylurea and diiso-
propyl-N-acylurea, are more soluble in organic solvents than the DCU by-product of a DCC
reaction. DIC reacts similarly to DCC, forming an active o-acylisourea intermediate with a
carboxylic acid group ( Figure 3.10 ). This active species may then react with a nucleophile such
as an amine to form an amide bond. Presumably, all the possible side reactions that DCC may
undergo are also possible with DIC, although it is not well documented.
Figure 3.10 The symmetrical carbodiimide DIC reacts with carboxylates to form active-ester intermediates able
to couple with amine-containing compounds to form amide bond linkages.
1. Carbodiimides 227
Figure 3.9 An azlactone reacts with amine groups through a ring-opening process, creating amide bond link-
ages with the attacking nucleophile.

228 3. Zero-Length Crosslinkers
2. Woodward ’ s Reagent K
Woodward ’s reagent K is N -ethyl-3-phenylisoxazolium-3-sulfonate, a zero-length crosslink-
ing agent able to cause the condensation of carboxylates and amines to form amide bonds
(Woodward and Olofson, 1961; Woodward et al., 1961). The reaction mechanism involved
in activating a carboxylate includes the conversion of the reagent under alkaline conditions
to a reactive ketoketenimine. This intermediate then reacts with a carboxylate to create an
enol ester. The enol ester is highly susceptible to nucleophilic attack. The reaction with an
amine proceeds to amide bond formation with loss of the inactive diketo derivative ( Figure
3.11 ). In aqueous solution, the major side reaction is hydrolysis which occurs rapidly (Dunn
and Affi nsen, 1974). Although Woodward ’s reagent K has been used successfully for conju-
gation applications with proteins and other molecules to form amide linkages (Boyer, 1986;
Pikuleva and Turko, 1989), its mechanism of reaction was called into question by Johnson and
Dekker (1996), who found that the compound reacted with cysteine and histidine groups in
E. coli L-threonine dehydrogenase, not the available aspartate or glutamate groups. Woodward ’s
reagent K is available from Fluka.
3. N,N-Carbonyldiimidazole
CDI (or N,N-carbonyldiimidazole) is a highly active carbonylating agent that contains two
acylimidazole leaving groups (Aldrich). The result is that CDI can activate carboxylic acids
or hydroxyl groups for conjugation with other nucleophiles, creating either zero-length amide
bonds or one-carbon-length N-alkyl carbamate linkages between the crosslinked molecules.
Carboxylic acid groups react with CDI to form N-acylimidazoles of high reactivity. The active
intermediate forms in excellent yield due to the driving force created by the liberation of carbon
dioxide and imidazole (Anderson, 1958). The active carboxylate then can react with amines to
form amide bonds or with hydroxyl groups to form ester linkages ( Figure 3.12 ). Both reaction

3. N,N-Carbonyldiimidazole 229
Figure 3.11 Woodward ’s reagent K undergoes a rearrangement in alkaline solution to form a reactive ketoket-
enimine. This active species can react with a carboxylate group to create another active group, an enol ester
derivative. In the presence of amine nucleophiles, amide bond formation takes place.
Figure 3.12 CDI reacts with carboxylate groups to form an active acylimidazole intermediate. In the presence
of an amine nucleophile, amide bond formation can take place with release of imidazole.
