Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

924 Reinhold Haberlandt
create
start situation
?
calculation:
new sites and
velocities
?
calculation:
fo rces
?
evaluation
?
end of the run ?
?
no
yes
end
Fig. 23.4. Schematic representation of the procedure of MD simulations (left) and
of the periodic boundary conditions. (right)
algorithms to solve the equations of motion (e. g. [4–7,10–13]). Fig. 23.4 (left)
shows the principal organization of an MD program (Verlet algorithm).
To calculate the velocities in each step by the often used velocity-Verlet-
Algorithm one needs the knowledge of the already calculated forces and also
of those corresponding to the new sites. Therefore it is suitable to calculate
the new velocities in two steps: considering first that part, which needs only
the former forces and second the final new velocities using the new calculated
forces.
Here – as an example only – this often used Verlet algorithm (cf. (23.20b))
and its velocity version (cf. (23.20c,23.20d)) are outlined. Adding to the Tay-
lor series
r
i
(t + h)=r
i
(t)+v
i
h +
h
2
2
¨
r
i
(t)+... (23.20a)
its analogue for r
i
(t −h), all terms with odd powers of the time increment h
will be cancelled and one gets up to terms of the fourth order
r
i
(t + h)=2r
i
(t) − r
i
(t − h)+h
2
¨
r
i
(t). (23.20b)
As the velocity version of the Verlet algorithm one obtains in a similar way
r
i
(t + h)=r
i
(t)+hv
i
(t)+
h
2
2m
i
F
i
(t), (23.20c)

23 Statistical Theory and Molecular Dynamics of Diffusion in Zeolites 925
v
i
(t + h)=v
i
(t)+
h
2m
i
[F
i
(t)+F
i
(t + h)]. (23.20d)
23.3.3 Methodical Hints
For the complete wealth of tricks in handling MD simulations one clearly has
to consult the relevant literature (see, e. g., [5, 6]). However, the following
methodical hints aim to summarize a few of the most basic procedures:
– Periodic boundary conditions – replication of the MD box by periodic
continuation (see Fig. 23.4 (right)) – can help to overcome artificial surface
effects due to spatially restricted systems.
– To avoid unnecessary calculations the interaction is taken into account
only in appropriate short distances (potential cutoff and shifted forces).
To take into account long-range interactions, special techniques (e. g. the
Ewald summation) must be used.
– To save computer time, the time steps should be as large as possible (but
the energy must be maintained) and as short as necessary (fast processes).
In some cases multiple step lengths are recommended (multiple time step
method).
– In order to avoid further time consuming calculations the number of pair
separations explicitly considered is reduced (Verlet neighbour list, up-
dated from time to time).
23.4 Simulation of Diffusion in Zeolites
23.4.1 Introduction
Diffusion processes in gases, liquids, solids and at interfaces have been studied
with different theoretical and experimental methods for a long time. The
present textbook provides a broad survey of this subject.
Bulk measurements of (self-) diffusion coefficients D
s
over wide ranges of
temperature T are often described generally by the Arrhenius relation (cf
Chap. 1)
D
s
= D
0
exp
−
E
A
k
B
T
(23.21)
with the pre-exponential factor D
0
, an activation energy E
A
(in a certain
sense). This relation can be explained using the transition theory of Eyring,
but its range of validity is not quite clear.
In this section, MD simulations will be applied in helping to understand
the features of molecular diffusion in more microscopic detail, namely under
the influence of the confined geometries in microporous materials. These mi-
croporous materials – especially zeolites – have attained a steadily increasing
926 Reinhold Haberlandt
role for both applications and fundamental research [14,20]. The study of dif-
fusion processes in zeolites is of great interest because these crystals contain
very regular internal surfaces and they have been used for many industrial
purposes [14].
Since the paper of Yashonath et al. [21], MD simulations (see Sect. 23.3,
[5, 6]) are applied to diffusion in zeolites to discuss the dynamics of kinetic
processes in zeolites in more detail. In the recent literature one finds some
reviews (e. g. [22–30]) and papers of several groups [39–81]).
MD simulations can use different models for guest molecules, lattices and
their interaction and allow, especially, variations in the system parameters
that are not possible in experiments. So, interrelations and dependences can
be examined.
In this section it will be discussed how MD simulations can answer ques-
tions like, e. g.:
1. Does there exist a macroscopic behaviour (i. e. thermalization, Maxwell-
Boltzmann distribution) of the few particles diffusing in the narrow holes
of zeolites?
2. What is the range of validity of the Arrhenius relation (23.21)?
3. How are the evaluated diffusion data influenced, e. g., by
a) small changes of parameter sets (structural, potential)?
b) idealization of the models used (neglecting of cations, fixed lattice)?
c) taking into account concentration gradients?
d) technical points with respect to the MD simulation?
4. How one can use additional theoretical tools – e. g. propagators – to
improve the understanding of diffusion processes in zeolites?
5. Can MD simulations at all describe experimental NMR data and/or
QENS data of diffusion?
6. What is the result for mixtures?
23.4.2 Simulations
To answer the above mentioned questions the calculations should at first be
restricted to simple model systems. Thus, in the beginning of this section,
the diffusion of (simple spherosymmetric) methane guest molecules through
idealized zeolites of LTA-type neglecting the exchangeable cations (for short:
ZK4) is examined. Then we will proceed with more complex guest molecules,
mixtures and take into account the cations of the zeolite lattice additionally.
Possible improvements of the model and application to mixtures of guest
molecules will be discussed later on.
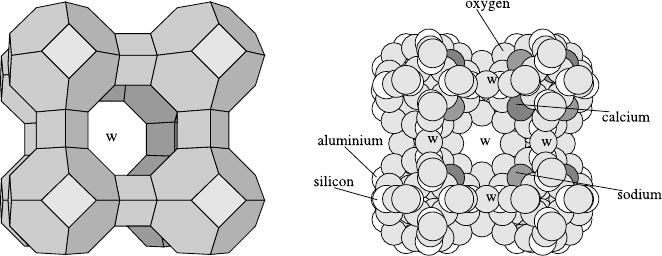
Fig. 23.5 (left) shows the general structure of zeolites of type LTA used
for the calculations. The sodalite units form a cubic lattice with large cavities
connected by so-called windows consisting of eight oxygen atoms.
23 Statistical Theory and Molecular Dynamics of Diffusion in Zeolites 927
Fig. 23.5. Structure of zeolites of type LTA (left: general view; right: interior view),
see text.
In Fig. 23.5 (right) the distribution of the lattice atoms around a large
cavity in the NaCaA zeolite is to be seen. Lattice atoms in front have been
removed in order to open the view into the interior of the cavity (windows
are marked by w).
One of the crucial points in determining diffusion coefficients in zeolites is
the knowledge of the inter- and intramolecular potentials. The forces acting
between the guest molecules and the zeolite lattice as well as among the guest
molecules must be known as good as possible in order to solve Newton’s
equations for the guest molecules in zeolites.
These forces can be derived from interaction potentials (for more details
see, e. g., [1, 22–24] and references therein). Potentials can be obtained from
quantum chemical calculations [24] and/or from fit procedures using experi-
mental results (for an illustrative example see [31] and [32–34]).
Alternatively – at least in the future –, the forces and potential energies
can be obtained by including quantum mechanical density functional calcu-
lations into an otherwise classical MD run [35–38]. However, such methods
are extremely computer-time consuming and can yield the trajectories only
for short times and/or small systems until now. So, they cannot be used for
long-time diffusion processes in large systems.
Here, for the interaction between the guest molecules CH
4
and the lattice
atoms of a cation-free analogue of LTA zeolites (‘ZK4’), we again use (23.18),
the well-known Lennard-Jones (LJ) (12,6) potential.
For NaCaA zeolites, additional polarization energy terms due to the
cations have been included. Using the conventional microcanonical MD en-
semble the self-diffusivity is calculated, while a generalized non-equilibrium
(NEMD) ensemble is used to determine the transport-diffusivities [48].
In order to get appropriate diffusion coefficients, long-run simulations were
carried out with up to 6 000 000 time steps with short step lengths of 5 and 10
fs, respectively. The basic MD box contains between 8 and 343 large cavities

928 Reinhold Haberlandt
with occupation numbers varying between 1 and 11 per cavity, respectively.
The velocity-Verlet-algorithm (see (23.20c), (23.20d)) is used [5, 6].
23.4.3 Results
Computer simulations not only yield the diffusion coefficients as the final
results, but, additionally, residence times, velocity autocorrelation functions
(vacf), potential surfaces, density distributions, and – last but not least –
propagators and Van Hove correlation functions. Thus a better understanding
of the diffusion processes is possible.
To demonstrate the influence of the potential the results of two sets of
potential parameters A and B (see Table 23.2, p. 932) are compared.
Applicability of the Diffusion Equation
First of all one must answer the non-trivial question whether or not systems
of a few particles in narrow-sized pores obey the diffusion equation. In the
kinetic theory of fluids this equation has been proven rigorously only in the
case of dilute gases. But, it is possible to check it here in the considered case
by computer experiments [42]. The validity of the diffusion equation (see,
e. g., Chaps. 1, 10, 18-20, and [2]) for an infinite system can be examined by
the propagator
P (r, r
0
,t,0) = (4πDt)
−3/2
exp
−(r − r
0
)
2
4Dt
. (23.22)
P (r, r
0
,t,0) is the (conditional) probability density to find a particle at a
site r at time t, which started at t =0atthesiter
0
. It is the solution of the
diffusion equation
∂P/∂t = D∆P ∆ : Laplacian (23.23)
with the initial condition P (r, r
0
,t=0)=δ(r − r
0
).
To examine the form of the solution of the diffusion equation, it is neces-
sary and sufficient that all moments f(r)
ν
of f(r)=(r − r
0
) (cf. (23.22,
23.24a)) will give the same diffusion coefficient. Applying the definition
f(r)
ν
=
P (r, r
0
,t)f(r)
ν
dr (23.24a)
we find for the first four moments ν =1, 2, 3, 4 of this distribution
| r − r
0
|
=4
&
Dt
π
, (23.24b)
(r − r
0
)
2
=6Dt , (23.24c)
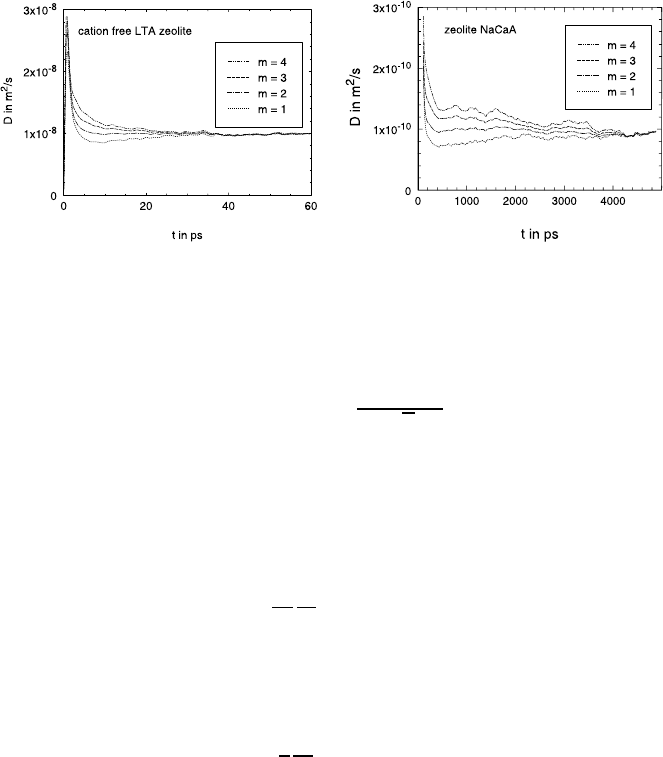
23 Statistical Theory and Molecular Dynamics of Diffusion in Zeolites 929
Fig. 23.6. D
s
from the first four moments of the displacement; left hand side:
methane in a cation-free LTA zeolite T = 300 K, parameter set A (see Table 23.2), 3
methane per cavity, right hand side: methane in a NaCaA zeolite, set B, T = 173 K,
7 methane per cavity.
| (r − r
0
)
3
|
=
32(Dt)
3/2
√
π
, (23.24d)
(r − r
0
)
4
= 60(Dt)
2
. (23.24e)
From each one of these equations an expression for Dt can be obtained, e. g.
for the first moment
Dt = π | r − r
0
|
2
/16 (23.24f)
or
D =
π
16
d
dt
| r − r
0
|
2
. (23.24g)
The differentiation (cf. (23.24g)) was used instead of simple division by t
(cf. (23.24f)) because one gets a faster convergence of the results. Corre-
spondingly one gets for the second moment – the mean square displacement
(MSD) –
D =
1
6
d
dt
(r − r
0
)
2
. (23.24h)
This equation is usually used to obtain the diffusion coefficient from MD
simulations. If the diffusion equation is valid then the D values derived from
the different moments should agree [42]. In Fig. 23.6 (left) an example of the
diffusivities in the cation-free analogue of NaCaA (‘ZK4’) is given. It can be
seen that there is good agreement for times larger than about 50 ps. That
means that in this case the hydrodynamical state is reached and we conclude
that (23.22) is then valid. The diffusion equation can be simply obtained by
comparing space and time derivatives of (23.22) and, hence, it must be valid,
too. In [42] this was also checked for other occupation numbers.
If the diffusion coefficients are smaller – as in the case of NaCaA (see
Fig. 23.6 (right)) – the hydrodynamical state is reached later (for NaCaA in
about 6 000 ps) [44]. This is probably also due to the additional cations.
930 Reinhold Haberlandt
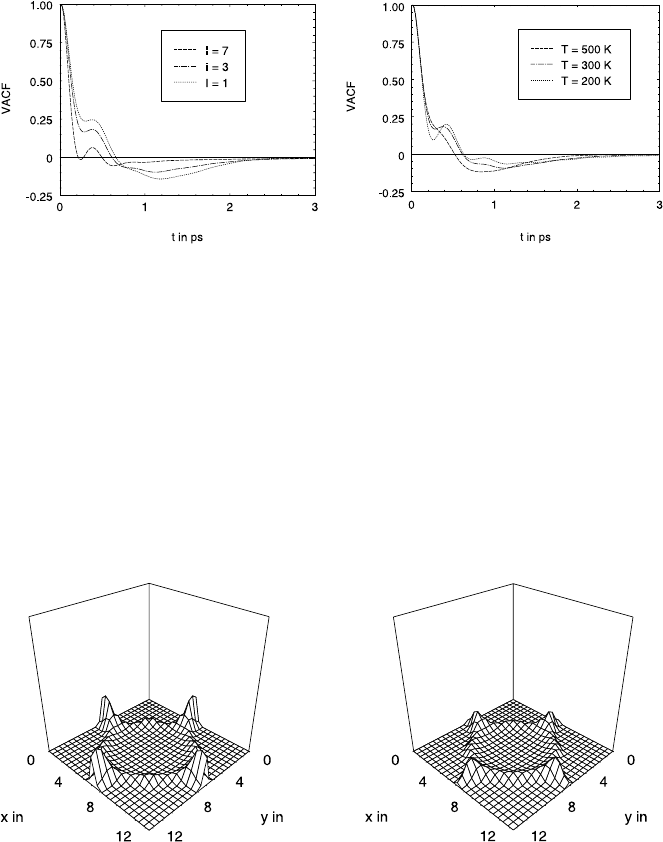
Residence Times and Velocity Autocorrelation Functions
In trying to understand the diffusion behaviour in more detail we define
the residence time for a given guest molecule as the time difference between
successive passages of limiting planes situated in the centre of each window
perpendicular to the window axis.
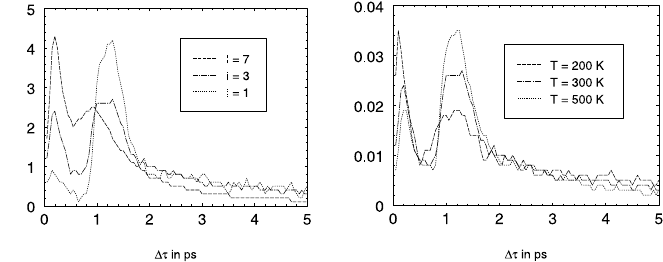
Fig. 23.7 shows the probability density of the thus defined residence times
within the individual cavities for three different sorbate concentrations, i. e.
loadings I (given in molecules per supercage), and temperatures, respectively
(parameter set A, see Table 23.2). The first maximum at ∼ 0.3 ps corresponds
to times which are too short to allow a passage through the cavity to one
of the five other windows [43]. Therefore, this maximum must be attributed
to trajectories, which are reversed immediately after the molecule has passed
the window.
It is interesting to note that the intensity of this first maximum increases
with increasing sorbate concentration. It may be concluded, therefore, that
the reversal in the trajectory is mainly caused by the influence of the other ad-
sorbate molecules. The second maximum corresponds to the passage through
the adjacent windows and is therefore responsible for diffusion.
These conclusions are confirmed by minima in the velocity autocorrelation
function (vacf) (see [43]) where the dependence of these minima on temper-
ature and loading are in agreement with the above reasoning (see Fig. 23.8).
Fig. 23.1 shows a typical situation in a system where methane molecules
are enclosed in cavities of the cation-free LTA zeolite. The large cavities and
the windows connecting them are indicated by grey isopotential surfaces.
To illustrate the time development all methane molecules that were initially
situated in the left hand lower cavity are marked by dark spheres while the
other ones are fair. So the exchange can be observed.
p (a.u.)
p (a.u.)
Fig. 23.7. Probability density of residence times of particles as function of time
for different loadings I (left) and different temperatures T (right).
23 Statistical Theory and Molecular Dynamics of Diffusion in Zeolites 931
Fig. 23.8. Velocity autocorrelation function (vacf) for different loadings (left) and
temperatures (right).
Density Distributions
The density distribution in cation-free LTA zeolites shown in Fig. 23.9 may
well be understood by isopotential lines (Fig. 23.10 and [43]) for the zeolites.
The density distributions – demonstrated in a plane through the centre of the
large cavity – show a remarkable structure. Corresponding to the potential
surface these distributions are different from zero practically only near the
cavity wall and have maxima in the window (set A, cf Table 23.2) and in
front of them (set B), respectively.
ÅÅ
ÅÅ
Fig. 23.9. Density distribution of CH
4
in a cation-free LTA with a loading of
I =3atT = 300 K monitored in a plane through the cavity centres (left: set
A; right: set B, cf Table 23.2) [51].
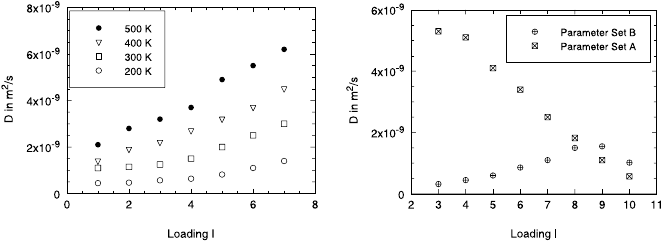
Influence of the Potential Parameters on D
The influence of potential parameters on the diffusion coefficients is remark-
able. Even small parameter changes may cause significant changes in the
932 Reinhold Haberlandt
Table 23.2. Parameter sets used for the LJ potential (cf. (23.18)).
zeolite σ in
˚
A in kJ/mol
LTA CH
4
− CH
4
3.817 1.232
LTA CH
4
− Si 2.14 0.29
LTA CH
4
− O (set A) 3.14 1.5
LTA CH
4
− O (set B) 3.46 0.81
LTA C
2
H
6
− O 3.775 1.536
silicalite CH
4
− CH
4
3.730 1.230
silicalite CH
4
− O 3.214 1.108
silicalite Xe − Xe 4.064 1.870
silicalite Xe − O 3.296 1.679
diffusion processes (see Fig. 23.11). It has been shown that the choice of
the σ parameter of the Lennard-Jones potential, (23.18), for the methane–
oxygen interaction has a dramatic influence not only on the value of the
diffusion coefficient but on its concentration dependence. In [43], two differ-
ent Lennard-Jones potentials – based on the sets A,B of potential parameters
shown in Table 23.2 – have been compared.
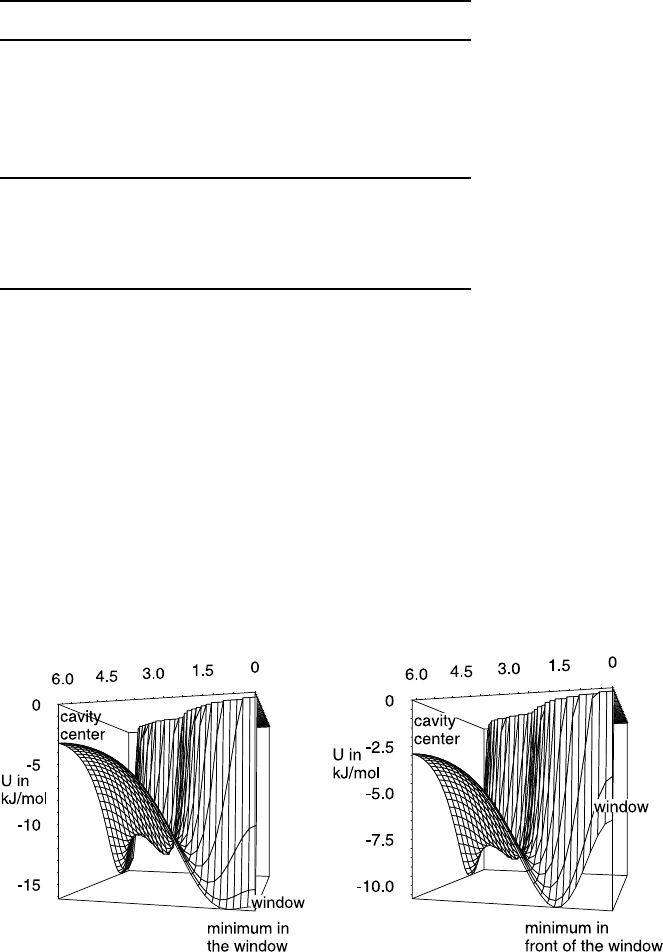
Analogously to the density distribution (Fig. 23.9), Fig. 23.10 shows the
shape of the potential surface and visualizes the different behaviour in the
vicinity of the window, resulting from the parameter set A (left) and set B
(right), respectively. It can be seen that the potential values are high in the
centre of the large cavity and, of course, at the repulsive walls. The potential
Fig. 23.10. Potential surface plotted through one quarter of the planes considered
in Fig. 23.9 (left: set A; right: set B, cf Table 23.2).
23 Statistical Theory and Molecular Dynamics of Diffusion in Zeolites 933
Fig. 23.11. Diffusion coefficient D for different loadings of methane in the cation-
free LTA zeolite (left: different temperatures, set B; right: T = 173 K, set A,B)
has a minimum in the window in set A, while for set B the maximum is in
front of the window and a saddle point in the centre of the window. This
threshold reduces the diffusivity in set B. From the larger value of σ in set
B, for the dominating oxygen–guest interaction in the zeolite there results a
narrower window between adjacent cavities. Especially for methane, which
has a similar size as the window, this causes dramatic changes. This effect
seems to be the consequence of the interrelation of two effects (reflection at
the inlet of the window, coming back after passing the window) with different
density dependence [43, 51].
Fig. 23.11 (left) shows that the diffusion coefficient D increases with in-
creasing mean number of guest molecules per cavity and temperature for set
B while this dependence for set A – demonstrated for T = 173 K – is re-
versed (right). Both cases show an Arrhenius-like behaviour (cf. (23.21)) (for
non-Arrhenius behaviour see, e. g., [67,68]).
For higher loadings this figure shows an interesting cross-over of the two
curves. Increasing diffusivities with increasing concentration is in fact the
behaviour found for paraffin in A type zeolites experimentally by PFG NMR
(see Chap. 10).
Influence of Lattice Vibrations on the Diffusion Coefficients
The influence of lattice vibrations on the diffusion coefficient in the cation-free
LTA zeolite is not very large for both of the parameter sets under consider-
ation as can be seen in Fig. 23.12 (left) [22, 58, 60, 66, 67]. Thus, the result,
initially given by Suffritti and Demontis [47] was corrected, who found a much
stronger effect using a parameter set beyond the region of A and B. (Since
their parameters lead to a practically closed window, it is most likely that an
occasional opening of the windows by vibrations may drastically increase D
in such cases.) Moreover, Fig. 23.12 again shows how the potential parameter
σ may affect the influence of another feature of the system (viz. the lattice
flexibility) on the diffusion coefficients.
