Hammond C. The Basics of Crystallography and Diffraction
Подождите немного. Документ загружается.


44 Crystals and crystal structures
(a) (b)
: Si at 0; : Si at
2
3
: Si at ;
1
3
Fig. 1.33. (a) Plan of the trigonal structure of α-quartz and (b) plan of the hexagonal structure of
β-quartz. Only the silicon atoms are shown, the oxygen atoms are tetrahedrally arranged about those
of silicon. The structures are clearly related by a displacive (shear) transformation. (From Crystal
Chemistry, 2nd Edn, by R.C. Evans, Cambridge University Press, 1966.)
two structural forms of diamond shown in Fig. 1.36 (Section 11.1.6). Figure 1.36(a)
shows the (common) face-centred cubic form of diamond; the positions of the carbon
atoms correspond to the positions of the silicon atoms in β-cristobalite. If we now add
the oxygen atoms half-way in between the silicon atoms then we generate the complete
β-cristobalite structure. Similarly, Fig. 1.36(b) shows the (uncommon) hexagonal form
of diamond (Lonsdaleite); the carbon atoms correspond to the positions of the silicon
atoms in β-tridymite and by adding the oxygen atoms half-way in between as before,
we generate the complete β-tridymite structure. In the α forms of these structures there
are small deviations from the cubic and hexagonal symmetries which give rise to lower-
symmetry enantiomorphous crystal forms.
Quartz is the best-known and most readily recognized mineral (as a visit to any ‘rock-
shop’ will show). Its common name is rock-crystal and it was thought by the ancients to
be a form of ice, frozen so hard that it did not melt. It was described in detail by Strabo
(d. ad 24), the Greek geographer who travelled widely throughout the Roman empire,
recording the geology and mining operations. He gave it the name Krystallos, which is
Greek for ice, and from which our word crystal is derived. The notion that rock-crystal
was a form of ice has a long history and persisted as late as the seventeenth century, but
what is very curious is that the structures of ice and water do have strong resemblances
to those of silica as described below.
In structural terms, the water molecule may be regarded as a sphere, or rather a
spherical envelope or radius 1.38 Å, with the large oxygen atom in the centre and two
(small) hydrogen atoms ‘embedded’ within it (Fig. 1.34).
These hydrogen atoms may be supposed to occupy two corners of a tetrahedron, the
other two corners being empty. However, it is not the location of the hydrogen atoms
which is important, but rather that the corners of the tetrahedron are oppositely charged,
1.11 Some more complex crystal structures 45
H
H
+
+
–
–
1.38 Å
1.01 Å
Fig. 1.34. The tetrahedral distribution of chargeon the water molecule and its effective radius (1.38 Å).
(From Crystal Chemistry, 2nd Edn, by R. C. Evans, Cambridge University Press, 1966.)
as indicated in Fig. 1.34. The water molecule, although it is neutral, is nevertheless polar
and the crystal structures of ice which arise are determined by the ways in which these
spherical, polar molecules, pack together. They are not close-packed, rather they form
tetrahedrally coordinated networks as in silica. In the common form of ice (ice-I
h
) the
pattern of H
2
O molecules is similar to that of the SiO
4
tetrahedra in β-tridymite except
that the hydrogen atoms are not disposed symmetrically between pairs of oxygen atoms
as indicated in Fig. 1.35(a) (Fig. 1.35(b) shows a model of an ice-I
h
snowflake crystal).
When water is frozen at very low temperatures, a structure (ice-I
c
) corresponding to
β-cristobalite is formed (and there are, as with silica, high pressure forms as well).
In liquid water, from about 4
◦
Cto150
◦
C (water II), the molecules are of course in a
state of flux, but at any moment small regions (10∼100 molecules in size) are arranged
in a β-quartz-like arrangement, denser, of course than the β-tridymite arrangement in
ice. Only at very high temperatures (>150
◦
C) and pressures do the molecules approach
random close-packing as in a liquid metal (water III).
Between 0
◦
C and 4
◦
C, as is known, the density of water increases. This is considered
to arise from the slow breakdown of the less-dense tridymite-like arrangement (water
I) to the more-dense quartz-like arrangement (water II). Above 4
◦
C, normal thermal
expansion is dominant.
Water is unique. It owes its properties—high melting and boiling points, latent heat
of boiling, etc.—to the divalency of oxygen, the polar nature of the molecules and the
hydrogen bonding between them. The distribution of charge, and the positions of the
hydrogen atoms (in both ice and water—Figs. 1.34 and 1.35(a)) is not fixed: the hydrogen
atom in any one bond may be associated with either of the two oxygen atoms each side
of it to give the alternative configurations O-H···OorO···H-O. In other words, there
is a multiplicity of ways in which a hydrogen atom can be arranged. In contrast, in H
2
S
the sulphur atom is insufficiently electronegative to form hydrogen bonds and H
2
S has
a low specific heat, a low boiling point (−62
◦
C) and forms close-packed structures, like
metals.
46 Crystals and crystal structures
: H;
x
y
z(a)
: O
(b)
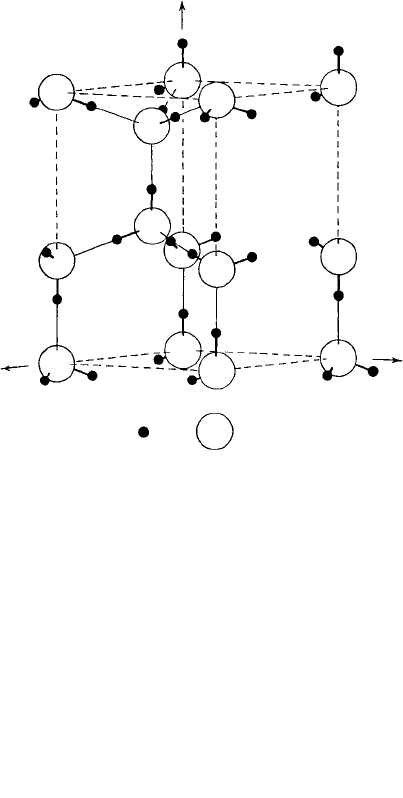
Fig. 1.35. (a) The hexagonal structure of ice-I
h
. The distribution of the hydrogen atoms is arbitrary.
(b) A model of an ice (ice-I
h
) crystal. The spheres represent water molecules tetrahedrally linked in the
hexagonal β-tridymite-like structure.
1.11.6 The structures of carbon
Of all the elements in the periodic table, only carbon and sulphur are capable of form-
ing elemental rings or chains whose stability is independent of length. However, the
chains formed by sulphur (with a higher valency than two), and those formed by
silicon, immediately below carbon in the periodic table, are thermally unstable and
1.11 Some more complex crystal structures 47
only short-length chain compounds are possible. Hence only carbon is capable of form-
ing the complex ring- and long-chain compounds which are the prerequisite of life itself:
the ‘sulphur man’ and ‘silicon man’ must remain figments of the imagination!
We will briefly survey only those structures of carbon itself, partly for their own
intrinsic interest and partly because they serve to illustrate some of the basic ideas we
have already met. These are diamond, graphite, ‘mesophase’, the fullerenes or ‘bucky
balls’ and nanotubes.
In diamond the basic structural unit is a carbon atom linked to four equidistant
neighbours in a tetrahedral coordination, which arises, in chemical terms, from the
sp
3
hybridization of the carbon atom. There are, however, two ways in which the tetra-
hedra can be linked together. In by far the commonest form of diamond the tetrahedra are
linked together to generate a cubic structure, the pattern of carbon atoms being precisely
the same as that in TiH (Fig. 1.14), or sphalerite or zinc blende, the cubic form of ZnS.
The analysis of this structure (by W. H. and W. L. Bragg in 1913) was indeed one of the
earliest triumphs of the X-ray diffraction technique. In the other, rare form of diamond,
called Lonsdaleite, after Kathleen Lonsdale
∗
, the tetrahedra are linked together to gen-
erate an hexagonal structure, the pattern of carbon atoms being precisely the same as
that in wurtzite, the hexagonal form of ZnS (Section 1.7). These structures are shown in
Fig. 1.36.
In graphite, the carbon atoms are linked together to form plane hexagonal nets or
graphene layers (sp
2
hybridization), the layers being stacked upon one another and held
together by weak (van der Waals) forces. As in the case of our close-packed layers of
metal atoms, the layers are not stacked immediately over each other (to give a simple
hexagonal structure) but are again displaced in either of two ways. In the commonest
form of graphite they are stacked in an ABAB sequence as in the hcp structure—carbon
atoms in the ‘B’ layers lying immediately above and below the hexagonal hollows of
the ‘A’ layers either side (Fig. 1.37(a)). This stacking pattern is precisely the same as
that of the cells in the two sides of a honeycomb—the corners of the cells on one side
corresponding with the centres of the cells on the other side.
5
In the uncommon form of graphite the layers are stacked in anABCABC … sequence
as in the ccp structure (Figs. 1.37(b)) and this is designated the rhombohedral form of
graphite on the basis of the simplest unit cell which can be drawn (see Fig. 1.7(c)).
However, just as in the case of metals ‘stacking faults’ doubtless occur particularly when
we shear the graphene layers over each other as we do when we write with a pencil! It
is also possible for the layers to be stacked in parallel layers but in a random orientation
(with no correlation between the atoms in each layer) and this so-called turbostratic form
appears to arise as an intermediate stage in the graphitization of pitch or some polymer
precursors.
Plan views of the stacking of the layers in the hexagonal and rhombohedral graphite
structures are shown in Fig. 1.37. These structures are related to the hexagonal and cubic
diamond structures respectively as shown in Exercise 1.10.
∗
Denotes biographical notes available in Appendix 3.
5
The dividing walls between the two sides of the honeycomb are not flat but are faceted at angles cor-
responding to those between the close-packed layers of atoms in the ccp structure (Section 1.3), and this
arrangement can be shown to result in the most economical use of beeswax.

48 Crystals and crystal structures
z
x
z
x
y
x
0
0
00
(a)
(b)
y
0
000
0
000
y
1
2
x
1
4
1
2
3
4
1
y
2
1
4
1
2
5
8
5
8
5
8
1
2
1
8
5
8
5
8
1
2
1
2
5
8
5
8
1
8
1
2
5
8
1
8
1
2
1
8
5
8
1
2
1
8
1
8
3
4
Fig. 1.36. (a) The cubic and (b) hexagonal structures of diamond in clinographic projection (left) and
in plan view (right). In both cases the carbon atoms are linked to four others in tetrahedral coordination
but the arrangement of the tetrahedra differs. The pattern of atom sites is precisely the same as in the
cubic (sphalerite) and hexagonal (wurtzite) forms of ZnS. (From Crystalline Solids by D. McKie and
C. McKie, Nelson, 1975.)
Perhaps the most remarkable of the structures of carbon—remarkable, that is, for
having been discovered so late—are the fullerenes or colloquially ‘bucky balls’. These
were postulated as an act of scientific imagination by D. E. H. Jones (who wrote under
the pen-name Daedalus) in 1966, but their actual existence and structures were not
established until 1985 by H. W. Kroto, R. W. Smalley, and their co-workers. If a hexagon
is replaced by a pentagon in a graphite-like net then, as model-building shows in a most
immediate way, thenetassumes a dome-like shape (Fig. 1.38). This principle was applied
in engineering design by the American architect and polymath R. Buckminster Fuller
∗
—
the distribution of pentagons and hexagons in the structure determining, of course, the
∗
Denotes biographical notes available in Appendix 3.

1.11 Some more complex crystal structures 49
A
B
A
B
A
C
(a) (b)
A-layer B-layer A-layer
B-layer C-layer
Fig. 1.37. (a) The hexagonal (layer sequence ABAB … ) and (b) the rhombohedral (layer sequence
ABCABC … ) structures of graphite. These are shown (below) in plan view with the layers shown
‘offset’ for clarity. A-layer—full lines; B-layer—dashed lines and C-layer—dotted lines.
overall shape and stability of what he called a geodesic dome
6
. In order to create a
complete closed sphere precisely 12 pentagons are needed and the smallest, simplest
sphere consists of 12 pentagons only, without any hexagons. The shape or polyhedron
which is formed is called a pentagonal dodecahedron (Fig. 1.39(a)) and is one of the
five ‘perfect’ polyhedra, or Platonic solids since all the 12 pentagonal faces and all the
20 vertices or ‘corners’ where the carbon atoms are situated are identical. [The other
Platonic solids are the cube (6 square faces, 8 vertices, Fig. 4.2(a)), the octahedron (8
triangular faces, 6 vertices, Fig. 4.2(b)), the tetrahedron (4 triangular faces, 4 vertices,
6
A geodesic, meaning ‘earth dividing’, is the shortest distance between two points across the surface of a
sphere—just as a straight line is the shortest distance between two points in a plane. A geodesic is part of the
circumference of what is called a great circle (see Chapter 12), a circle which has its centre at the centre of the
sphere and which divides the sphere into two equal halves. In the design of geodesic domes, the constructional
struts, the ‘geodesics’, follow the lines of great circles such that the stresses are distributed evenly. The edges
of the Platonic, and many other polyhedra, also follow the lines of great circles (see Appendix 2).

50 Crystals and crystal structures
(a)
(b)
Fig. 1.38. Model-building with simple linked straws or sticks, (a) The plane graphite-like or graphene
net formed by linking hexagons and (b) the dome-like net formed when a hexagon is replaced by a
pentagon.
Fig. 4.2(c)), and the icosahedron, Fig. 1.39(b) (20 triangular faces, 12 vertices)]. Hence,
the simplest and smallest of the ‘bucky-balls’ consists of 20 carbon atoms. It is called
dodecahedrene, but it has not been isolated and is doubtless thermally unstable.
As an increasing number of hexagons are included in the structure a family of
fullerenes of increasing thermal stability and an increasing number of carbon atoms
is developed (Fig. 1.40). The most interesting of these is Buckminsterfullerene itself
with 60 carbon atoms (C
60
) consisting of 12 pentagons separated from each other by 20
hexagons (Fig. 1.40 (a)) precisely in the same way as a football (or US soccer ball). This
polyhedron is called a truncated icosahedron and is one of the thirteen ‘semi-regular’ or
Archimedean polyhedra in which every face is a regular polygon, though not all faces
are of the same kind. In crystals of C
60
the spheres arrange themselves in an fcc pattern

1.11 Some more complex crystal structures 51
(a) (b)
Fig. 1.39. (a) the pentagonal dodecahedron (12 pentagonal faces) and (b) the icosahedron (20 trian-
gular faces)—two of the five ‘perfect’or ‘Platonic’polyhedra (the others being the cube, the octahedron
and the tetrahedron—see Fig. 4.2 and Appendix 2).
C
28
C
32
C
50
C
60
C
70
(a)
(b)
Fig. 1.40. (a) Buckminsterfullerene, C
60
, consisting of 12 pentagonal and 20 hexagonal faces (from
Perfect Symmetry by Jim Baggott, Oxford University Press, 1994) and (b) fullerenes C
28
–C
70
all of
which have 12 pentagonal faces and an increasing number of hexagonal faces (from Science 242, 1142,
1988: reproduced by courtesy of Prof. Sir Harry Kroto).

52 Crystals and crystal structures
Fig. 1.41. The silicaceous skeleton of Aulonia hexagona, a deep sea-water radiolarian, drawn by E.
Haeckel (from ‘On Growth and Form’ by D’Arcy Wentworth Thompson, Cambridge University Press,
1942).
but doubtless faulting can also occur. The next fullerene, C
70
, with 70 carbon atoms,
has the shape of a Rugby ball (US football) and fullerenes with much larger numbers of
carbon atoms also occur. These structures have their counterparts in the structures of the
spherical skeletons of the radiolaria (deep sea-water creatures) where pentagons (and
octagons and heptagons) occur in a predominantly hexagonal network (Fig. 1.41). Here
we see nature obeying the dictates of geometry both on an atomic and on a biological
scale!
Finally, the graphene layers may not lie flat as in graphite, but may ‘roll up’ with the
edges joined together to form carbon ‘nanotubes’. These may consist of a single rolled-up
layer (single walled nanotubes) or many such tubes, one inside the other (multi-walled
nanotubes) the spacing between the graphene layers being rather larger than that for
graphite. These structural forms of carbon were first identified by Iijima in 1991 and
Bethune et al. in 1993, but again were suggested as a possible form of carbon by D.E.H.
Jones in 1972. The tubes may also be ‘capped’ with fullerene hemispheres, to form
close-ended nanotubes.
Carbon nanotubes also exhibit different geometries or patterns depending upon the
orientations of the carbon hexagons with respect to the tube axis. Figure 1.42 shows
nanotube models made from sheets depicting graphene layers rolled up in different
ways and overlapped such that the hexagons match along the join. In Fig. 1.42(a) the
carbon atoms show an ‘armchair’ pattern along the axis of the tube; in Fig. 1.42(b)
a ‘zig-zag’ pattern and (perhaps of most interest) in Fig. 1.42(c) the carbon hexagons
form a helical pattern along the axis of the tube. Clearly, there are many more helices
possible, of different pitches, both right and left handed. These different patterns are not

Exercises 53
(a)
(b)
(c)
Fig. 1.42. Models of single-walled carbon nanotubes showing different orientations of the graphene
cells along the tube axis (a) ‘armchair’, (b) ‘zig-zag,’ and (c) helical conformations.
just of crystallographic model-making interest but give rise to entirely different thermal,
conductivity and strength properties and which make carbon nanotubes of such potential
importance in nanotechnology.
A note on boron nitride—boron and nitrogen, which occur each side of carbon in the
periodic table, form BN compounds with strikingsimilaritiesto those of carbon described
above. The boron and nitrogen atoms are linked alternately in a planar (sp
2
) conforma-
tion, giving rise to graphite-like structures, or in a tetrahedral (sp
3
) conformation giving
diamond-like structures—the latter possess very high hardness and melting-points and
are of great importance in materials engineering. It is not known whether BN also
exhibits Buckminster fullerene structures—this may be unlikely in view of the necessity
for adjacent boron or nitrogen atoms in the 5-ring components. However, open-ended
‘nanotube’ structures, which do not require 5-ring components, may occur.
Exercises
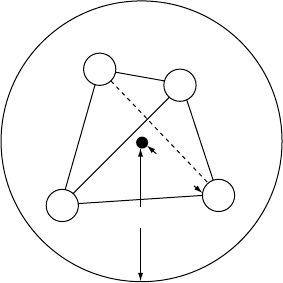
1.1 With reference to Fig. 1.5(b) or Fig. 1.11(a), determine the c/a ratio for the hcp structure.
(Hint: a is equal to d , the atomic diameter and edge length of the tetrahedron, and c is
twice the height of the tetrahedron. Determine also the height of the tetrahedron above the
triangular base.
1.2 Determine the radius ratio, r
X
/r
A
, for the tetrahedral interstitial sites in the bcc structure.
1.3 Determine the radius ratio, r
X
/r
A
, for the six-fold coordinated interstitial site in the simple
hexagonal structure.
1.4 Examine your crystal models and find:
(a) the number of different (non-parallel) close-packed planes and close-packed directions
in the ccp and hcp structures; and
(b) the number of closest-packed planes and close-packed directions in the bcc structure.
