Hammond C. The Basics of Crystallography and Diffraction
Подождите немного. Документ загружается.

34 Crystals and crystal structures
.... Stacking of the Zn (or S) atoms in zinc blende and the ∇∇ ... stack-
ing of the Zn (or S) atoms in wurtzite. Many carbides, including silicon carbide,
SiC, also possess such close-packed structures with the carbon atoms occupying the
tetrahedral sites and the metal or metalloid atoms stacked in various combinations of
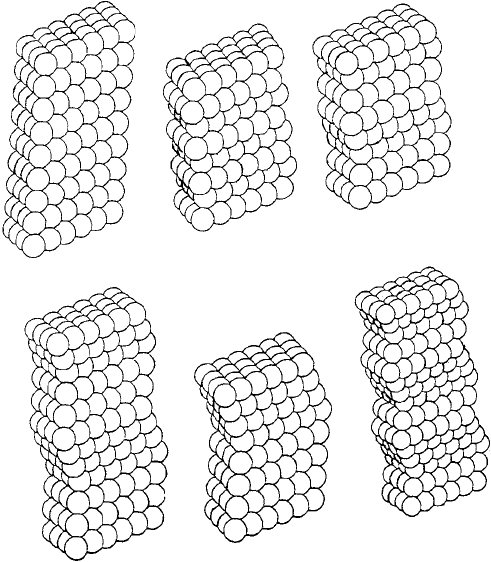
... and ∇∇ .... Figure 1.26 shows five structures or polytypes of silicon
carbide (B3–B7) in which the silicon atoms are represented as solid spheres and the
carbon atoms as small open spheres. The low-temperature form, β-SiC (B3) has the fcc
structure, isomorphous with zinc blende. There are a number of high-temperature poly-
types known collectively as α-SiC, the simplest structure of which, B4, is isomorphous
with wurtzite. The other polytypes of α-SiC, three of which (B5, B6, B7) are shown in
Fig. 1.26, have more complex stacking sequences, resulting in longer unit cell repeat
distances.
For example, the stacking sequence in B5 (Carborundum III) is (reading up) ABA-
CABAC giving a four-layer repeat distance. In the Frank notation this is represented
as ∇∇∇∇... i.e. by inversions in the stacking sequence every two layers, rather
than every layer as in B4, the wurtzite structure. Similarly, for B6 (Carborundum II)
the stacking sequence is ABCACBA… giving a six-layer repeat distance which in the
Frank notation is ∇∇∇...i.e. an inversion every three layers.
Figure 1.27 shows an alternative representation of the polytypes of SiC showing (for
clarity) only the close-packed layers of the metalloid (Si)atoms. The number below each
polytype refers to the number of layers in the unit cell repeat distance and the letter
refers to the type of unit cell (C—cubic; H—hexagonal; R—rhombohedral). All these
polytypes of silicon carbide should not be thought of as distinct ‘species’, rather they
should be regarded as interrelated as a result of different sequences of stacking faults and
the transformations β α SiC appear, from electron microscopy evidence, to occur
as the result of the passage of partial dislocations across the close-packed planes in the
same way as for the generation of a twinned crystal, as shown in Fig. 1.20. For example
6H, a common form of α-SiC, may be regarded as a ‘microtwinned’ form of 3C, the
three-layer thick twins being generated by the passage of three partial dislocations on
successive close-packed planes as shown in Fig. 1.20(d).
In aluminium oxide, A1
2
O
3
, the large oxygen anions occur in close-packed or nearly
close packed layers with the aluminium cations occupying two-thirds of the octahedral
interstitial sites. We now have an added complexity; not only may the oxygen anions
be packed in different sequences but also the aluminium cations may be distributed
differently throughout the interstitial sites—i.e. there may be different distributions
of the one-third ‘empty’ sites. In the well-characterized form, α-Al
2
O
3
(corundum-
isomorphous with α-Fe
2
O
3
), the oxygen anions are stacked in the hcpABAB … stacking
sequence and the aluminium cations between them are stacked in a rhombohedral
sequence in exactly the same pattern as the carbon atoms in the rhombohedral form
of graphite (see Fig. 1.37(b)). Hence the structure of α-Al
2
O
3
is rhombohedral with a
six-layer unit cell repeat distance of oxygen anions. The other polytypes of alumina,
called the transition aluminas, are not so well characterized, particularly with respect
to the distribution of the aluminium cations. A common form, γ -Al
2
O
3
is based on an
ABCABC … (fcc) stacking of oxygen anions with a distribution of aluminium cations
which gives rise to a maghemite structure, described in Section 1.11.3.
1.11 Some more complex crystal structures 35
C
C
B
B
B
B
B
B
C
A
A
C
B
B
B
B
B
A
A
A
A
A
A
A
B
B
B
B
B
B
B
B
B
B
B
B
C
C
C
C
C
C
C
C
C
C
C
A
A
A
A
A
A
A
A
A
A
A
A
(a) (b) (c)
(d) (e) (f)
3C
6H 8H
15R
2H
4H
C
Fig. 1.27. The crystal structures of six SiC polytypes. 3C is β-SiC, the fcc low temperature form and
the others with 2, 4, 6 and 8-layer repeat hexagonal cells or a 15-layer repeat rhombohedral cell are
the α-SiC high temperature forms (from Ceramic Microstructures by W. E. Lee and W. M. Rainforth,
Chapman & Hall, 1994).
1.11.3 The oxides and oxy-hydroxides of iron
Iron is remarkable for the range of oxides and hydroxides which can be formed and is
one of the few elements which form compounds intermediate between these two—the
oxy-hydroxides, the crystal structures of which are also of interest, and we will now
describe them (as far as we are able) and trace their interrelationships.
We will begin with the simplest oxide, wustite, ferrous oxide, FeO and consider
the structural changes which take place in the progressive oxidation of FeO to Fe
3
O
4
(ferroso-ferric oxide) and Fe
2
O
3
(ferric oxide). FeO has the NaCl structure—the Fe
2+
ions are situated in the octahedral interstitial sites between the oxygen anions
3
. How-
ever, FeO is rarely stoichiometric but has vacant Fe
2+
sites in the face-centred cubic
3
The terms ‘anion’and ‘cation’are used when we want to draw specific attention to the charge on the ionic
species, otherwise the more general term ‘atom’ is used—it being understood that the term encompasses both
neutral and charged species.
36 Crystals and crystal structures
structure, electrical neutrality being preserved by the presence of equal numbers of Fe
3+
ions (some of which are situated in the tetrahedral interstitial sites). The ‘oxidation’ of
FeO to Fe
3
O
4
proceeds, not by the ‘addition’of oxygen atoms to the structure, but by the
migration of Fe atoms to the surface (to combine with atmospheric oxygen there)—to a
first approximation the close-packed oxygen atoms in the original FeO structure remain
undisturbed. Within the structure the Fe
2+
ions are progressively replaced by Fe
3+
ions,
half of which are situated in the tetrahedral interstitial sites. The structure of Fe
3
O
4
(mag-
netite) thus formed is that of an inverse spinel with the general formula AB
2
O
3
in which
the ‘A’ tetrahedral sites are occupied by Fe
3+
ions and the ‘B’ octahedral sites by equal
numbers of Fe
3+
and Fe
2+
ions. Fe
3
O
4
is therefore better represented by the formula
Fe
3+
(Fe
3+
Fe
2+
)O
4
. However, the occupancy of the interstitial sites is not random, but
ordered such that the unit cell of Fe
3
O
4
has twice the side-edge (eight times the volume)
of the original FeO unit cell and contains 32 O
2−
ions, 8 Fe
2+
ions and 16 Fe
3+
ions.
The distinction betweenan‘inverse’and a ‘normal’spinel (bothbadnames!) is simple:
spinel is the mineral MgAl
2
O
4
. The Mg
2+
ions occupy the ‘A’ tetrahedral sites and the
Al
3+
ions occupy the ‘B’ octahedral sites—with regard to the ‘A’ sites only the inverse
of Fe
3
O
4
. Again, we see that the occupancies of the interstitial sites are determined not
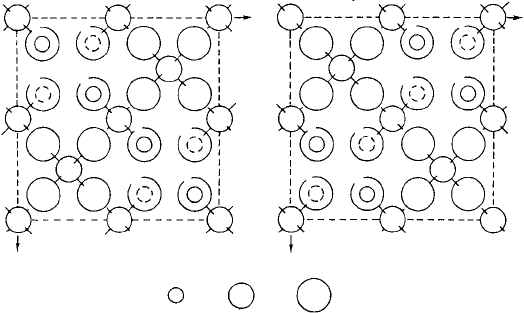
simply by the valencies of the ions but also their sizes. Figure 1.28 shows a projection,
or crystal plan of the spinel structure (see Section 1.8), split into two halves to make the
atom/ion positions more clear.
As oxidation proceeds further the remaining eight Fe
2+
ions in the unit cell are
replaced by two-thirds of their number by Fe
3+
ions (i.e. to maintain electrical neutrality)
giving a total of 16 +5
1
/
3
= 21
1
/
3
Fe
3+
ions, 32 O
2−
ions and the overall composition
Fe
2
O
3
. Except for a small reduction in volume (on account of the increased number
of vacant lattice sites) the large cubic unit cell is unchanged. The structure is called
maghemite, γ -Fe
2
O
3
and is isostructural with the γ -Al
2
O
3
structure (see Section 1.11.2).
1
0
040
0
55
5
5
5
5
5
5
5
5
5
5
6
6
7
7
7
7
44
400
7
7
7
77
7
77
3
1
3
4
4
0
3
2
2
3
3
3
3
4
4
0
0
: B;: A;: X
0
xx
3
3
33
3
1
1
1
yy
1
1
1
1
11
1
Fig. 1.28. Plan view of the unit cell of the cubic spinel structure AB
2
X
4
projected on to a plane
perpendicular to a cube axis. The heights of the atoms are indicated in units of one-eighth the cubic
cell edge length. For clarity, the upper and lower halves of the cell are shown separately and only the
tetrahedral coordination of the A ions is indicated. (From Crystal Chemistry, 2nd Edn, by R. C. Evans,
Cambridge University Press, 1966.)
1.11 Some more complex crystal structures 37
However, γ -Fe
2
O
3
(like γ -Al
2
O
3
) is not the stable structural form and its conversion to
α-Fe
2
O
3
(haematite) occurs essentially by the rearrangement of the oxygen atoms from
the fcc to the hcp stacking sequence.
Similar relationships exist in the oxy-hydroxides in which iron is in the Fe
3+
(ferric)
form. The formulae are best written FeO·OH (rather than Fe
2
O
3
·H
2
O) to emphasize the
replacement of oxygen ions by hydroxyl, (OH)
−
groups rather than the presence in the
structure of discrete ‘molecules’ of water. In γ -FeO·OH (lepidocrocite), the oxygen and
hydroxyl ions are approximately cubic close-packed and in α-FeO·OH (goethite), they
are approximately hexagonal close-packed. The deviation from perfect close-packing
arises from the formation of directed hydrogen bonds between the hydroxyl groups
in different layers; the structures are not cubic or hexagonal but have orthorhombic
symmetry (see Chapter 3). On heating, these oxy-hydroxide structures dehydrate to
γ -Fe
2
O
3
and α-Fe
2
O
3
, respectively.
Lepidocrocite and goethite are not the only oxy-hydroxides which occur. Of perhaps
more biological or technological importance is ferrihydrite, a poorly crystallized mineral
which is ubiquitous across the Earth’s surface. It is a common product of weathering of
iron-bearing minerals and of the microbial oxidation of ferrous ions and doubtless of the
rusting of iron itself.
The determination of its crystal structure and composition is a matter of considerable
difficulty because of the very small crystallite size, typically in the range 2–10 nm. How-
ever, it appears that the oxygen-hydroxyl groups are arranged in an hcp (2H, 4H or 6H)
stacking sequence (see Fig. 1.27), i.e. closer to goethite than lepidocrocite and possibly
isostructural with a natural alumina hydrate phase, akdaleite. The composition is com-
monly given as Fe
5
(OH)
8
.4H
2
O but the water content appears to depend on particle size.
Ferrihydrite is an example of a naturally occuring nanocrystalline material—upon
which a whole new science and technology is now being built. Their importance consists
in the simple fact that at these sizes a large proportion of the atoms or ions are at, or
near, the surface and the conditions for the stability of the structures are found to be (as
might be expected) quite different at the centres of the crystals and at their surfaces.
Such nano-sizedcrystals of ferrihydrite alsoappear to constitute theinorganic ‘core’of
the ferritin molecule—the iron storage molecule that occurs principally within the liver.
The core is enclosed within a protein ‘shell’ (Fig. 1.29) which has a cubic symmetry not
found in any inorganic crystals (see Chapter 4).
The mechanisms by which iron leaves and enters the ferritin molecule, and how
it is taken up, and released, from the ferrihydrite, are matters of much research. But
undoubtedly the crystallography—the valency and distribution of iron ions across the
interstitial sites both at and below the crystal surfaces—will emerge as factors of great
importance.
1.11.4 Silicate structures
Silicates—which constitute by far the most important minerals in the earth’s crust—
are based on the different ways in which SiO
4
tetrahedra
4
may be joined together, each
4
The SiO
4
tetrahedra may be referred to more generally as Si–O tetrahedra since, as described in sub-
sections (a)–(g), the silicon–oxygen ratio depends on how they are linked.
38 Crystals and crystal structures
Fig. 1.29. The cubic (point group 432) structure of the ferritin protein shell viewed along a cube axis.
(From Mineralization in Ferritin: An Efficient Means of Iron Storage by N Dennis Chasteen and Pauline
M Harrison, Journal of Structural Biology 126, 182–, 1999.)
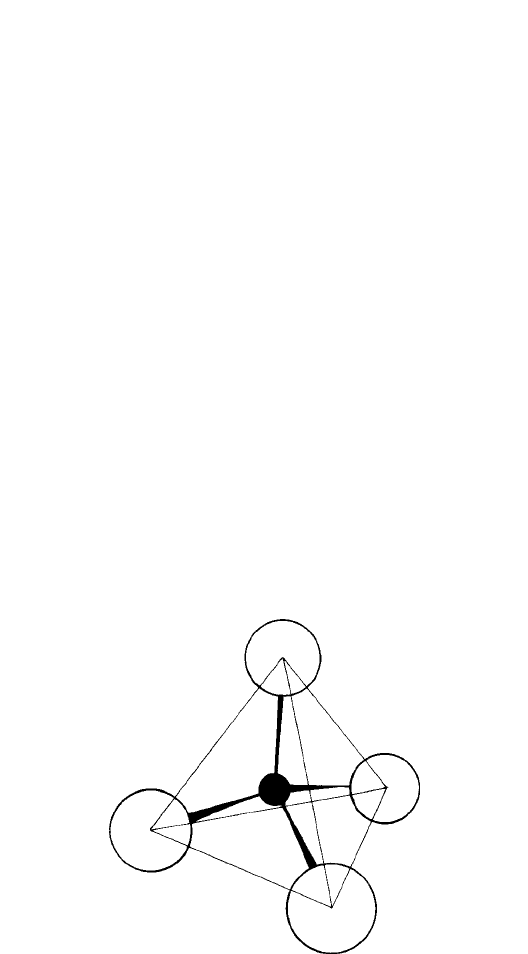
Fig. 1.30. A perspective of the SiO
4
tetrahedron. The oxygen anions at the corners of the tetrahedron
are nearly close packed, as shown in the models, e.g. in Fig. 1.7 (the radius of the oxygen anion,
r
n
= 0.132 nm and that of the silicon cation, r
X
= 0.039 nm, give a radius ratio, r
X
/r
n
= 0.296,
slightly larger than the ideal value r
X
/r
n
= 0.225, Fig. 1.10(a) and (b)).
1.11 Some more complex crystal structures 39
tetrahedron being made up of four oxygen anions with the silicon cation in the tetrahedral
interstice in the centre (Fig. 1.30). Silicate chemistry is based on the linking of the SiO
4
tetrahedra, i.e. whether they occur separately, or whether they are linked by common
oxygen anions to form chains, rings, sheets or complete frameworks. This provides the
initial basis for the classification of silicate structures. If the Si–O bond is considered
to be purely ionic, there are four positive charges associated with each silicon cation
and two negative charges associated with each oxygen anion; hence there are four net
negative charges associated with each SiO
4
tetrahedron. The ‘charge balance’in silicates
may be achieved in seven possible ways (see Fig. 1.31):
(a) Separate SiO
4
tetrahedra (nesosilicates): the charge balance (four net negative
charges) is achieved with metal cations, e.g. Mg
2+
,Fe
2+
, which also link the tetra-
hedra together. Typical minerals are forsterite, Mg
2
(SiO
4
), or fayalite, Fe
2
(SiO
4
),
the end-members of the olivine group, MgFe(SiO
4
).
(b) Two tetrahedra linked together sharing one oxygen anion (sorosilicates): the Si:O
ratio is now Si
2
O
7
giving six net negative charges which are balanced with
metal cations. Typical minerals are melilite, Ca
2
Mg(Si
2
O7), or hemimorphite,
Zn
4
(OH)H
2
O(Si
2
O
7
).
(c) Three or more tetrahedra linked together to form rings, each tetrahedron sharing
two oxygen anions (cyclosilicates): the Si:O ratio is Si
n
O
3n
, where n is the number
of tetrahedra in the ring. A typical mineral is beryl, with a ring of six tetrahedra,
Be
3
Al
2
(Si
6
O
18
).
(d) Many tetrahedralinked together toform single chains(inosilicates): each tetrahedron
shares two oxygen anions, as in the ring structures above, and which therefore give
rise to the same Si:O ratio. This is the basis of the group of minerals called the
pyroxenes. Typical examples are enstatite, Mg
2
(Si
2
O
6
), or diopside, CaMg(Si
2
O
6
).
(e) Tetrahedra linked together to form double chains (inosilicates), each tetrahedron
sharing alternately two and three oxygen anions, giving the Si:O ratio Si
4
O
11
. This
is the basis of the group of minerals called the amphiboles. Typical examples are
anthophyllite, Mg
7
(OH)
2
(Si
4
O
11
)
2
, or tremolite, Ca
3
Mg
5
(OH)
2
(Si
4
O
11
)
2
.
(f) Tetrahedra linked together to form sheets (phyllosilicates), each tetrahedron sharing
three oxygen anions giving the Si: O ratio Si
2
O
5
. This is the basis of the micas,
chlorites and the clay minerals.
(g) Tetrahedra linked together such that all the oxygen anions are shared giving a three-
dimensional framework (tectosilicates). The Si:O ratio is now SiO
2
and there is an
overall charge balance without the necessity of any linking cations.
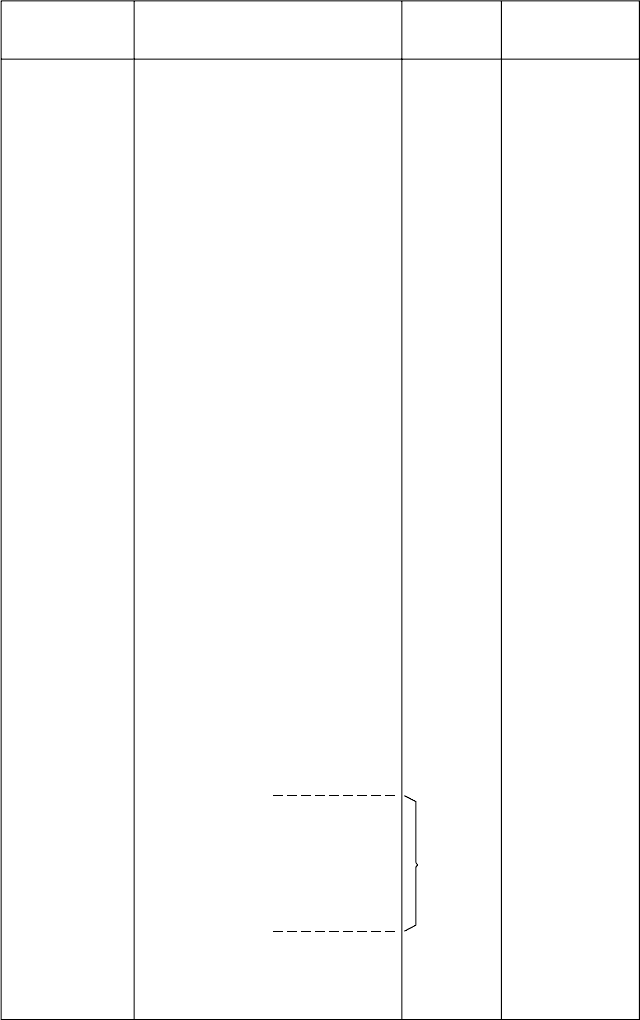
Figure 1.31 shows the arrangements of SiO
4
tetrahedra in these seven silicate
structures. However, having established the basic pattern there are very important com-
plications both in the chemistry and the arrangements of the tetrahedra which must not
be overlooked. In all the silicates, and in the chain, sheet and framework silicates in par-
ticular, the silicon in the centre of the tetrahedron can be substituted by aluminium—a
trivalent rather than a tetravalent ion. For each such substitution an additional positive
charge by way of a ‘linking cation’is required. All sheet silicates or phyllosilicates show
40 Crystals and crystal structures
Class
(a)
Nesosilicates
Oxygen
(SiO
4
)
4–
Olivine,
(Mg, Fe)
2
SiO
4
Hemimorphite
Zn
4
Si
2
O
7
(OH)·H
2
O
Beryl,
Be
3
Al
2
Si
6
O
18
Pyroxene
e.g. Enstatite,
MgSiO
3
(Si
2
O
7
)
6–
(Si
6
O
18
)
12–
(Si
2
O
6
)
4–
(b)
Sorosilicates
(c)
Cyclosilicates
(d)
Inosilicates
(single chain)
Arrangement of SiO
4
tetrahedra
(central Si
4+
not shown)
Unit
composition
Mineral example

1.11 Some more complex crystal structures 41
(e)
Inosilicates
(double chain)
Amphibole
e.g. Anthophyllite,
Mg
7
SiO
8
O
22
(OH)
2
(Si
2
O
5
)
2–
(SiO
2
)
0
Mica
e.g. Phologopite,
KMg
3
(Alsi
3
O
10
)(OH)
2
High cristobalite,
SiO
2
(f)
Phyllosilicates
(g)
Tectosilicates
(Si
4
O
11
)
6–
Fig. 1.31. The arrangements of the SiO
4
tetrahedra and Si:O ratios in the seven main types of silicate
structures, with examples of typical minerals (from Manual of Mineralogy, 21st edn, by C. Klein and
C. S. Hurlbut Jr., John Wiley & Sons Inc., 1993).
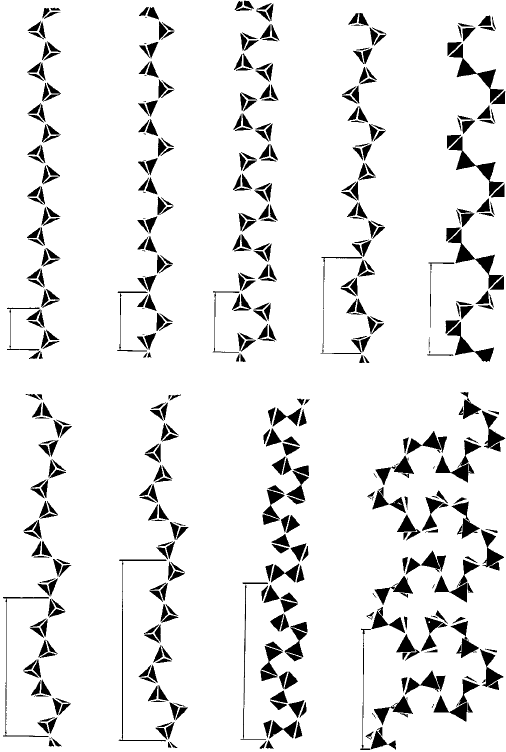
42 Crystals and crystal structures
(a)
(f)
(b)
(c)
(d)
(e)
(g)
(h) (i)
Fig. 1.32. The arrangements of the tetrahedra in the inosilicates. They are described (in German)
according to the number of the tetrahedra in the repeat distance: a (zweier); b (dreier); c (vierer);
d (fünfer); e (sechser); f (siebener); g (neuner); h (zwölfer); i (24er) (from Structural Chemistry of
Silicates by F. Liebau, Springer-Verlag, 1985).
such substitution to a greater or lesser extent, for example phlogopite in which one sil-
icon is replaced by one aluminium cation giving the formula KMg
3
(OH)
2
(Si
3
AlO
10
).
In framework silicates (tectosilicates), substitution gives rise to an important new class
of minerals—the feldspars, the most abundant minerals in the earth’s crust, e.g. albite,
Na(Si
3
A1O
8
), or orthoclase, K(Si
3
A1O
8
) (one silicon cation substituted), or anorthite,
Ca(Si
2
Al
2
O
8
) (two silicon cations substituted).
1.11 Some more complex crystal structures 43
With respect to the arrangements of the tetrahedra, one example—that of the
inosilicates—will suffice to show the principles involved. Figure 1.32 shows nine pos-
sible patterns (a)–(i) or conformations of the (unbranched) single chains, giving rise
to different repeat distances as indicated: (a) (the simplest—known as zweier single
chains because there are two tetrahedra in the repeat distance) is that for diopside and
enstatite; (b) (drier single chains with three tetrahedra in the repeat distance) is that for
wollastonite, Ca
3
(Si
3
O
9
), and so on.
Clearly, there are also many possible arrangements of the tetrahedra in the cyclo-
silicates, phyllosilicates and tectosilicates, and it is these which give rise (in part) to
the many structural differences in silicate minerals. For example, in the tectosilicates
the three different crystallographic forms of silica—quartz, tridymite and cristobalite—
simply correspond to different ways in which the SiO
4
tetrahedra are linked together.
1.11.5 The structures of silica, ice and water
Of the three structural forms of silica—quartz, tridymite and cristobalite (not counting
the high-pressure forms, coesite and stishovite)—quartz is by far the most common and
is structurally stable at ambient temperatures, whereas tridymite is stable between 857
and 1470
◦
C and cristobalite is stable from 1470
◦
C to the melting point. At ambient
temperatures, these latter two forms of silica are therefore metastable but they do not
transform to quartz because in order to do so, a rearrangement of the linking of the
SiO
4
tetrahedra needs to take place—in short, a reconstructive phase transformation
must occur in contrast to a displacive transformation in which atomic bonds are not
broken. However, displacive transformations occur in all three forms of silica by small
rotations of the SiO
4
tetrahedra, giving rise to the ‘more open, high temperature’ β
forms and the ‘more closed, low temperature’ α forms. This is illustrated, for quartz, in
Fig. 1.33. Figure 1.33(b) is a plan view or projection of the hexagonal β-quartz structure
perpendicular to the c-axis. For simplicity, only the Si atoms are indicated and their
relative heights along the c-axis: white (0), grey (1/3) and black (2/3). Figure 1.33(a)
is the corresponding projection for α-quartz; the structure is ‘twisted’ but no bonds are
broken. The symmetry also changes from hexagonal to trigonal, as will be described in
Section 3.3.
However, it is worth noticing at this stage one very important structural feature of
quartz: the silicon atoms (and hence the SiO
4
tetrahedra) are arranged in a helical pattern
along the c-axis. If we imagine a spiral staircase in the centre of a hexagon then the steps
go: 0, 1/3, 2/3 … in a clockwise fashion giving rise to a left-handed screw or helix. Now,
we can interchange the positions of the grey and black atoms such that the steps go 0,
1/3, 2/3 … in an anticlockwise fashion giving rise to a right-handed screw or helix. In
short, quartz (both α and β) has two forms, one ‘left-handed’ and one ‘right-handed’and
is an example of an enantiomorphous crystal structure (see Table 3.1 and Section 4.5).
Quartz is the densest structural form (not counting the high-pressure form, coesite),
(∼2.66 g/cm
3
); tridymite and cristobalite have much more ‘open’ structures (∼2.33
g/cm
3
). In their β forms, these two structures are similar to those of wurtzite and zinc
blende (the two structural forms of zinc sulphide), respectively, the SiO
4
tetrahedra
being in the positions of the Zn and S atoms. More specifically, they correspond to the
