Hammond C. The Basics of Crystallography and Diffraction
Подождите немного. Документ загружается.


24 Crystals and crystal structures
AAA
AA
BB
C
Fig. 1.19. On deformation, a B layer atom glides not in a straight line, e.g. B → B (left to right) but
in two steps via a C site across the ‘saddle points’ between the underlying A layer atoms, i.e. B → C
(first step), C → B (second step).
B
A
C
B
B
B
B
A
A
A
(a) (b)
(c) (d)
C
C
C
C
C
C
B
B
B
A
A
A
C
C
C
B
B
B
B
A
C
C
C
B
B
B
B
A
A
A
A
A
A
A
Fig. 1.20. (a)-(d) the sequence of faulting in a ccp (or fcc) crystal which leads to the formation of
a twinned crystal as in Fig. 1.18. In Fig. 1.20(a) the close-packed layer to be faulted is an ‘A’ layer
(arrowed) which in Fig. 1.20(b) becomes a ‘B’layer and the layers above are relabelled accordingly; then
the next layer above (the ‘C’ layer, arrowed) is faulted to become an ‘A’ layer (Fig. 1.20(c)) and so on.
for the generation of a twinned crystal by deformation is illustrated sequentially in
Fig. 1.20. Figure 1.20(a) shows the close packed layers of a single fcc crystal ‘edge
on’. Let us now slip an A layer (arrowed) into the sites of the B layer atoms (partial
slip), as shown in Fig. 1.20(b). In doing so all the layers above the A layer move too:
B → C; C → A; A → B and so on. Figure 1.20(b) also shows these re-labelled lay-
ers of atoms. Now let us slip the next layer up (arrowed), C → A; and again A → B,
B → C and so on (Fig. 1.20(c)). Again, slip the third layer up (arrowed) and re-label
the layers—as shown in Fig. 1.20(d). As we can see we will ultimately generate the
1.9 Stacking faults and twins 25
twinned crystal as shown in Fig. 1.18. This mechanism for deformation-twin formation
may appear to be complicated; it occurs in practice (as in nearly all material deformation
processes) through the movement of (partial) dislocations, a subject of great importance
in materials science. The arrows in Fig. 1.19 represent the slip vectors for dislocations
passing through the crystal; a slip vector such as B→B, in which the stacking sequence
is unchanged, represents the passage of a whole dislocation; a slip vector such as B→C
or C→B in which, as described above, the stacking sequence is changed, represents
the passage of a partial dislocation. Such whole and partial dislocations also occur in
bcc metal (and other) crystals. In the particular example above (Fig. 1.19) the partial
dislocation vectors B → C and C → B (which lie in the close-packed slip plane) are
called Shockley partials, after William Shockley
∗
who first identified them.
The twinned crystal shown diagrammatically in Fig. 1.18 is just one particular exam-
ple of very general phenomenon, which occurs in crystals with much more complex
structures and in which the two parts of the twinned crystal may not simply be related by
reflection across the twin plane (reflection twins). In general a twinned crystal is one in
which the two parts are related to each other by a rotation (usually, but not necessarily,
180˚) about some particular direction called a twin axis. For example, we could create
the twinned crystal in Fig. 1.18 by folding the figure 180˚ along the dashed line (the
twin axis) which lies in the twin plane; alternatively we could ‘cut’ the single crystal in
Fig. 1.20(a) in two along the c layer and create the twinned crystal by rotating the upper
half 180˚ about a vertical direction (another twin axis) which in this case is normal to
the twin plane. In our crystal all these actions (reflection in the twin plane, rotation 180˚
about an axis in the twin plane, rotation 180˚ about an axis normal to the twin plane)
are all equivalent, but this is not necessarily the case as we will discover when we have
learned about the symmetry of crystals.
Some examples of twinned crystals from geology and metallurgy are shown in Fig.
1.21. The left–right-hand character of the twinned crystal in Fig. 1.21(a) is easily seen;
in practice the twins may be interpenetrated and twinning may occur not just in one
but in several different planes, as shown in Figs 1.21(b) and (c). In metals and alloys,
the presence of twins may be seen in polished and etched surfaces (Fig. 1.21(d)). Old
cast brass doorknobs provide a good and homely example. The stacking fault energy of
α-brass (Cu ∼ 30% Zn) is very low (30 mJ m
−2
) and almost all the grains or crystals
will contain twins. The corrosive contact of human hands reveals the irregular outlines
or boundaries between the grains and also the perfectly straight lines or traces of the
twin boundaries which terminate (unlike scratches) at the grain boundaries, as shown in
Fig. 1.21(d).
Twinning is very common in minerals, frequently occurring as a result of phase
transitions during cooling. They may be observed, like the brass, on the polished surfaces
of minerals, where they may give rise to beautiful iridescent textures as a result of the
diffraction and interference of light (Chapter 7). They may be more readily seen in
petrographic thin sections under the polarizing light microscope. For the petrologist
they constitute one of the most important means of mineral identification.
∗
Denotes biographical notes available in Appendix 3.

26 Crystals and crystal structures
(b)(a)
(c)
(d)
s
s
ma
a
a
s
m
m
m
s
a
Fig. 1.21. Examples of twinned crystals: (a) rutile (TiO
2
) twinned on a {101} plane (from Rutley’s
Elements of Mineralogy, 25th edn, revised by H. H. Read, George Allen and Unwin Ltd, 1962); (b)
multiple twinning in rutile (from Introduction to Crystallography, 3rd edn, by F. C. Phillips, Longmans,
1963); (c) interpenetrant twin in mercurous chloride (HgC1), twin plane {101} (from F. C. Phillips loc.
cit); (d) a photomicrograph ofthe etched surface ofα-brassshowing grainboundaries and (straight-sided)
annealing twins, twin plane {111}.
1.10 The crystal chemistry of inorganic compounds
The newcomer to crystallography is often dismayed by the daunting level of complexity,
or immensity of all the different crystal structures—not only inorganic but also organic.
The subject may seem to depend so much on rote-learning and the committing to memory
of a vast number of facts. The subject is certainly immense, but it is not arbitrary, and the
1.10 The crystal chemistry of inorganic compounds 27
rules or criteria determining the simplest structures described in the preceding sections
are the stepping-stones to an understanding of more complex structures.
We will consider the types of bonding which apply to inorganic crystals and the main
differences to organic crystals (which are considered in more detail in Chapter 4). We
have already seen how crystals may be represented as plans or projections (Section 1.8):
now we will see how they can be represented in terms of coordination polyhedra, which
lead naturally to a (brief) discussion of Pauling’s rules which describe the conditions
for the stability (and therefore the existence of) predominantly ionic crystal structures.
Then, in Section 1.11, we describe some rather more complex crystal structures which
either illustrate the principles we have already learned or for their own intrinsic interest
and topicality.
1.10.1 Bonding in inorganic crystals
So far in this book we have described some simple crystal structures solely in geometrical
terms—i.e. the various arrangements in which ions and atoms of different sizes can pack
together. Now we must consider the forces which hold the ions and atoms together. This
is of course a subject of profound importance in physical chemistry and we can only
summarize, in a very simple way, the main features of the bonding mechanisms.
In inorganic crystals, the dominant bonding forces are ionic or heteropolar bonds
and covalent or homopolar bonds, with lesser contributions from van der Waals and
hydrogen bonds.
Ionic bonding dominates in inorganic crystals as a result of the high electronegativity
difference between the atomic species—the (positive) metal cations on the one hand
and the (negative) F
−
,Cl
−
,O
2−
anions on the other. The simple compounds NaCl,
CsCl and CaF
2
which we have already described are examples of almost ‘pure’ ionic
bonding. Moreover, the ionic bond is non-directional, an important characteristic which
it shares with the metallic bond which is the dominant cohesive force in metals with
a high conductivity: the positive metal ions are held together by a ‘sea’ of conduction
electrons which are wholly non-localized, i.e. the electrons are not bound to individual
atoms.
As the electronegativity difference between the atomic species decreases, the covalent
bond begins to dominate: the bonding electrons are ‘shared’between the atoms instead of
being transferredbetween the atomic orbitals. In sulphides, e.g. ZnS, thebonding is partly
covalent. Unlike the ionicbond, the covalentbond is directional, the configuration around
the atoms corresponds to, or arises from, the configuration of the atomic orbitals. An
example of‘pure’covalent bondingis diamond (thehardest material known), described in
more detail in Section1.11.6. The carbon atomsindiamond are tetrahedrally coordinated,
corresponding to the pattern of the sp
3
orbitals. The metallic bond, mentioned above,
may be regarded as a ‘special case’ of a covalent bond—an atom shares electrons with
its nearest neighbours and the empty orbitals permit the flow of conduction electrons.
In many inorganic compounds, the bonding is a ‘mixture’ of (dominantly) ionic and
(dominantly) covalent bonding. For example, in calcite, CaCO
3
, the bonding between
the Ca
2+
and (CO
3
)
2−
ions is dominantly ionic but the bonds which hold the (CO
3
)
2−
groups together is dominantly covalent. The structure of calcite strongly resembles that

28 Crystals and crystal structures
: Ca; : O
Fig. 1.22. The rhombohedral structure of calcite, CaCO
3
. The cell shown is not the smallest unit cell
but corresponds to the cleavage rhombohedra which occur in natural crystals of calcite. The smallest
unit cell may be outlined by linking together the eight innermost Ca
2+
ions, following the pattern shown
in Figure 1.7(c). (From An Introduction to Crystal Chemistry, 2nd Edn, by R. C. Evans, Cambridge
University Press, 1964.)
of NaCl, except that the presence of the planar (CO
3
)
2−
groups, all orientated the same
way, parallel to the ABCABC … stacking sequence (see Fig. 1.6(b)), ‘distorts’the cubic
symmetry, or shape of the unit cell, to rhombohedral as shown in Fig. 1.22.
It is the high strength of ionic and covalent bonds which gives rise to the most char-
acteristic mechanical property of inorganic compounds—their high hardness. However,
many inorganic compounds are highly anisotropic in their mechanical properties, being
‘strong’in some directions and ‘weak’ in others. Examples are the ‘layer-structure’phyl-
losilicates, talc and mica (see Section 1.11.4) and graphite (see Section 1.11.6). Within
the layers the ions/atoms are strongly ionically or covalently bonded, but the layers are
only held together by much weaker van der Waals bonds which arise from the contin-
uously changing dipole moments within atoms or molecules. An analogous situation
applies to solid organic compounds; the atoms in the molecules themselves are strongly
covalently bonded but the molecules are held together by weak van der Waals bonds and
solid organic compounds are, in comparison, generally soft materials.
The concept of a molecule, introduced above, is of course fundamental in chemistry,
but it can be an obstacle in understanding the arrangements and proportions of atoms
and ions in inorganic compounds in the solid state. For example, it is not possible to
identify a ‘molecule’ of NaCl in a crystal (Fig. 1.14(a)); each Na ion is bonded equally
to six surrounding Cl ions and vice versa; the Na and Cl ions are not ‘paired’ to each
other in any special way. W. L. Bragg records (in The Development of X-ray Analysis)
that his chemical colleagues were loath to give up the idea of molecules in inorganic
compounds and even as late as 1927, Henry E.Armstrong, an eminent chemist, published
the following letter in Nature.
1.10 The crystal chemistry of inorganic compounds 29
‘Poor Common Salt’
“Some books are lies frae to end” says Burns. Scientific (save the mark) speculation would seem
to be on the way to this state!... Prof. W.L. Bragg asserts that “In sodium chloride there appear to
be no molecules represented by NaCl. The equality in number of sodium and chlorine atoms is
arrived at by a chess-board pattern of these atoms; it is a result of geometry and not of a pairing-off
of the atoms.”
This statement is more than repugnant to common sense. It is absurd to the n..th degree, not
chemical cricket. Chemistry is neither chess nor geometry, whatever X-ray physics may be. Such
unjustified aspersion of the molecular character of our most necessary condiment must not be
allowed any longer to pass unchallenged … It were time that chemists took charge of chemistry
once more and protected neophytes against the worship of false gods; at least taught them to ask
for something more than chess-board evidence.
However, perhaps the letter was written with a pinch of salt!
In addition, many inorganic compounds are non-stoichiometric. FeO, for example,
which has the same structure as NaCl, rarely has the exact formula FeO, but a smaller
proportion of Fe than in the ratio 1:1. This simply indicates the existence of vacant Fe
2+
lattice sites and equal numbers of Fe
3+
ions on other sites to ensure electrical neutrality
and does not indicate a distinct chemical compound (see Section 1.11.3).
However, as mentioned above, the concept of a molecule is of vital importance
in understanding the structures of solid organic compounds. The strong covalent bonds
which bindtogether the carbon, nitrogen, oxygen, etc. atoms within theorganic molecule,
and which cause it to retain its identity when the compound is melted, dissolved, or even
vaporized, are much stronger than the van der Waals bonds between the molecules
in the solid state. The symmetries of the crystal structures which occur are, to a first
approximation, determined by the overall shape or envelope of the molecules and the
ways in which they pack together in the most efficient manner. However, we defer any
further consideration of organic compounds until Chapter 4 when we have learned about
symmetry, lattices and the methods needed to describe the geometry of crystals.
1.10.2 Representing crystals in terms of coordination polyhedra
2
As we have seen in Sections 1.6 and 1.7, inorganic crystal structures may be described in
terms of (small) atoms or cations, surrounded, or coordinated by, (large) atoms or anions.
An alternative and very fruitful way of representing such structures is to concentrate
attention on the coordinating polyhedra themselves rather than the individual atoms
situated at their corners. We can then view the crystal structure as a pattern of linked
polyhedra, without our view being obscured, as it were, by the large atoms or ions
themselves.
The most important coordination polyhedra in inorganic crystals are the tetrahedron,
r
X
/r
A
= 0.225; the octahedron, r
X
/r
A
= 0.414 (see Section 1.6) and the cubeoctahe-
dron and hexagonal cubeoctahedron, r
X
/r
A
= 1.000, all of which occur in close-packed
2
See Appendix 2 – Polyhedra in crystallography.
30 Crystals and crystal structures
structures. The last two polyhedra represent the pattern of 12 atoms surrounding, or
co-ordinating, an atom in face-centred and hexagonal close-packing respectively.
The polyhedra may be separate or linked together in various ways – corner-to-corner,
edge-to-edge, face-to-face or in various combinations. Geometrically, the possibilities
are almost endless, but in terms of the stability of inorganic crystals, they are not. The
criteria which govern the stability, and hence possible structures, in (dominantly) ionic
crystals, were set out by Linus Pauling
∗
in a series of empirical rules. Essentially, these
rules express the requirement for a charge balance between a cation and its surround-
ing polyhedron of anions and also between an anion and the cations that immediately
surround it. The chemical law of valency is satisfied not by ‘pairing off’ individual
anions and cations but by a ‘sharing’, or distribution of (say) the positive charge of
a cation among the surrounding polyhedron of anions. This is further evidence of the
(c)
(a) (b)
Fig. 1.23. Co-ordination polyhedra in three simple crystal structures: (a) edge-sharing octahedra in
sodium chloride; (b) corner-sharing tetrahedra in zinc blende; and (c) corner-sharing octahedra and the
(central) cubeoctahedron in perovskite. (From An Introduction to Mineral Sciences by Andrew Putnis,
Cambridge University Press, 1992.)
∗
Denotes biographical notes available in Appendix 3.
1.11 Some more complex crystal structures 31
non-existence of identifiable ‘molecules’ in inorganic ionic structures. Pauling’s rules
also determine, or limit, the ways in which the polyhedra can be linked together. The
octahedra and cubeoctahedra surrounding larger and weaker cations may share corners,
edges or even faces, but the tetrahedra surrounding the smaller and more highly positive
cations tend only to share corners, such that the cations are as far apart as possible.
We have already encountered this aspect of Pauling’s rules in our discussion (Section
1.7) of the ‘non-existence’ of hcp structures in which all the tetrahedral sites are filled;
the sites occur in pairs with the tetrahedra arranged face-to-face and the cations are too
close to ensure stability. This also applies to the (Si,Al)O
4
tetrahedra which comprise the
building blocks of the silicate minerals (see Section 1.11.4), the tetrahedra are isolated
or share one, two, three or four corners, never edges or faces. However, this is not true of
all synthesized ceramic materials. The new nitrido-silicates and sialons are based on the
linkage of (Si, Al)(N, O)
4
(predominantly SiN
4
) tetrahedra which share edges as well as
corners and in addition, Si is octahedrally coordinated by N.
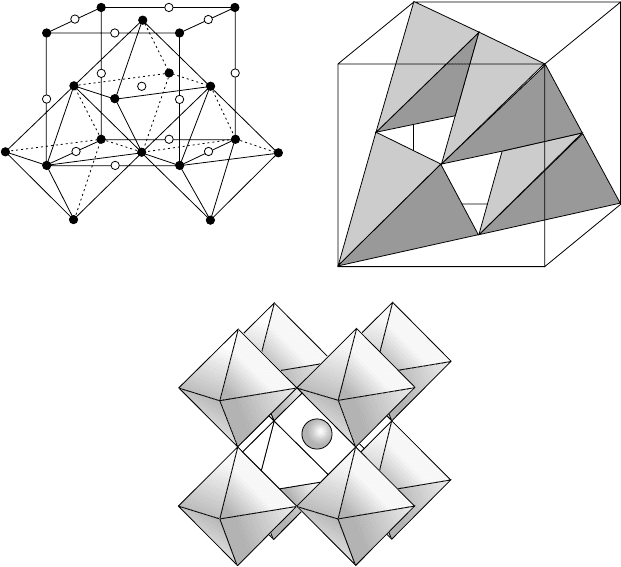
Figure 1.23 shows the coordination polyhedra in three simple crystal structures: (a)
the edge-sharing octahedra in sodium chloride, NaCl; (b) the corner-sharing tetrahedra
in zinc blende, ZnS; and (c) the corner-sharing octahedra and the central cubeoctahedron
in perovskite, CaTiO
3
. The unit cell shown is the same as that in Fig. 1.17(a).
1.11 Introduction to some more complex crystal structures
1.11.1 Perovskite (CaTiO
3
), barium titanate (BaTiO
3
) and
related structures
Perovskite is an important ‘type’ mineral (in the same way as sodium chloride, NaCl)
and is the basis of many technologically important synthetic ceramics in which the Ca
is replaced by Ba, Pb, K, Sr, La or Co and the Ti by Sn, Fe, Zr, Ta, Ce or Mn. The
general formula is ABO
3
(see Fig. 1.17), the A ion being in the large cubeoctahedral
sites and the B ion being in the smaller octahedral sites (Fig. 1.23(c)). In perovskite
itself, A is the divalent ion and B the tetravalent ion. This however is not a necessary
restriction; trivalent ions can, for example, occupy both A and B sites; all that is needed
is an aggregate valency of six to ensure electrical neutrality. It is, in short, a working
out of Pauling’s rules again. Of much greater importance are the sizes of these ions
because they lead, separately or in combination, to different distortions of the cubic cell.
In perovskite itself, the Ca cation is ‘too small’ for the large cubeoctahedral site and so
the surrounding octahedra tilt, in opposite senses relative to one another, to reduce the
size of the cubeoctohedral site. This is shown diagrammatically in Fig. 1.24(a). The unit
cell is now larger, as outlined by the solid lines, the unit cell repeat distance now being
between the similarly oriented octahedra (see Section 2.2). The symmetry is tetragonal,
rather than cubic. The tilts are also slightly out of the plane of the projection which
further reduces the symmetry to orthorhombic (see Chapter 3 for a description of these
non-cubic structures).
In barium titanate, BaTiO
3
, the Ti cation is ‘too small’for the octahedral site and shifts
slightly off-centre within the octahedron (Fig. 1.24(b)); the cubic unit cell is distorted to
tetragonal. These shifts may occur along any of the three cube-edge directions such that a

32 Crystals and crystal structures
(a) (b)
Fig. 1.24. Two modifications to the perovskite structure: (a) octahedra tilted in opposite senses, the
cubic cell is outlined by dashed lines and the tetragonal cell by full lines; (b) displacements of the B
cations from the centres of the octahedra resulting in a distortion of the original cubic cell to a tetragonal
unit cell (fulllines). (FromAn Introduction to Mineral Sciences byAndrew Putnis, Cambridge University
Press, 1992.)
Fig. 1.25. A polarized-light micrograph (crossed polars) of a single crystal of barium titanate which
reveals the domains in each of which the tetragonal distortion is in a different cube-edge direction.
single crystal of BaTiO
3
may be divided into domains; within each domain, the shift is in
the same direction (Fig. 1.25) (the idea of domains is discussed, with respect to ordered
crystals, in Section 9.7). The consequence is that within a crystal (or within a domain)
there is a net movement of charge, resulting in a structure with a dipole moment, and this
spontaneous electrical polarization leads to the property of ferroelectricity which is of

1.11 Some more complex crystal structures 33
such great importance in many electronic devices and is the basis of much research
on the titanates—some of which are antiferroelectric (e.g. PbZrO
3
), ferromagnetic
(e.g. LaCo
0.2
Mb
0.8
O
3
) or antiferromagnetic (e.g. LaFeO
3
). Barium titanate itself under-
goes further transformations at lower temperatures (to orthorhombic and rhombohedral
forms) which are, as with the tetragonal form, ferroelectric.
At higher temperatures (120
◦
C for barium titanate), due to the increased thermal
movement of the atoms, these structures revert to the cubic forms. This is an example
of a displacive transformation: no bonds are broken. Such displacive transformations
also characterize the ‘high temperature’ (α) and ‘low temperature’ (β) forms of quartz,
tridymite and cristobalite (see Section 1.11.5) and also relate the polymorphous forms
of many organic compounds—the structures only differing in the way in which the
molecules are packed together under the influence of the weak van der Waals forces.
1.11.2 Tetrahedral and octahedral structures—silicon carbide
and alumina
Consider, for example, the crystal structures which consist of close-packed or nearly
close-packed layers of large atoms or ions with the smaller atoms or ions occupy-
ing some or all of the tetrahedral interstitial sites. Zinc blende and wurtzite are two
such structures (Section 1.7) which are re-drawn in Fig. 1.26 so as to emphasize the
B4 (2H)
Wurtzite
c = 2
a
√2
B3 (3C)
Zinc blende
B5 (4H)
Carborundum III
B6 (6H)
Carborundum II
B7 (15R)
Carborundum I
√3
c = 3
a
√2
√3
c = 4
a
√2
√3
c = 6
a
√2
√3
c = 15
a
√2
√3
Fig. 1.26. Five common tetrahedral structure types. The bracketed symbols refer to the number of
layers in the repeat sequence and the structure type: H (hexagonal), C (cubic) and R (rhombohedral)
(after E. Parthe, Crystal Chemistry of Tetrahedral Structures, Gordon and Breach, New York, 1964,
reproduced from The Structure of Metals, 3rd edn, C. S. Barrett and T. B. Massalski, Pergamon, 1980).
