Guozhong Cao. Nanostructures & Nanomaterials: Synthesis, Properties & Applications
Подождите немного. Документ загружается.


236
Nanostructures and Nanomaterials
Fig.
6.5.
(a) An SEM micrograph showing the radially grown nanotubes on the surface of
a 125 pm-diameter optical fiber. (b) A close-up micrograph showing the conformally per-
pendicular nature of the nanotube grown on the fiber. (cF(0 are examples
of
nonplanar,
complex surfaces where nanotubes can be conformally grown perpendicular to the local
surface. [C. Bower,
W.
Zhu,
S.
Jin, and
0.
Zhou,
Appl.
Phys.
Lett.
77,
830
(2000).]
Fig.
6.6.
(a) An SEM micrograph and (b) a schematic showing the straightkurled nan-
otube structure produced by an alternating plasma and thermal process (a 2 min plasma
process followed by a
70
min thermal process), indicating both the field induced alignment
effect and the base growth mechanism. (c) TEM micrograph showing a bundle of nan-
otubes with the upper portion straight and the lower portion curled. [C. Bower,
W.
Zhu,
S.
Jin, and
0.
Zhou,
Appl.
Phys.
Lett.
77,
830
(2000).]

Special
Nanomaterials
237
It should be noted that the catalyst growth mechanism of carbon
nanotubes is similar to that of VLS growth of nanowires or nanorods
discussed in Chapter
4.
Baker and Harris proposed this model for the cat-
alytic carbon filament growth.42 Atomic carbon dissolves into the metal
droplets, then diffuses to and deposits at the growth surface, resulting
in
the growth of carbon nanotubes. The catalyst growth offers an additional
advantage; it is relatively easy to prepare patterned carbon nanotube films
by standard lithographic
technique^^^,^^
and to grow aligned carbon nan-
otubes with or without the ~ubstrate.~~?~~ Methods of CVD growth of
carbon nanotubes, assisted by the transition metal catalysts, are also con-
sidered as the method for the mass produ~tion.~~ CVD methods also allow
the growth of carbon nanotubes at much lower growth temperatures such
as
700
or
800"C.48949
The as-grown nanotubes generally show poor crys-
tallinity, but can be much improved by a heat treatment at
2500-3000°C
in argon.50
For the catalytic growth, two models have been proposed to explain
the experimental observations: the base growth and tip growth, which
were originally developed for the catalytic growth of carbon
filament^.^'
Both models are used to explain the growth
of
carbon nanotubes. In the
case of
PECVD
and pyrolysis growth, the catalytic particles are usually
found at the tip and explained by the tip growth m~del.~*-~~ The base
model has been used to explain the vertically aligned carbon nanotube
growth by thermal CVD using iron as ~atalyst.~~-~~ However, experi-
ments showed that the vertical growth of aligned carbon nanotubes does
not necessarily follow the base-growth model.60 The growth of aligned
carbon nanotubes is possible through both tip-growth and base-growth
models, depending on the catalyst and substrate used in the deposition
method. Furthermore, the diffusion of precursor molecules to the cata-
lyst at the bottom of the growing nanotubes would be difficult, particu-
larly considering the high density and large length (up to
100
pm)
of the
grown carbon nanotubes. However, no research has been done
to
address
this issue yet.
Another proposed mechanism for the carbon nanotube growth assumes
that the nanotubes are always capped.61 The growth is nucleated at active
sites of a vapor-grown carbon fiber and the growth involves C2 dimer
absorption near a pentagon at the cap of the nanotube. Subsequent restruc-
turing would result in the formation
of
an additional carbon hexagon,
which is added into the nanotube and leads to the growth of the tube.
In almost all the synthesis methods, carbon nanotubes are found along
with other carbon materials, such as amorphous carbon and carbon
nanoparticles. Purification is generally required and refers to the isolation

238
Nunostructures and Nanomuterials
of carbon nanotubes from other entities. Three basic methods have been
used for purification: gas phase, liquid phase and intercalation methods.62
The gas phase purification method removes nanoparticles and amorphous
carbon in the presence of nanotubes by an oxidation pro~ess.~~?~~ The gas
phase purification process also tends to burn off many of the nanotubes,
particularly the smaller diameter nanotubes. Liquid phase removal of
nanoparticles and other unwanted carbons is achieved using a potassium
permanganate, KMn04 treatment.65 This method retains most of carbon
nanotubes, a higher yield than gas phase purification, but with shorter
length. Carbon nanoparticles and other carbon species can be intercalated
by reacting with CuCl2-KC1, whereas the nanotubes would not intercalate
since they have closed cage structures. Subsequent chemical reactions can
remove the intercalated species.66
Properties of carbon nanotubes have been extensively studied. Langer
et
aZ.67
were the first to study the transport properties of carbon nanotubes,
and further measurements were done by many research gro~ps.
Carbon nanotubes are excellent candidates for stiff and robust structures,
since the carbon-carbon bond in graphite is one of the strongest in nature.
TEM observation revealed that carbon nanotubes are flexible and do not
break upon bending.71 Thermal conductivity of carbon nanotubes could be
extremely high, considering the fact that thermal conductivity
of
diamond
and graphite (in-plane) are extremely high,72 and thermal conductivity of
individual carbon nanotubes was found much higher than that of graphite
and
bulk nan~tubes.~~ Carbon nanotubes have a wide spectrum of poten-
tial applications. Examples include use in ~atalysis,’~ storage of hydrogen
and other gases,75 biological cell
electrode^,^^
quantum resistors,77
nanoscale electronic and mechanical devices,7g electron field emission
tips,79 scanning probe tip,*O flow sensorsg1 and nanocomposites.g2
6.3.
Micro and Mesoporous Materials
According to the classification made by IUPAC,s3 porous solids can be
grouped into three categories, depending on their pore diameter: microp-
orous
(d
<
2
nm), mesoporous
(2
nm
<
d
<
50
nm), and macroporous
(d>
50
nm) materials. Almost all of zeolites and their derivatives are
microporous, whereas surfactant templated mesoporous materials and
most xerogels and aerogels are mesoporous materials. In this section, we
will briefly introduce these meso and microporous materials and their
respective synthesis techniques. This field has been extensively covered
with excellent review
article^.^^,^^

Special
Nanomaterials
239
6.3.1.
Ordered mesoporous structures
Ordered mesoporous materials are made with a combination of using self-
assembled surfactants as template and simultaneous sol-gel condensation
around template. Mesoporous materials may have many important tech-
nological applications as supports, adsorbents, sieves or nanoscale chem-
ical reactors. Such materials have uniformly sized and shaped pores with
diameters ranging from
3
nm to several tens nanometers and microns long,
and often have a very large pore volume (up to
70%)
and very high sur-
face area (>700m2/g). Before we discuss the details of the synthesis of
ordered mesoporous materials, a brief introduction to surfactants and the
formation of micelles is given.
Surfactants are organic molecules, which comprise two parts with dif-
ferent polarity.86 One part is a hydrocarbon chain (often referred
to
as
polymer tail), which
is
nonpolar and hence hydrophobic and lipophilic,
whereas the other is polar and hydrophilic (often called hydrophilic head).
Because of such a molecular structure, surfactants tend to enrich at the
surface of a solution or interface between aqueous and hydrocarbon sol-
vents,
so
that the hydrophilic head can turn towards the aqueous solution,
resulting in a reduction of surface or interface energy. Such concentration
segregation is spontaneous and thermodynamically favorable. Surfactant
molecules can be generally classified into four families, and they are
known as anionic, cationic, nonionic and amphoteric surfactants, which
are briefly discussed below:
(1)
Typical anionic surfactants are sulfonated compound with a general
formula R-S03Na, and sulfated compounds of R-OS03Na, with R
being an alkyl chain consisting of
11
to
21
carbon atoms.
(2)
Cationic surfactants commonly comprise
of
an alkyl hydrophobic
tail and a methyl-ammonium ionic compound head, such as cetyl
trimethyl ammonium bromide (CTAB), C16H33N(CH3)3Br and cetyl
trimethyl ammonium chloride (CTAC), C16H33N(CH3)3C1.
(3)
Nonionic surfactants do not dissociate into ions when dissolved in a
solvent as both anionic and cationic surfactant. Their hydrophilic head
is a polar group such as ether, R-0-R, alcohol, R-OH, carbonyl,
R-CO-R, and amine, R-NH-R.
(4)
Amphoteric surfactants have properties similar to either nonionic sur-
factants or ionic surfactants. Examples are betaines and phospholipids.
When surfactants dissolve into a solvent forming a solution, the surface
energy of the solution will decrease rapidly and linearly with an increasing
concentration. This decrease is due to the preferential enrichment and the
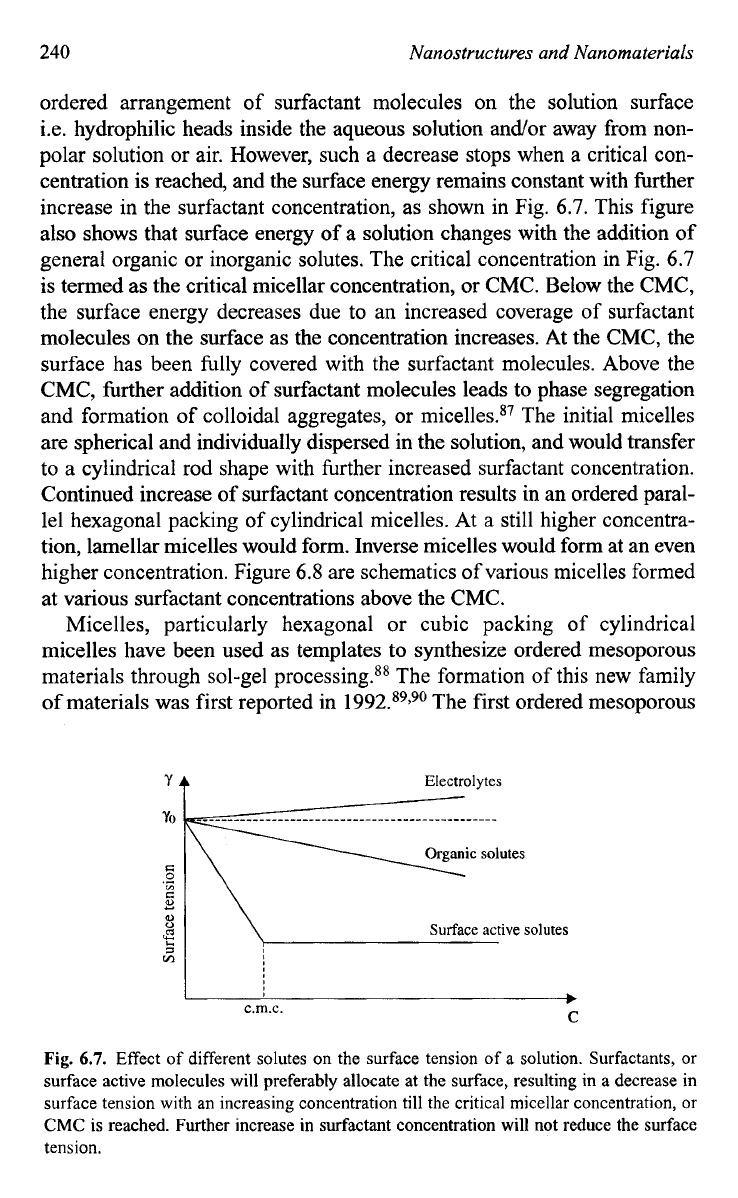
240
Nanostructures and Nanomaterials
ordered arrangement of surfactant molecules on the solution surface
i.e. hydrophilic heads inside the aqueous solution andor away from non-
polar solution or air. However, such a decrease stops when a critical con-
centration is reached, and the surface energy remains constant with further
increase in the surfactant concentration, as shown in Fig.
6.7.
This figure
also shows that surface energy of a solution changes with the addition
of
general organic or inorganic solutes. The critical concentration in Fig.
6.7
is termed as the critical micellar concentration, or CMC. Below the CMC,
the surface energy decreases due to an increased coverage of surfactant
molecules on the surface as the concentration increases. At the CMC, the
surface has been fully covered with the surfactant molecules. Above the
CMC, further addition of surfactant molecules leads to phase segregation
and formation of colloidal aggregates, or micelle~.~~ The initial micelles
are spherical and individually dispersed in the solution, and would transfer
to a cylindrical rod shape with further increased surfactant concentration.
Continued increase of surfactant concentration results in an ordered paral-
lel hexagonal packing of cylindrical micelles. At a still higher concentra-
tion, lamellar micelles would form. Inverse micelles would form at an even
higher concentration. Figure
6.8
are schematics of various micelles formed
at various surfactant concentrations above the CMC.
Micelles, particularly hexagonal or cubic packing of cylindrical
micelles have been used as templates to synthesize ordered mesoporous
materials through sol-gel processing.88 The formation of this new family
of materials was first reported in
1992.89>90
The first ordered mesoporous
ys
Electrolytes
k
C
c.m.c.
Fig.
6.7.
Effect
of
different solutes on the surface tension
of
a solution. Surfactants, or
surface active molecules will preferably allocate at the surface, resulting in a decrease in
surface tension with an increasing concentration till the critical micellar concentration,
or
CMC
is
reached. Further increase in surfactant concentration will not reduce the surface
tension.

Special
Nanomaterials
24
I
Anionic
surfactant
Fig.
6.8.
(a) Spherical micelle forms first as the concentration of surfactants is above the
CMC.
(b) Individual cylindrical micelle forms as the concentration of surfactants increases
further. (c) Further increased concentration of surfactants results in the formation of
hexagonally packed cylindrical micelles, (d) Lamellar micelles would form when the
concentration
of
surfactants rises even further.
materials synthesized were denoted as
MCM-41
and
MCM-48. MCM-41
is an aluminosilicate possessing hexagonally arranged one-dimensional
pores with diameters ranging from 1.5 to lOnm, and
MCM-48
is an alu-
minosilicate with a three-dimensional pore system and diameters of order
of
3
nm. It should be noted that the inorganic portion of mesoporous mate-
rials
MCM-4
1
and
MCM-48
are amorphous aluminosilicates.
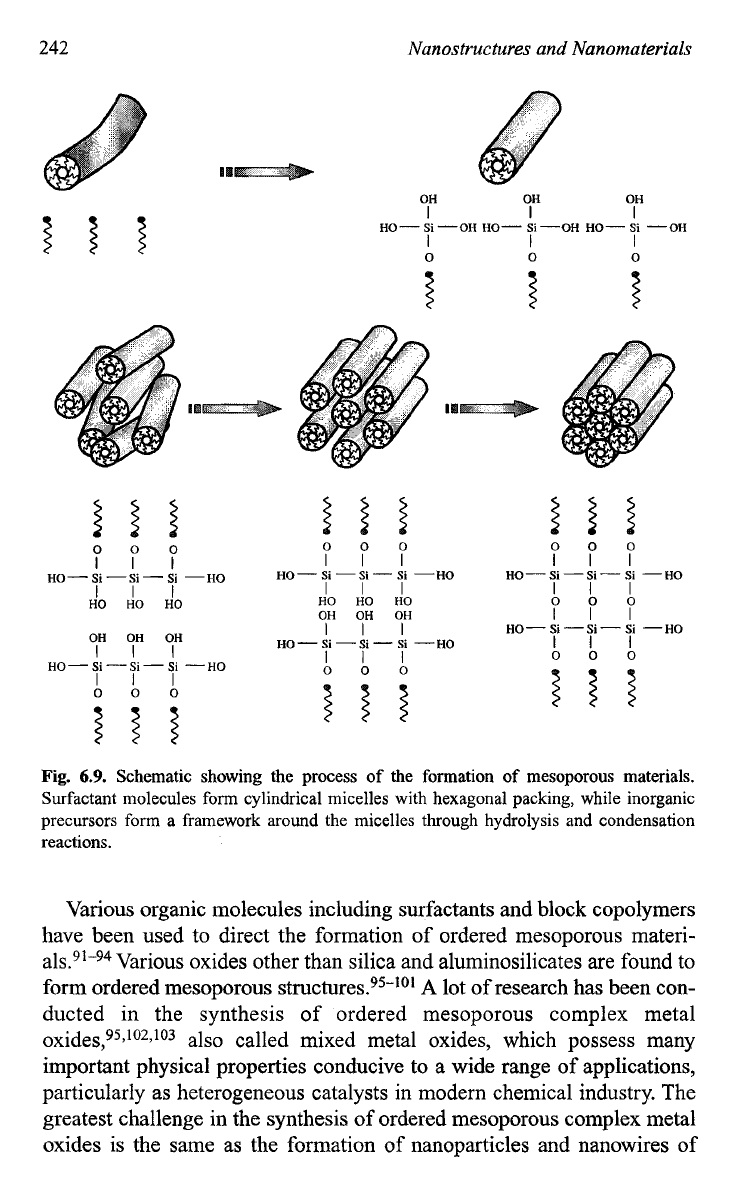
The process is conceptually straightforward and can be briefly
described below. Surfactants with a certain molecule length are dissolved
into a polar solvent with a concentration exceeding its
CMC,
mostly at a
concentration, at which hexagonal or cubic packing of cylindrical micelles
is formed. At the same time, the precursors for the formation
of
desired
oxide(s) are also dissolved into the same solvent, together with other nec-
essary chemicals such as a catalyst. Inside the solution, several processes
proceed simultaneously. Surfactants segregate and form micelles, whereas
oxide precursors undergo hydrolysis and condensation around the
micelles simultaneously, as schematically shown in Fig.
6.9.
242
Nanostructures
and
Nanomaterials
I
333
OH
OH
OH
HO-
Si -OH
HO-
Si-OH
HO-
Si
-OH
I
I
I
I
I
I
0
0 0
3
3
333
000
HO-Si-Si-Si
-HO
Ill
I
d0
€!O
HO
OH
OH
OH
HO-Si-Si-Si
-HO
000
Ill
Ill
333
553
000
HO-Si-Si-Si
-HO
A0
€!O
HO
Ill
I
OH
OH
OH
HO-Si-Si-Si
-HO
000
Ill
Ill
535
533
000
HO-Si-Si-Si
-HO
Ill
Ill
bbo
I
.I,
HO-Si-Si-Si -HO
000
Ill
535
Fig.
6.9.
Schematic showing the process of the formation of mesoporous materials.
Surfactant molecules form cylindrical micelles with hexagonal packing, while inorganic
precursors form a framework around the micelles through hydrolysis and condensation
reactions.
Various organic molecules including surfactants and block copolymers
have been used to direct the formation of ordered mesoporous materi-
al~.~~-~~ Various oxides other than silica and aluminosilicates are found
to
form ordered mesoporous
structure^.^^-^^'
A lot
of
research has been con-
ducted in the synthesis
of
ordered mesoporous complex metal
OX~~~S,~~J~~,~~~
also called mixed metal oxides, which possess many
important physical properties conducive
to
a wide range
of
applications,
particularly as heterogeneous catalysts in modern chemical industry. The
greatest challenge in the synthesis of ordered mesoporous complex metal
oxides is the same as the formation
of
nanoparticles and nanowires of

Special Nanomaterials
243
complex metal oxides by sol-gel processing, which is to ensure the for-
mation of homogeneous desired stoichiometric composition through hetero-
condensation. All the general considerations that have been discussed
previously are applicable here. However, the situation here is even more
complex, since the presence of surfactants in the solution would compli-
cate the reaction kinetics of hydrolysis and condensation reactions. Some
surfactants would act as catalysts to promote hydrolysis and condensation
reactions. The presence of relatively large surfactant molecules and
micelles in the solution would certainly have a steric effect on the diffu-
sion process. Although all these surfactant effects are present in the syn-
thesis of single metal oxide mesoporous materials, a given surfactant may
have varied degree effects on different precursors. Therefore, the influ-
ences of surfactants on the hydrolysis and condensation reactions in the
formation of ordered mesoporous complex metal oxides should be care-
fully considered. Table 6.1 summarizes some physical properties of meso-
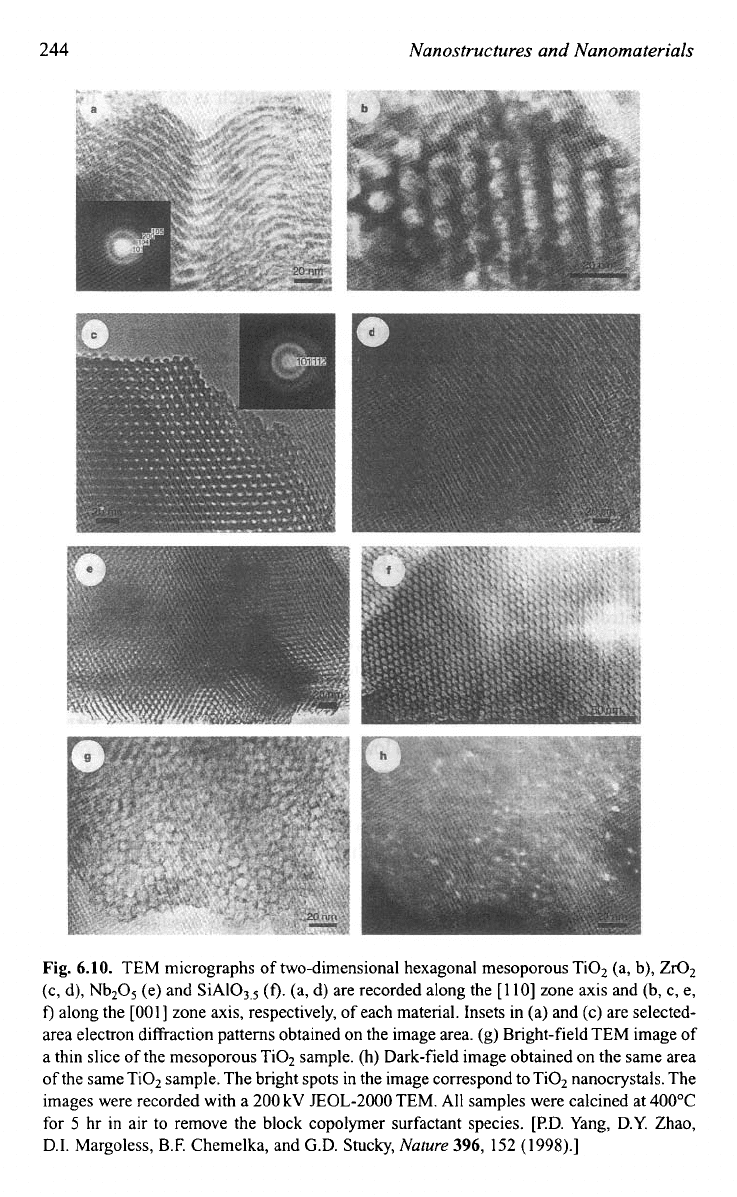
porous complex oxides and Fig. 6.10 shows TEM images of various
mesoporous materialsg5
Optically transparent and electronically conductive complex oxide,
indium tin oxide (ITO), has also been studied to form mesoporous struc-
ture.’@ In the fabrication of mesoporous ITO, a prime impediment is used
to control the competing hydrolysis and condensation reactions, which
is achieved by employing atrane complexes as precursors to slow the
kinetics of hydrolysis. Indium acetate and tin isopropoxide with desired
stoichiometric ratios were dissolved in a 10-fold molar excess of tri-
ethanolamine under an inert nitrogen atmosphere. Approximately 10 vol%
dry formamide was also added to lower the viscosity. After the solution was
mixed for
4
hr, CTAB in a
3.5
:
1 molar ratio with respect to the total metal
concentration was admixed to the solution, and the pH was adjusted to 8
with
4
M sodium hydroxide. The mixture was held at 80°C for 96 hr prior
to filtering off the product. The resultant IT0 powder with a In
:
Sn molar
Table
6.1.
Physical properties
of
mesoporous complex metal oxides.
Oxide Pore size
(nm)
BET surface BETsurface Porosity
(“A)
area
(m2/g)
area
(m2/cm3)
SiA103,,
6 310 986 59
Si2AI05,5
10 330
965
55
A12Ti05
8 270 1093 59
ZrW208
5
170 1144 51
SiTi04
5
495 1638 63
ZrTi04
8 130
670
46
244
Nanostructures and Nanomaterials
Fig.
6.10.
TEM micrographs of two-dimensional hexagonal mesoporous Ti02 (a, b), Zr02
(c, d), Nb2O5 (e) and SiAI03,5
(f).
(a, d) are recorded along the [110] zone axis and (b, c,
e,
f)
along the
[OOI]
zone axis, respectively, of each material. Insets in (a) and (c) are selected-
area electron diffraction patterns obtained on the image area.
(g)
Bright-field TEM image of
a thin slice of the mesoporous Ti02 sample. (h) Dark-field image obtained on the same area
of
the same Ti02 sample. The bright spots in the image correspond to Ti02 nanocrystals. The
images were recorded with a 200
kV
EOL-2000 TEM.
All
samples were calcined at 400°C
for
5
hr in air to remove the block copolymer surfactant species. [P.D. Yang, D.Y. Zhao,
D.I. Margoless, B.F. Chemelka, and G.D. Stucky,
Nature
396,
152
(1998).]

Special Nanomaterials
245
ratio of
1
:
1 has a BET surface area of 273 m2/g and a pore diameter of
-2
nm
as determined by nitrogen adsorption isotherms.
XRD
indicates the
formation of crystalline IT0 after calcinations at unspecified temperatures,
and TEM image shows a worm-hole topography. However, electrical con-
ductivity measurements taken on a water-free pressed pellet showed an
average value of
u
=
1.2
X
1
0-3
S/cm at room temperature, which is about
3
orders of magnitude lower than that of IT0 thin films under the same
condition.
In addition to manipulating chemical compositions, crystal and
microstructures, physical and chemical properties can also be introduced
into order mesoporous materials through various surface modifications,
including coating, grafting and self-assembly.
lo5-l
Typical mesoporous materials are in the form of powders (or bulk meso-
porous materials) and films. Bulk mesoporous materials comprise a collec-
tion of macroscopically sized grains (up to several hundreds micrometers).
In each grain, there is crystallographically ordered mesoporous structure,
however, all grains are randomly packed. This hinders diffusional accessi-
bility to the mesopore structure, and thus limits the applications of ordered
mesoporous materials in practice. Several groups have been successful in
aligning mesoporous films parallel to a substrate surface over large areas,
or within microchannels.'
12-11'
However, there is limited accessibility to the
pores due to parallel alignment to the surface rather than the ideal perpen-
dicular alignment.
Efforts
have also been made in achieving the alignment
of mesoporous silica perpendicular to a surface (i.e. dead end pores), but
this was done with a strong magnetic field on a small sample size and has
very limited practical possibilities. The synthesis of oriented or hierarchi-
cally structured mesoporous materials has also been reported.
'
19-12'
6.3.2.
Random
mesoporous
structures
Mesoporous structures can be created by a variety of other methods.
Examples include leaching a phase separated glass,
122
anodic oxidation of
thin metal foils in an acidic ele~trolyte,'~~ radiation-track etching,124 and
sol-gel processing.125 In this section, the discussion will be focused on
sol-gel derived mesoporous materials. Depending on the conditions
applied for the removal of solvent during drying, two types of mesoporous
materials can be obtained. One is xerogel, when solvents are removed
under ambient conditions. Another is aerogel, which refers to mesoporous
material with very high porosity and surface area and is generally made
with supercritical drying. Both xerogels and aerogels are highly porous
