Gross R., Sidorenko A., Tagirov L. Nanoscale Devices - Fundamentals and Applications
Подождите немного. Документ загружается.

Thermodynamic Principles of Artificial Nose
Based on Supramolecular Receptors
V. V. Gorbatchuk, M. A. Ziganshin
Kazan State University, A. M. Butlerov Institute of Chemistry, Kazan
420008, Russia
Abstract: The cooperative effects in the substrate vapor binding by solid
receptors and their relevance for structure-property relationships in
the odor sensor applications are discussed and reviewed.
Keywords: calixarenes, cyclodextrins, cross-linked hydrophilic polymers,
proteins, vapor binding cooperativity, molecular recognition, odor
sensors, headspace GC analysis, model sensor systems, structure-
property relationship.
Introduction
Application of supramolecular receptors such as calixarenes, cyclodextrins
and proteins in the odor recognition devices provides the enhanced vapor
binding selectivity [1, 2]. The answer on the question, why a given receptor
is more or less selective, is necessary for a design of the effective odor
sensors that can substitute for this purpose the living beings. In this review,
the effect of cooperativity in the substrate vapor binding by solid receptors
on the structure-property relationships of this process is discussed.
Cooperativity is one of the major factors defining the receptor selectivity.
However, this effect is often underestimated and even neglected in the
sensor applications.
In this review, we analyze two types
of the binding cooperativity. One
of them can be observed in binary systems. For another to be revealed, a
third component is necessary. These two types of cooperativity are of
thermodynamic nature (i.e. can be seen on sorption isotherms) and relate to
the
well-known cooperative effects of molecular biology and biochemistry.
23
R. Gross et al. (eds.), Nanoscale Devices - Fundamentals and Applications, 23–34.
© 2006 Springer. Printed in the Netherlands.
24 V. V. Gorbatchuk, M. A. Ziganshin
The first was observed nearly a hundred years ago by Hill [3]. It is a
homotropic cooperative binding of oxygen by hemoglobin. The second is a
cooperative hydration effect on the enzyme activity [4]. The structural
requirements for synthetic supramolecular receptors (hosts) to mimic these
types of cooperativity are discussed in this paper.
Being cooperative, a substrate-receptor binding has a bundle of related
cooperative phenomena. These are the memory of the receptor preparation
history, the cooperative effect of the third component, and the temperature
effect on the binding threshold. Most of these effects are hard to be seen
using sensor devices. So, the data obtained for the model systems with
receptor powder, using headspace GC analysis, where these cooperative
effects can be controlled, are regarded here together with the corresponding
experimental approaches.
Because of the complicated interference of cooperative effects, they can
have a strong influence on the observed structure-property relationships.
Here several simple relationships of this kind are discussed, which were
obtained in standard conditions removing the memory effects, so that some
objective basis is provided for an aimed molecular design of sensitive
materials for “electronic nose” devices being capable to match the best
receptors of living nature.
Cooperative Binding in Binary Substrate-Receptor
Systems
Cooperative binding in the system with two relevant components: substrate
vapor and solid receptor, or vaporous guest and solid host, were observed in
a lot of works [5-11] for calixarenes and other clathrate forming
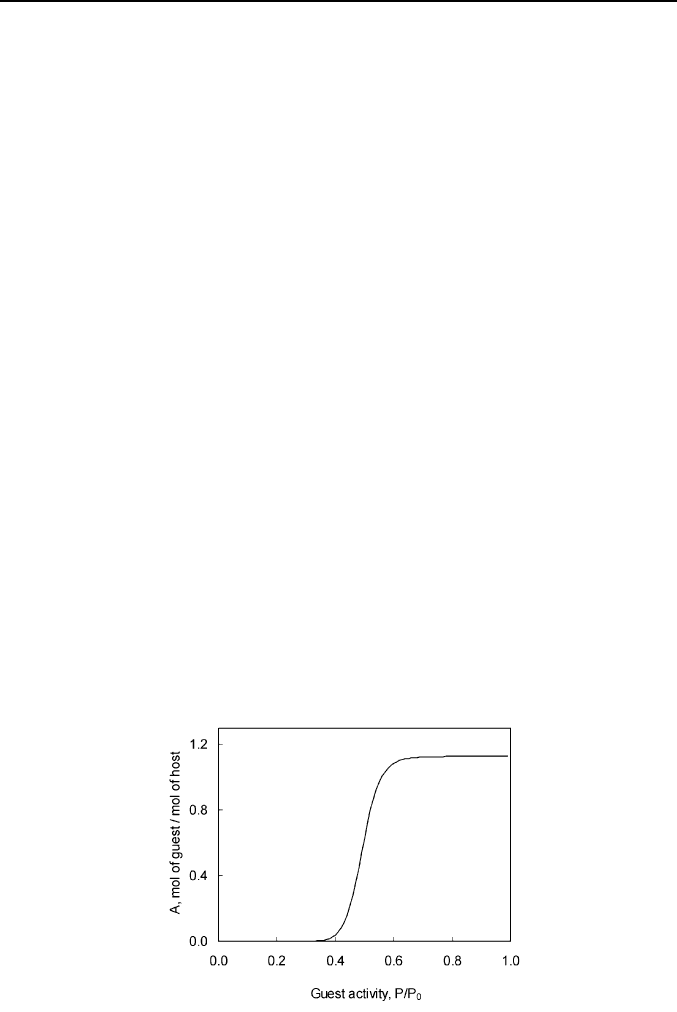
Fig. 1. A typical sorption isotherm of guest vapor by solid host in the binary
system.
Thermodynamic Principles of Artificial Nose 25
receptors. The typical shape of guest binding isotherm observed for
nitromethane vapor – solid tert-butylthiacalix[4]arene
is shown on Fig. 1.
This isotherm has a threshold of guest thermodynamic activity, or
relative vapor pressure, P/P
0
, below which no significant binding can be
seen. Above this threshold the guest-host binding capacity sharply increases
up to a saturation level, which corresponds to the formation of the saturated
clathrate.
The same shape of sorption isotherms was observed for the binding of
oxygen by hemoglobin in water solution [12]. While this cooperative effect
for a single hemoglobin molecule in solution is not very simple to explain,
mostly because the crystals of this protein, for which the structural X-ray
data were obtained, do not perform a significant binding cooperativity
[12, 13], for organic hosts like calixarenes a sigmoidal sorption isotherm
has rather trivial explanation. In terms of the Gibbs phase rule it is a result
of phase transition in solid host phase at the binding of the guest vapor that
gives a host-guest inclusion compound, or clathrate [5, 6, 9, 14, 15]. This
conclusion was confirmed by the comparison of the powder X-ray
diffractograms of the initial host and of the host saturated by guest [6, 16].
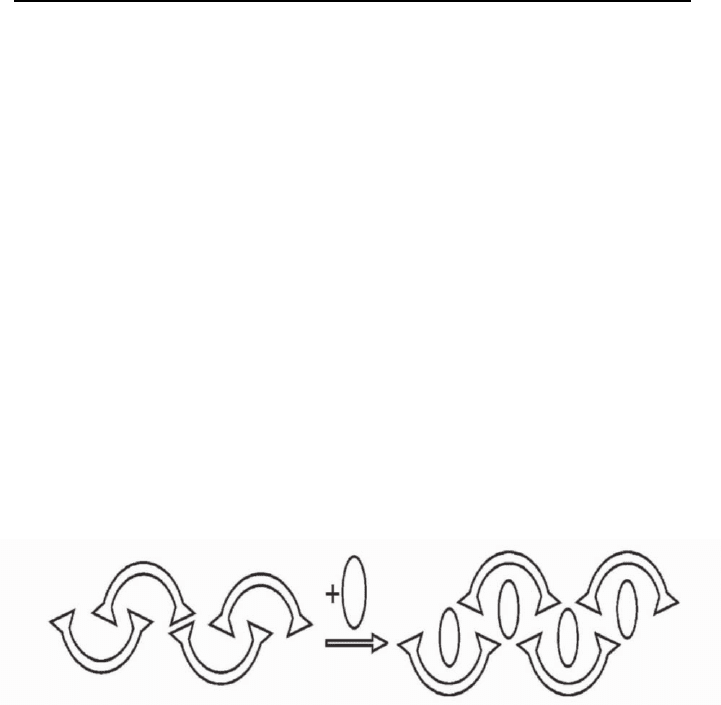
A structural illustration of the phase transition at the formation of inclusion
compound with solid host is shown on Figure 2.
Fig. 2. A structural illustration of the phase transition at the formation of inclusion
compound with solid host.
For description of sigmoidal sorption isotherms the Hill equation can be
used:
A = SC(P/P
0
)
N
/ (1 + C(P/P
0
)
N
), (1)
where S – inclusion stoichiometry, C – sorption constant, N – cooperativity
constant, A – experimentally determined solid phase composition (mol of
guest per mol of host). The fitting of the sigmoidal sorption isotherms with
the Hill equation (1) gives two stable solutions: the stoichiometry S and the
ratio (lnC)/N. The last value directly relates to the threshold activity of the
guest at the half saturation of host A = 0.5S:
a
0.5S
= exp(-(lnC)/N). (2)
26 V. V. Gorbatchuk, M. A. Ziganshin
The Gibbs energy of the guest inclusion can be calculated by the
integration of the sorption isotherms having a saturation part:
SOc
aRTdYPPRTG
5.0
1
0
ln)/ln(
∫
==∆ .
(3)
Here, Y = A/S is the host saturation extent. The inclusion free energy
∆
G
c
is
the free energy of transfer of 1 mole of guest from the standard state of pure
liquid to the saturated solid phase (inclusion compound). The right part of
equation (3) is valid if the ln(P/P
0
) value is given by equation (1) as a
function of Y.
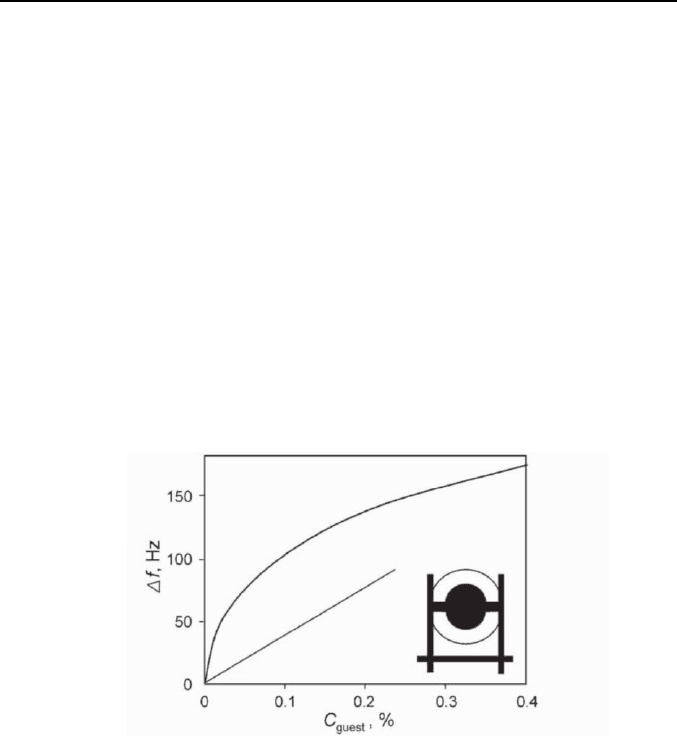
The problem is that no sorption isotherms, obtained up-to-date using thin
layer of solid receptors on QCM sensors, have a shape shown on Figure 1.
The initial part of these isotherms have rather Langmuir [1, 17-19] or the
linear [1, 20] shape, Figure 3. For the same toluene vapor – solid tert-
butylcalix[6]arene pair the thin layer of this host on QCM sensor gives a
BET isotherm [17], while its powder gives a sigmoidal isotherm [21].
Fig. 3. Typical isotherms of the guest vapor sorption by the host thin layer on QCM
sensor with linear and BET shape.
The reason for that may be the different history of the host samples. The
low-temperature decomposition of clathrates, which occurs on the surface
of QCM sensors, may produce the zeolite-like material with empty cavities
in the host keeping the packing of clathrate. Such transition was observed
using X-ray method [22]. The zeolites with the fixed surface of gas–solid
interface have the sorption isotherms with the Langmuir shape like shown
on Figure 3 [23]. The host polymorphism as a function of thermal history
was also observed in the other studies [24-26]. The sigmoidal isotherms
(Figure 1) were observed for the host powder, where the clathrate memory
effect was removed by heating [5 ,6 ,9 ,14 ,15].
Thermodynamic Principles of Artificial Nose 27
Cooperative Hydration Effect on the Substrate Vapor
Binding
The molecular design of biomimetic receptors for the sensor applications is
often confined to the synthesis of molecularly imprinted polymers (MIP)
with the binding sites similar to those of antibodies and enzymes [27]. For a
MIP to be a genuine analogue of antibodies, it should have also a
biomimetic hydration effect on a substrate binding [28]. Hydration is a
crucial factor for the protein receptor properties. It cooperatively enhances
the rates of enzymatic reactions in low water conditions [4]. Proteins in
contact with some water-organic mixtures show a cooperative increase both
in water uptake [29, 30] and uptake of hydrophobic organic components
[31, 32] above a certain hydration threshold. Antibodies also need a
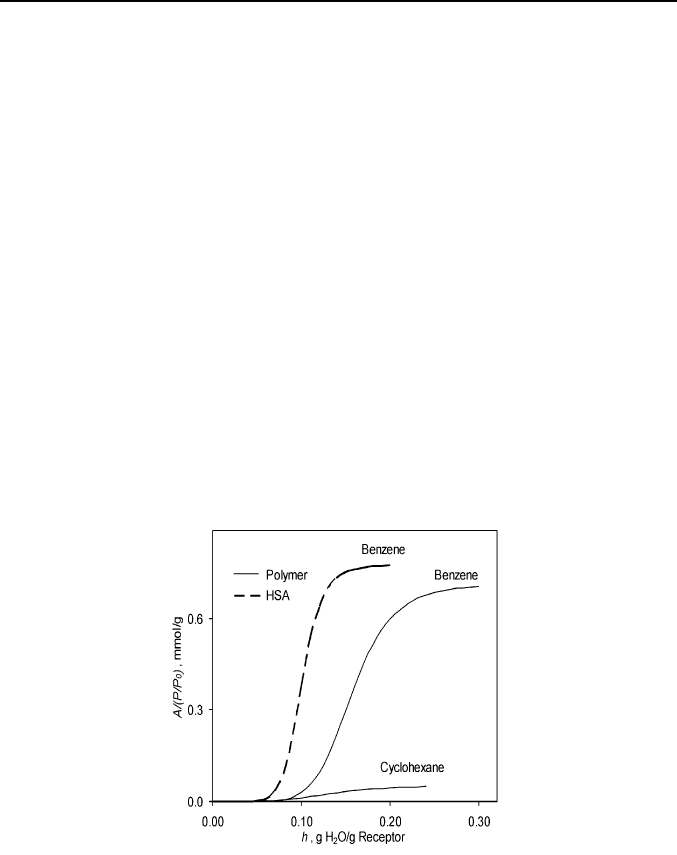
sufficient hydration to bind antigens [2]. The typical sigmoidal sorption
isotherm, where the partition coefficient A/(P/P
0
) between the pure liquid
sorbate and solid protein phase is plotted against the protein hydration, is
shown on Figure 4.
Fig. 4. The hydration effect on the binding affinity of cross-linked poly(N-6-
aminohexylacrylamide) (data from Ref. [28]) and human serum albumin (data from
Ref. [31]) for benzene and cyclohexane.
The protein hydration is favorable for the binding of hydrophobic
compounds, like benzene, but only above a threshold value of water
contents. Above this threshold the protein binding affinity for a sorbate
(substrate) goes up to the saturation level that is approximately equal to the

28 V. V. Gorbatchuk, M. A. Ziganshin
value observed for the same protein-substrate pair in water solutions
[31, 32]. For this favorable hydration effect to be seen, a synthetic polymer
should be hydrophilic to let water penetrate inside its bulk phase and have a
rather rigid structure preventing the sorption of hydrophobic or large
substrates without hydration [28]. The cross-linked poly(N-6-
aminohexylacrylamide) (PNAHAA), studied in [28], fits rather well to
these requirements. The sorption isotherm of benzene on this polymer has
almost the same shape as for human serum albumin, Figure 4.
Cooperative phenomena in binary protein-water systems were
described as a result of protein microheterogeneous structure [33].
The
clathrates of water were observed around hydrophobic groups of amino acid
residues [34]
and bound hydrophobic compounds [35, 36]
in protein
crystals.
Since the same cooperative hydration effect is observed for the
binding of hydrophobic compounds by an amorphous non-protein
macromolecular material, like PNAHAA [28], and proteins that do not
perform significant cooperativity at the binding of organic vapors in
absence of water [31, 32],
one can conclude that a source of protein
hydration cooperativity may be rather the properties of bound water itself
than the special protein structure.
Water bound by hydrophilic macromolecular receptor contributes
much to its binding selectivity. The hydration of PNAHAA increases its
selectivity for the pair benzene-cyclohexane up to almost that of liquid
water [28]. But this polymer as well as proteins [31, 32] becomes much less
selective for the pairs of more hydrophilic sorbates when hydrated. Strong
dependence of the substrate binding selectivity on the receptor hydration
can be a powerful tool in the applications of the odor recognition devices.
A further justification of the clathrate nature of the cooperative
hydration effect and the role of hydration in the substrate binding by
hydrophilic receptors comes from the sorption studies for beta-cyclodextrin
(BCD) [37]. Dry beta-cyclodextrin does not bind monofunctional
compounds larger than ethanol. Hence, being hydrophilic, it fits to the
above-mentioned requirement for the receptor to have the biomimetic
hydration effect. BCD does bind benzene up to hydration of 0.06 g H
2
O/g
BCD and benzene activity P/P
0
=0.8 as shown in Figure 5. But when BCD
is almost completely hydrated, it forms 1:1.3 clathrate with benzene [37]
(see Figure 5). The shape of benzene sorption isotherm on BCD hydrated to
0.172 g H
2
O/g BCD has the same shape as for the guest vapor binding by
solid hydrophobic hosts as shown by Figure 1.
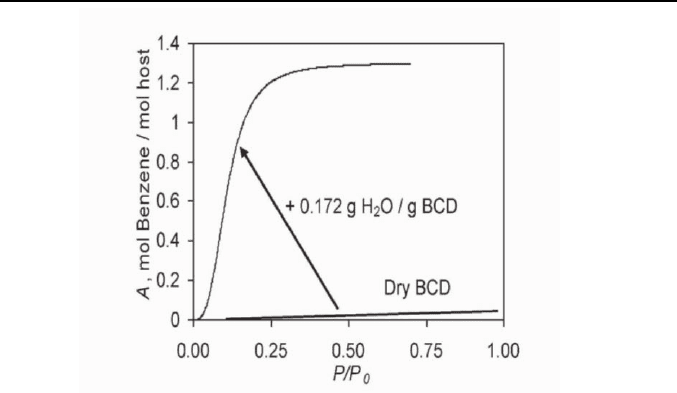
Thermodynamic Principles of Artificial Nose 29
Fig. 5. The hydration effect on the sorption of benzene by beta-cyclodextrin
(BCD). Data taken from ref. [37].
Secondary Cooperative Effects for the Substrate Vapor
Binding
The main two types of cooperative effects described above define the odor
sensing technique based on the substrate-receptor (host-guest) binding with
the formation of clathrates, or inclusion compounds, in the binary systems
with the homogeneous initial host, where the memory effects are removed,
and in ternary systems, where the change of receptor hydration is a
dominating factor. Generally, in the sensor applications more complex
systems and/or conditions may be used. In these cases, the secondary
cooperative effects may be observed, which, in essence, relate to the main
two, but may significantly change the substrate-receptor affinity and
binding selectivity, or even mask the system cooperativity as it is observed
in the systems with memory effects (Figure 3).
The cooperative effect of the third component (second guest) on the
guest binding by solid host is one of such secondary effects. This effect can
reduce the inclusion threshold by the guest activity so that the sorption
isotherm acquires a Langmuir shape (Figure 6) due to the addition of a
small amount of the third component [8]. This change may occur when
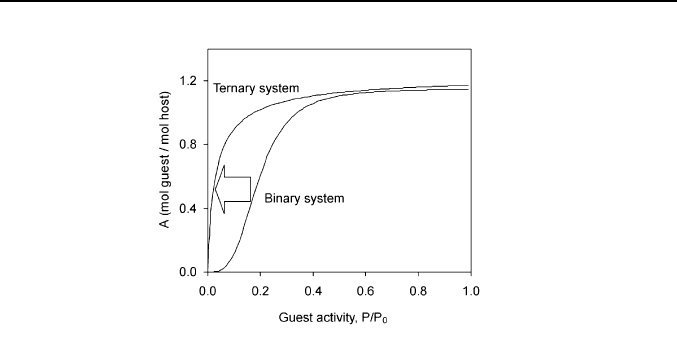
30 V. V. Gorbatchuk, M. A. Ziganshin
Fig. 6. The cooperative effect of third component (0.1 mol toluene/mol host) on the
sorption of acetonitrile by tert-butylcalix[4]arene at 298 K. Data from ref. [8].
both guests included can coexist in a single crystal [5]. Otherwise, the
threshold activity of the guest binding increases a little [8], probably
because of the competition between two guests for the binding sites in solid
host phase.
Formally, the reduction of the guest threshold activity in the presence of
a small amount of the third component (Figure 6) corresponds to the
increase of the host-guest binding affinity below the host saturation level.
Nearly the same effect was observed for hydrated beta-lactoglobulin, which
in the presence of 1.2 % of lipids shows more than a double binding affinity
for decane and terpenes than the defatted preparation [32]. Lipids behave as
included in the protein solid phase, because the combined effect of lipids
and hydration perform an apparent synergism.
The cooperative effect of the third component may have a profound
effect on the structure-energy relationships of the host-guest binding,
making the host selectivity in ternary systems with the binary mixtures of
guest vapors much different from the value calculated from the binding
Gibbs energies in binary “guest vapor – solid host” systems.
Another significant secondary cooperative effect is a temperature effect
on the receptor hydration threshold. It was observed for beta-lactoglobulin,
which shows a reduction of the hydration threshold value on almost 0.1 g
H
2
O/g BLG at relatively small temperature increase from 298 to 309.5 K
(Figure 7) [32].
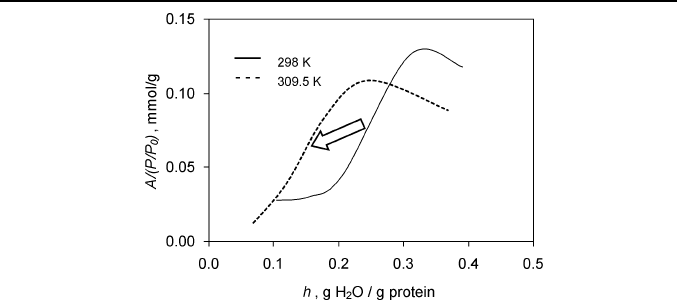
Thermodynamic Principles of Artificial Nose 31
Fig. 7. The temperature effect on the hydration threshold of alpha-terpinene
Besides, the value of the protein hydration threshold depends on the
protein hydration history: preliminary hydration pushes the hydration
threshold of hydrophobic sorbate (substrate) binding to the higher values, as
compared with the value of threshold hydration observed when protein is
hydrated in situ – in the presence of the hydrophobic sorbate [31]. Such
hydration history or memory effects were observed in studies of enzymatic
reactions with enzymes suspended in organic solvents [38].
The described secondary cooperative effects can give a larger variety of
structure-affinity relationships for the same set of receptors prepared or
used in different conditions, so that a more specific “fingerprint” can be
obtained for a given organic component or a complex organic vapor
mixture than in the case, when liquid sensitive material is used.
Structure-Energy Relationship
The secondary effects, especially the receptor memory of previous
treatment and clathrate structure are detrimental for the predictability of the
receptor behavior in the odor sensor applications. Moreover, the apparent
structure-energy relationship depends much
on the choice of substrate
(guest) standard state or concentration scale in the estimations of the sensor
effect or selectivity. When the substrate vapor with a certain pressure or
concentration is chosen as a standard state, the difference in Gibbs energies
of the substrate vapor condensation is often a major contribution in the
observed sensor selectivity, no much matter what is the nature of the
receptor used [39]. In this case, the sensor selectivity for organic vapors
follows the selectivity of their solvation in liquid solvents, like
sorption by initially dried defatted beta-lactoglobulin. Data taken from Ref. 32.
32 V. V. Gorbatchuk, M. A. Ziganshin
(Linear Solvation Energy Relationship) approach, which may be used for
the characterization of unknown vapors by array of polymer-coated sensors
[40].
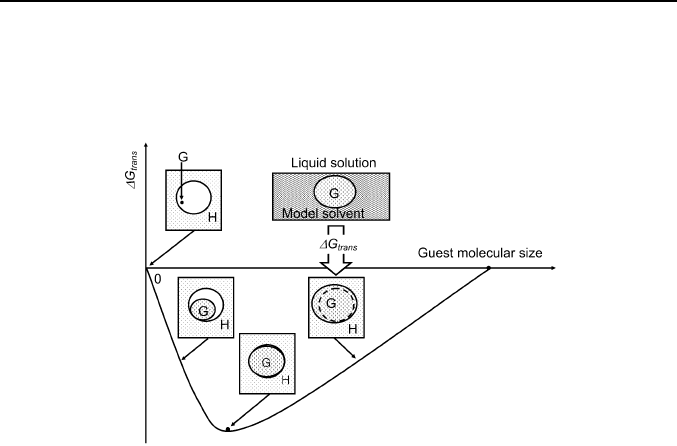
Fig. 8. Nonlinear influence of the guest molecular size on the excessive Gibbs
energy of guest inclusion by solid host from the infinitely dilute solution in model
liquid solvent.
Still the supramolecular receptors (hosts) exist that are able to bind a very
restricted number of guests, when the host memory of the former clathrate
structure is removed by heating [7, 10]. This phenomenon corresponds to
the essentially nonlinear structure-energy relationship for clathrate
formation. A scheme of such relationship for the case, where only guest
(substrate) size is important, is given on Figure 8. This scheme describes
the guest size effect on the inclusion Gibbs energy ∆G
trans
determined for
the standard state: an infinitely dilute liquid solution of the guest in a model
solvent having the same energy of pair-wise molecular interactions with the
guest as the host cavity interior. This approach allows extracting a pure
contribution of supramolecular effect in inclusion Gibbs energy ∆G
c
calculated using equation (3) from the sorption data.
The minimum on the V-like dependence corresponds to the guest size
such that its further infinitely small increase gives the same cavity energy
costs in the host phase as in the model solvent. Above this point an ordinary
size exclusion effect should be observed. It was found as a linear structure-
energy relationship for the binding of organic vapors by 2,2’-bis(9-
hydroxy-9-fluorenyl)biphenyl [7] and tert-butylthiacalix[4]arene [10]
powders with toluene chosen as a model solvent, and the guest molar
refraction MR
D
used as a guest molecular size parameter.
hexadecane. The last process can be easily quantified in terms of LSER
