Goldfarb D. Biophysics DeMYSTiFied
Подождите немного. Документ загружается.

42 Biophysics D emystifieD
Fluorescence Spectroscopy
Fluorescence is the opposite of absorption. In absorption, light is converted
into the kinetic energy of electrons in an atom or molecule; this bumps the
electrons into a higher or excited energy state. In fluorescence, the electrons
drop down from their excited state, emitting light in the process. Fluores-
cence is caused by absorption, although not all absorption results in fluores-
cence. The wavelength of the emitted light is typically longer than that of the
absorbed light.
Fluorescence spectroscopy, as with absorption spectroscopy, can be used to
characterize (i.e., identify) molecules and to measure and follow conforma-
tional transitions and ligand binding. A fluorophore is a small molecule or the
specific part of a molecule that is responsible for the fluorescence. Fluorescent
tagging is a technique in which a fluorophore is attached to another molecule
in order to track that molecule through some biological process. Fluorescent
tagging is one of the techniques used to determine the sequence of residues
in DNA.
50 55 60 65
Temperature (°C)
Increase in Absorbance at 260 nm
70 75 80
0%
50%
100%
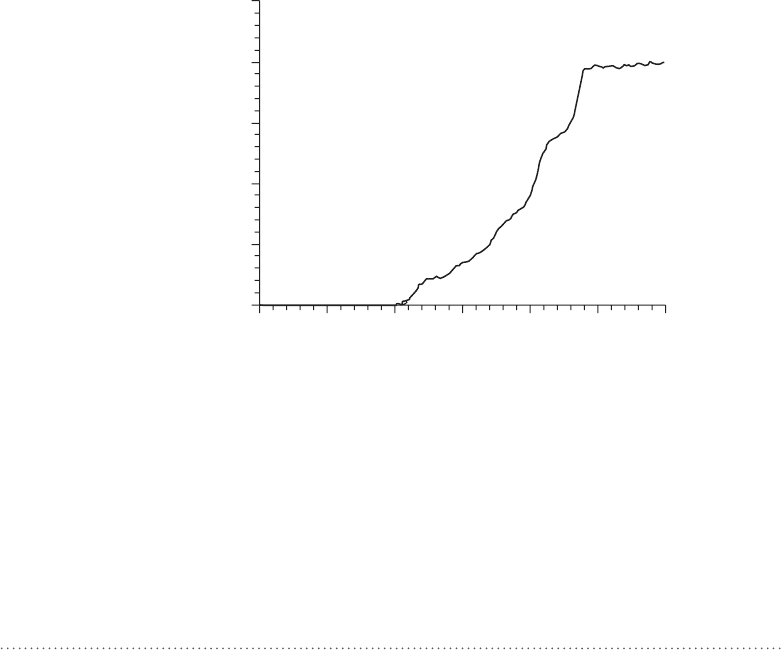
FigUrE 3-2 • A single-stranded DNA helix absorbs more light at
260 nm than a double helix. This makes it possible to use absorption
at 260 nm to measure unwinding of the DNA double helix into indi-
vidual single strands of DNA. The conformational transition from
double helix to single-stranded DNA can be induced by increasing
temperature. This makes temperature-scanning absorption spec-
troscopy a convenient tool for studying these conformational transi-
tions in
dna.

chapter 3 Biophysical Techniques and applicaTions 43
Mass Spectrometry
Mass spectrometry is a technique in which molecules or parts of molecules are
ionized and then passed through a magnetic field. By measuring the movement
of the charged molecules in the magnetic field, it is possible to get a very accu-
rate measurement of the mass or molecular weight of the molecules.
Mass spectrometry is used both for determining molecular weights and for
identifying molecules (once the molecular weight is known). When combined
with other methods, mass spectrometry can also reveal information about the
structure of molecules. The very large molecules typically found in biological
systems present particular challenges. Large molecules can be difficult to ionize
in a quantifiable way, and it can be difficult to cause them to fly through a
vacuum (a requirement for mass spectrometry). However, recent breakthroughs
are making the use of mass spectrometry in biophysics quite fruitful.
X-ray Crystallography
X-ray crystallography is a technique for determining the relative positions of
atoms within a crystal. A crystal is an orderly, three-dimensional, repeating
arrangement of atoms or molecules. Many substances can be crystallized; this
means that conditions can be arranged so the intermolecular forces cause the
molecules to line up in an organized, repeating manner.
The technique of X-ray crystallography provides very precise, high-resolution
structural information for molecules in a crystal. For example, X-ray crystallog-
raphy was used to discover that DNA is a double helix. The great advantage of
X-ray crystallography is the high resolution of structural detail that it can provide
(see Fig. 3-3). The disadvantage is that the molecules must be in crystalline form
in order for the technique to work. Fortunately, over the years, crystallographers
have become quite adept at manipulating chemical conditions in order to cause
many biomolecules to crystallize. Still, not all molecules can be crystallized, in
which case other techniques must be used to obtain structural information.
X-ray crystallography operates on the principle of diffraction. Diffraction occurs
when light waves pass through an ordered arrangement of openings (as one finds
in a crystal) and interfere with each other on the other side. Each opening acts as a
new starting point for the waves. The waves from each opening then meet on the
other side. When waves meet in phase with one another, the peaks from one wave
come together with peaks from the other wave, and troughs from one wave come
together with the troughs from the other wave. The result is constructive interference;

44 Biophysics DemystifieD
the peaks become higher and the troughs become lower. But when waves meet out
of phase, a peak and a trough come together.The result is destructive interference;
the waves flatten out and lower the intensity of radiation.
The technique takes advantage of the behavior of electromagnetic waves
when they encounter atoms or molecules that are arranged in a regular, repeat-
ing structure. The atoms in general change the direction of the electromagnetic
wave fronts, scattering them in all directions. The result is that each atom in the
crystal acts as a starting point for the scattered waves. The scattered waves then
interfere with each other, resulting in a pattern of constructive and destructive
interference at various locations.
The regular repeating pattern of atoms simplifies that mathematics. If we
measure the angle of the incoming radiation, the wavelength of the radiation,
FigUrE 3-3 • DNA double helix. On the left is the original sketch by Francis Crick on discover-
ing the DNA double helix in 1953. On the right is a space-filling model showing the various
atoms. Both were determined using X-ray crystallographic data.

chapter 3 Biophysical Techniques and applicaTions 45
and the distances and angles to the points where we observe constructive inter-
ference, we can then extrapolate back to the source of scattering (the atoms)
and calculate the distances between the atoms.
X-rays, as you know, are a type of electromagnetic radiation, as is light. But
X-rays are not visible to the eye. Instead we use photographic film and other
detection devices in order to “see” X-rays and measure their intensity.
still struggling?
Mathematically, electromagnetic radiation can be treated as particles, called
photons, or waves (electromagnetic waves). This is sometimes referred to as the
dual nature of light: light appears to behave as particles in some situations and
as waves in others. We use the particle model or the wave model, depending on
which best explains the phenomenon we are discussing. In some cases either
will do. But in other cases one is clearly more convenient than the other. as par-
ticles, photons exhibit elastic collisions, bouncing, and scattering off of objects.
(They can also be absorbed. See absorption spectroscopy.) As waves, however,
electromagnetic radiation exhibits reflection (bouncing off a surface), refraction
(bending while passing through a surface), and interference (the combining of
two or more waves in a specific location, resulting in increased or decreased
wave height at that location).
?
Nuclear Magnetic resonance Spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy is a type of EM spectroscopy
that differs from most forms of EM spectroscopy in the following significant
and practical ways:
NMR involves interaction of light (EM) with the nuclei of atoms in a
•
molecule, whereas most forms of EM spectroscopy involve interaction of
light with the electrons in the molecule.
Although most forms of spectroscopy do provide some structural informa-
•
tion, NMR can provide much more structural details (higher resolution) than
other forms of spectroscopy.
NMR involves the application of a strong magnetic field to the sample
•
being studied. This magnetic field alters and limits some of the energy

46 Biophysics DemystifieD
states available to the nuclei; this is what makes it possible to measure the
absorption and emission of EM by the nuclei in the sample.
NMR uses EM in the radio frequency (RF) portion of the spectrum (whereas,
•
for example, absorption and fluorescent spectroscopy typically rely on EM
in ultraviolet, visible, and infrared regions of the EM spectrum).
NMR works on the basic principle that a spinning charge (such as the nucleus
of an atom) generates a magnetic field. In other words the nucleus is like a small
magnet. Under normal conditions, the spins of the various nuclei are randomly
oriented in any direction. However, under the influence of a strong magnetic field
the nuclear spins are constrained to only certain orientations with respect to the
external magnetic field (typically parallel and antiparallel to the magnetic field).
When a nucleus jumps from one spin orientation to another, it will absorb or
emit EM radiation. The frequency of this EM radiation will be proportional to
the energy difference between the two spin states. By scanning the EM spectrum,
we can find all the specific frequencies at which the nuclei are absorbing and
emitting radiation, and thus determine all the energy differences between spin
states. Each of these energy differences depends on the strength of the magnetic
field in the local region of the molecule surrounding the nucleus. Nuclei that are
shielded by electrons and other atoms will experience less of the applied mag-
netic field, so the energy difference between their spin states will be smaller. The
smaller energy difference between spin states means that these nuclei will reso-
nate with lower frequencies (less energy) of EM. On the other hand, the nuclei
that are less shielded by electrons and other atoms will be more exposed to the
magnetic field. These nuclei resonate with higher-frequency EM. Therefore, by
observing which frequencies of light are absorbed and emitted as a result of the
magnetic field, we can infer structural information about molecules.
Electron Microscopy
The most powerful light microscopes provide only enough magnification to view
objects larger than 200 nm. This limitation is due to the wavelength of visible
light. In 1928 physicist Ernst Ruska was experimenting with magnetic lenses for
focusing electron beams and realized that it was possible to take advantage of the
smaller wavelength of electrons to create an imaging device theoretically capable
of greater magnification than a light microscope. In 1931 Ruska and fellow engi-
neer Max Knoll built the first electron microscope. Although it was no more
powerful than a light microscope, they had proved the concept of using focused

chapter 3 Biophysical Techniques and applicaTions 47
electron beams for microscopic imaging. By 1933 they built an electron micro-
scope that surpassed the resolving power of light microscopes. Today electron
microscopes are capable of viewing objects 1000 to 2500 smaller than what can
be seen with even the most powerful light microscope.
Whereas a light microscope focuses a beam of light on the specimen to be
magnified, electron microscopes use a beam of highly accelerated electrons.
There are several types of electron microscopy. The two most common types
are transmission electron microscopy and scanning electron microscopy. Trans-
mission electron microscopy (TEM) is similar to light microscopy in that the
beam passes through a very thinly sliced sample to provide an image on the
other side. Since electrons are not visible to the eye, the image is made by focus-
ing the electron beam onto a view screen coated with some material that fluo-
resces (emits visible light) in response to the incoming electrons. The image can
be further enhanced using detectors similar to those found in digital cameras.
In scanning electron microscopy (SEM) the electron beam is gradually scanned
across the surface of the specimen. At each point on the surface some of the
intensity of the electron beam is lost. This decrease of intensity can be measured
in a variety of ways and can be converted into a surface image of the object (see
Fig. 3-4). SEM is only about 1/10 as powerful as TEM, but it provides a more
three-dimensional image instead of a cross-sectional slice of the specimen.
10 µm
FigUrE 3-4 • Scanning electron micrograph of helianthus annuus
(sunflower) pollen. (Courtesy of Louisa Howard)
48 Biophysics DemystifieD
Atomic Force Microscopy
Atomic force microscopy (AFM) provides magnified images similar to those of
SEM, but with resolution similar to those of TEM (i.e., about 10 times better
than SEM). AFM works by moving a mechanical probe across the surface of
the object being scanned. AFM therefore provides true, three-dimensional
information about the object. There are several variations of AFM that vary
somewhat in how data are collected and in how the probe is manipulated. All
are part of the more general class of techniques called scanning probe micros-
copy (SPM). The more advanced techniques of SPM and AFM keep the probe
a constant distance from the object’s surface in order to avoid the possibility
of the probe damaging or deforming the surface of the object being
scanned.
Optical Tweezers
An optical tweezers is an instrument that uses focused laser beams to create
piconewton (10
212
N)-size forces that can be used to hold and manipulate
microscopic particles, even as small as a single molecule or atom. The phenom-
enon of focused laser beams holding a single particle in place in three dimen-
sions is called an optical trap. For this reason, an optical tweezers is also
sometimes referred to as an optical trap.
Optical tweezers can be used to hold and manipulate particles anywhere
from 0.1 nm (about the size of an atom) to 10,000 nm in size (about the
size of a bacterium), and have been used to trap single viruses, DNA mol-
ecules, bacteria, living cells, and organelles. Optical traps are particularly
useful for investigating the mechanics of and forces associated with molecu-
lar motors. They can be used for sorting and separating cells of various types
or for measuring the forces necessary for bending or breaking a DNA
molecule.
Voltage Clamp
A voltage clamp is a technique used in electrophysiology to measure and char-
acterize electric currents in cells, particularly in excitable tissue cells such as
neurons (nerve cells). The basis of the technique is the ability to insert a very
fine microelectrode into the cell, with another electrode in contact with the

chapter 3 Biophysical Techniques and applicaTions 49
fluid around the outside of the cell. The electrodes can be used to measure
(and manipulate) the voltage or current across the cell membrane. In the volt-
age clamp technique, the voltage is clamped, or held constant, through a
feedback mechanism. The value of the voltage that is held constant is called
the command voltage. The feedback mechanism detects even the slightest
change in the voltage and immediately pumps current across the membrane
through the electrode to keep the voltage at the command voltage. To do this,
the current generated by the electrode needs to be equal and opposite to the
current generated by the cell. We record the current generated by the feed-
back mechanism and use it to calculate the current generated by the cell.
A voltage clamp allows us to measure the cell’s electric current under a
variety of conditions. If we do this for a range of command voltages, then we
can also measure how voltage affects the current. This is useful to discover and
characterize voltage-gated ion channels which are proteins found in some cell
membranes that allow ions to pass through the membrane only when the volt-
age is within a particular range.
Current Clamp
The current clamp is a technique similar to the voltage clamp, except that the
electrode feedback mechanism is used to keep the current (across the mem-
brane) constant while allowing the voltage to vary. In this way we can measure
how the cell varies the voltage across its membrane in response to a particular
current or other conditions.
Patch Clamp
The patch clamp is an alternative technique for applying the electrode to the
cell. Instead of poking a sharp, metal electrode through the cell membrane, the
electrode is placed inside a micropipette (a very thin glass tube) filled with an
electrolyte solution, and the micropipette is placed against the cell membrane.
Gentle suction is applied, forming a tight seal between the micropipette and
the cell membrane (see Fig. 3-5). This tight seal has a high electrical resistance
[1 to 10 megaohms (M)] electrically isolating the small patch of membrane
under the electrode. This allows us to investigate the behavior of a single ion
channel within the membrane.

50 Biophysics DemystifieD
still struggling
The terms voltage clamp, current clamp, and patch clamp all sound like devices
rather than techniques. The device, however, is the same for all three. The device
consists of an electrode, some wires, and an electronic feedback unit that is
capable of simultaneously measuring and manipulating voltages and currents.
The techniques differ primarily in the settings on the device: whether to mea-
sure current or voltage, which to hold fixed (and at what value), and which to
manipulate via the feedback mechanism. In the case of the patch clamp, there
is the addition of a micropipette (a thin glass tube, commonly found in any
physiology laboratory) into which the electrode is placed.
?
Calorimetry
Calorimetry is the measurement of energy changes in the form of heat. An
instrument designed to measure heat energy is called a calorimeter. In biophys-
ics, calorimetry is typically used to measure the amount of energy absorbed or
Cell
Electrolyte solution
Micropipette
Metal electrode
FigUrE 3-5 • Patch clamp technique:
Instead of poking a sharp metal electrode
through the cell membrane, the electrode
is placed into a micropipette (a thin glass
tube) and the micropipette is held against
the cell membrane.
chapter 3 Biophysical Techniques and applicaTions 51
released in the course of biochemical reactions, conformational transitions, or
ligand binding. For example, calorimetry can be used to determine the amount
of energy necessary to unwind a piece of DNA helix. Calorimetry can also be
used to measure the binding strength of various drugs to a particular protein.
The results are then used to determine which drug is most effective at a par-
ticular task. There are various types of calorimeters. In particular, microcalorim-
eters are useful for measuring the very small amounts of energy associated with
many biophysical processes.
There are numerous other biophysical techniques not yet discussed here.
The goal of this chapter has been to present and define some of the more com-
mon techniques.
