Goldfarb D. Biophysics DeMYSTiFied
Подождите немного. Документ загружается.

32 Biophysics De mystifieD
Allosterics results from a combination of ligand binding and conforma-•
tional transitions at the same time. And allosteric regulation is detectable
by observing the kinetics of the process.
Molecular transport often involves investigating how certain molecules
•
get from one side of a membrane to another. And, the mechanisms of
electrophysiology always involve molecular transport across membranes
within excitable tissue.

chapter 2 Biophysical Topics 33
Quiz
Refer to the text in this chapter if necessary. Answers are in the back of the
book.
1. What are the three major divisions of biophysics when categorizing the branches
of biophysics according to size of what is being studied?
a. small, medium, and large biophysics
B.
Microscopic, tissue, and organism biophysics
c. subcellular, physiological, and environmental biophysics
D. Subcellular, structural, and macromolecular biophysics
2. A conformational transition is
a. measurable in a variety of ways.
B.
a change in the shape of a molecule.
c. a way that biological molecules carry out their function.
D. all of the above
3. Which of the following statements is most true?
a. secondary structure is two dimensional, whereas tertiary structure is three dimen-
sional.
B.
primary structure is one dimensional, specifying only the sequence in which atoms or
groups of atoms are connected to one another.
c. Biological molecules are rarely polymers.
D. Residues are the parts of a molecule left behind after the molecule carries out its
function.
4. A ligand is
a. connective tissue that holds bones together.
B. connective tissue that ties muscles to bones.
c. an element in the periodic table.
D. a smaller molecule or atom that binds to a larger molecule.
5. Name three branches of biophysics within the division of molecular and subcel-
lular biophysics.
a. Kinetics, membrane biophysics, and histology
B. sensory biophysics, radiation biophysics, and allosterics
c. allosterics, conformational transitions, and statistical thermodynamics
D.
Protein biophysics, orgone biophysics, and electrophysiology
6. Statistical mechanics falls into which division of biophysics?
a. Molecular and subcellular biophysics
B. physiological biophysics
c. Environmental biophysics
D.
All of the above
34 Biophysics DemystifieD
7. Cell membranes are primarily made of
A. DNA.
B. protein.
c. polysaccharides.
D.
lipids.
8. Bioenergetics falls into which division of biophysics?
a. Molecular and subcellular biophysics
B. physiological biophysics
c. Environmental biophysics
D.
All of the above
9. Which of the following statements is most true?
a. statistical mechanics uses statistics to calculate the average force animals use to acceler-
ate to their top speed.
B. statistical mechanics is the application of statistical methods to biomechanics.
c. statistical mechanics is a misnomer because in practice it has nothing to do
with statistics.
D. Statistical mechanics uses statistical averages of populations of molecules to calculate
measurable thermodynamic quantities.
10. A biopolymer is a
a. type of allosteric interaction.
B. large collection of biomolecules within the cell.
C.
biological molecule made up of many smaller molecules linked together.
D. lipid bilayer

35
In this chapter we present an overview of the various techniques and applica-
tions of biophysics. These are important primarily for two reasons. First, we
need to know what we can learn from each technique and how each technique
can be useful in studying the various topics of biophysics. Second, some of the
techniques are branches of biophysics unto themselves.
CHAPTEr OBJECTiVES
In this chapter, you will
learn to define the most common biophysical techniques.
•
understand what information each biophysical technique can provide.
•
Be able to list and briefly describe several applications of biophysics in medi-
•
cine and in other areas.
Quar
k
w
B
E
De Broglie’s photon
sin
sin
S
D
ec
ec
2
B
B
Electr yclosity
E
lec
ty closit
y
Relativist
Ong
in
ca
a
a
e
a
e
ev
m
m
k
e
chapter
3
Biophysical Techniques
and Applications
36 Biophysics DemystifieD
As we mentioned previously, biophysical techniques and applications are some-
times considered as branches of biophysics. That is, some biophysicists dedicate
their focus of study to a single technique or application, with the following
goals in mind: (1) to research and better understand the physical principles
underlying the technique, and (2) to work toward extending the technique’s
abilities.
Biophysical techniques fall into two major categories: preparative and ana-
lytical. Preparative techniques are those that purify or isolate biological speci-
mens (organisms, cells, and molecules) or otherwise get them ready for use in
some other process or further experimentation. Analytical techniques are those
used to measure physical aspects of a biological system. Many biophysical tech-
niques fall into both categories at the same time.
Ultracentrifugation
A centrifuge is a machine used to spin a sample of material around in circles. The
circular motion places a force on the sample. The force is similar to, but typi-
cally much larger than, the normal force of gravity. An ultracentrifuge is a cen-
trifuge specially designed to spin at an extremely high rate of speed. Some
ultracentrifuges can exert forces as much as 1 million times that of gravity.
Why do we do this? Centrifuges operate on the principle of sedimentation.
Sedimentation describes the motion of particles in a fluid under the application
of a force. Take for example a snow globe; you know, those glass or clear plastic
containers filled with water and some sparkly snowlike particles. Typically the
globe also contains some kind of winter scene. You shake up the particles inside
and then put the snow globe down. The force of gravity causes the particles to
slowly descend in the water, making it appear as though it is snowing inside the
globe. This is sedimentation. In fact, real snow showers result from sedimenta-
tion of snowflakes in the atmosphere.
The physics of sedimentation shows that the sedimentation rate of a particle
or molecule depends on several things, including the force, the density of the
fluid, and the size and density (or concentration) of the particles in the fluid.
Applying a force stronger than gravity can increase the sedimentation rate. It
can also magnify differences in sedimentation behavior between different mol-
ecules. This makes ultracentrifugation a convenient technique for separating
molecules of different sizes. Ultracentrifugation is used as both a preparative
and an analytical technique. For example, the ultracentrifuge is commonly used
to isolate samples of pure DNA.
chapter 3 Biophysical Techniques and applicaTions 37
An analytical ultracentrifuge contains special optical devices and sensors that
can track the movement of molecules as they are being centrifuged. Sedimenta-
tion rates can be measured directly, under various conditions. We can use the
formulas that describe the physics of sedimentation to calculate the size and
approximate shape of the molecules. An analytical ultracentrifuge can also be
used to detect conformational transitions (see Chap. 2) and to determine the
number of subunits making up a molecular complex.
Electrophoresis
Electrophoresis is another technique that relies on the principle of sedimenta-
tion. However, in electrophoresis the force results from an electric field applied
to electrically charged particles or molecules. Many biomolecules such as DNA
and proteins have an electric charge. We can take advantage of this by applying
a strong electric field to the molecules in solution. Under these conditions, for
example, negatively charged DNA will move (sediment) through a solution
toward the positive side of the applied electric field.
A very common type of electrophoresis is gel electrophoresis. A gel is a fluid
that has a molecular structure that gives it properties similar to a solid. Jellies
and jams are gels. The molecular structure that gives the gel its solid-like prop-
erties also acts to obstruct the movement of molecules dissolved in the gel.
Larger molecules are more easily obstructed than smaller molecules, so the gel
actually increases the differences in sedimentation rates between molecules of
different sizes within the gel.
Think of balls rolling down a hill and imagine there are many obstacles on
the hill (fence posts, trees, bushes, boxes, etc., anything that would obstruct
the balls freely rolling down the hill). The balls bounce off the obstacles but
eventually find their way to the spaces between the obstacles and continue rolling
down the hill. But if the balls are of various sizes, then the larger balls—being
larger and taking up more space—are more likely to bang into the obstacles
along the way. This is especially true if the gaps between obstacles are not
much bigger than the largest balls. Every time a ball hits an obstacle, it slows
its descent.
The smaller balls, however, sail through the gaps between the obstacles and
move to the bottom of the hill faster. In the same way smaller, more compact
molecules move through a gel much faster than larger molecules.
Sedimentation in gel electrophoresis is affected not only by the density of
the gel and the size and shape of the molecules but also by the charge on the

38 Biophysics DemystifieD
molecules. Molecules with more charge will experience a stronger force gener-
ated by the electric field.
Size Exclusion Chromatography
Size exclusion chromatography (SEC) is another technique that relies on sedi-
mentation. SEC, however, uses gravity, or sometimes pressure, to sediment a
solution through a gel. The gels used in SEC, however, differ from those used in
electrophoresis in the following way. SEC gels are not one solid piece of gel, but
rather a tightly packed suspension of gel beads or particles with spaces between
them. There are pores on the surface of the gel particles through which molecules
pass to get into the gel matrix on the inside each particle or bead. These pores,
however, are very small, excluding larger molecules. Smaller molecules enter
these pores, but when they do it can take them some time to pass through or
otherwise exit the gel bead. This slows down the smaller molecules. Larger mol-
ecules bump into the gel particles (as we saw with electrophoresis) but move
around the spaces between the gel beads much faster than the smaller molecules
that are temporarily trapped inside the gel beads. The end result is similar to
electrophoresis in that molecules are separated based on their size. However, in
SEC it is the larger molecules that pass through the gel faster and the smaller
molecules that lag behind. Using gels with different size pores can “exclude” dif-
ferent size molecules from the insides of the gel particles.
Spectroscopy
As a general class of investigative techniques, spectroscopy typically involves
sending some form of electromagnetic radiation into a sample and measuring
various properties of the electromagnetic radiation that emerges from the sam-
ple. For example, one can measure the intensity of the emergent radiation.
Other common properties include the direction of the emitted radiation and
its polarization. The measured property is then plotted as a function of the
wavelength or frequency of the radiation; the resulting plot is called a spectrum
(plural: spectra).
Just as a reminder, all electromagnetic radiation can be characterized by
wavelength or frequency. The two are inversely proportional. If we know one,
we can calculate the other.
υ 5 c/λ (3-1)
chapter 3 Biophysical Techniques and applicaTions 39
where is the frequency, c is the speed of light (2.9979 3 10
8
m/s), and l is the
wavelength. So, plotting a spectrum by wavelength or frequency is more or less
the same thing. It certainly contains the same information. The frequency, how-
ever, has the advantage that it is proportional to the energy; thus
E 5 hυ (3-2)
where h is Planck’s constant (6.626068
3 10
234
m
2
kg/s).
Originally, the techniques of spectroscopy were developed using only the
visible light portion of the electromagnetic spectrum [wavelengths of approxi-
mately 380 to 750 nanometer (nm)], and later grew to include ultraviolet,
infrared, and a much broader range of wavelengths.
Over the years, additional techniques have been developed which do not
necessarily involve electromagnetic radiation but which nonetheless produce a
spectrum of sorts. For example, electron spectroscopy measures the kinetic
energy of electrons that emerge from the sample. Mass spectrometry produces
a spectrum as a function of mass. By and large, however, most spectroscopic
techniques involve electromagnetic radiation. Therefore, when necessary in
order to distinguish those forms of spectroscopy that use electromagnetic radia-
tion from other uses of the word spectroscopy, we will specifically use the term
EM spectroscopy.
There are dozens of spectroscopic techniques used in the biophysical sci-
ences. In the following sections we briefly describe a few of them. Each type of
spectroscopy teaches us something different about the biological sample, with
some overlap in what we can learn from each technique. Commonly, spectro-
scopic techniques provide information about the identity of biological mole-
cules, about their structure, conformational transitions, binding, and kinetics.
The various spectroscopic techniques are classified according to the type of
light (electromagnetic radiation) used and according to the properties of the
emergent light measured. Additionally, some spectroscopic techniques are fur-
ther distinguished according to the conditions of the experiment that are con-
trolled. For example, if we measure the amount of light absorbed as we control
and slowly vary the temperature of a sample, we would call this temperature-
scanning absorbance spectroscopy.
A note on the use of the word light: Throughout this book we will often use
the word light rather freely to mean electromagnetic radiation. Strictly speak-
ing, the word light is meant to distinguish those parts of the electromagnetic
spectrum that are visible to living organisms. In practice, light is something we
are very familiar with, and use of the word light to mean electromagnetic
40 Biophysics DemystifieD
radiation emphasizes the fact that all forms of EM radiation have the same basic
nature that light has. That is, they are rapidly oscillating electric and magnetic
fields of various frequencies (which we perceive as color) and amplitutdes
(which we perceive as intensity or brightness). Therefore you can assume within
context that light is meant to convey in general any portion or all of the elec-
tromagnetic spectrum, from gamma rays and X-rays, through visible light,
microwaves, and even radio waves. Of course, wherever it is important to dis-
tinguish one portion of the electromagnetic spectrum from another or to make
very clear which type of light we are talking about, then we will use specific
qualifiers such as infrared, visible, ultraviolet, X-rays, and so on.
Absorption Spectroscopy
Absorption spectroscopy (also called absorbance spectroscopy) is one of the more
common (and easy to understand) forms of spectroscopy. In absorption spectros-
copy, we shine light of a specific wavelength through a sample and measure the
intensity of the light that comes out the other side. Really we are most inter-
ested in measuring the absorbance, or how much light does not come through
to the other side (i.e., how much light is absorbed).
The absorbance of a given sample depends on three things: (1) the intrinsic
ability of the molecules in solution to absorb light, (2) the concentration of the
molecules in solution, and (3) the path length of the light as it passes through
the sample (i.e., if the sample container is larger, then the light has to pass
through more of the solution before getting to the other side, and so more light
will be absorbed). Since our goal is to know a given molecule’s ability to absorb
light, we need to somehow account for the concentration of the solution and
the path length of the light. The molar extinction coefficient is a measure that
accounts for both concentration and thickness of the sample being studied. It
does this by expressing the absorbance in units that are per concentration and
per length (M
21
cm
21
).
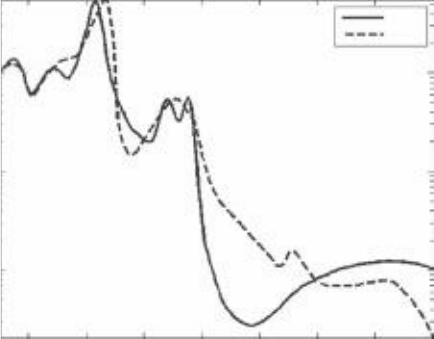
It is common to measure absorbance at many different wavelengths and plot
a graph of the absorbance versus the wavelength or frequency of light. Such a
graph is called the absorption spectrum (Fig. 3-1). Many molecules have unique
or characteristic absorption spectra, so an absorption spectrum can be used to
identify types of molecules in a sample. Absorption spectroscopy can also be
used to measure the concentration of molecules in solution (once the identity
of the molecule is known).
chapter 3 Biophysical Techniques and applicaTions 41
Notice in Fig. 3-1 that the difference in absorbance between the two forms of
hemoglobin is greatest at a wavelength of about 690 nm. This means we can
measure absorbance at 690 nm as a convenient way to determine the proportion
of hemoglobin with oxygen bound to it. For example, let’s say we have a sample
of hemoglobin. Now say we measure the difference in absorbance between our
sample and oxygen-free hemoglobin to be only 3/4 of the difference we expect
for fully oxygenated hemoglobin. Then we can say that 75% of the oxygen bind-
ing sites in our hemoglobin sample have oxygen bound to them, while 25%
remain unoccupied.
In many molecules, absorption of light also varies with conformation, as well
as with the presence or absence of bound ligands. So absorption spectroscopy can
be used to follow conformational transitions and ligand binding. Temperature-
scanning absorption spectroscopy measures absorbance (usually at a single wave-
length) across a range of temperatures. This is useful for studying
temperature-induced conformational transitions, that is, changes in molecular
shape that can be brought about by changes in temperature. This is a common
technique for studying conformational transitions in membranes, proteins, and
DNA (nucleic acids). See Fig. 3-2.
Molar Extinction Coefficient vs. Wavelength
Wavelength (nm)
300
10
3
10
4
10
5
400 500 600 700 800 900 1000
HbO
2
Hb
Molar Extinction Coefficient (cm
–1
M
–1
)
FigUrE 3-1 • Absorption spectra of hemoglobin with oxygen bound to
the hemoglobin (solid line) and without oxygen bound to the hemoglo-
bin (dashed line). (Courtesy of Wikimedia Commons.)
