Goldfarb D. Biophysics DeMYSTiFied
Подождите немного. Документ загружается.

272 Biophysics De mystifieD
somehow has a very large kinetic energy, then it may be able to penetrate into
the bilayer, at which point moving out of the bilayer to either side is highly
favorable.) Large molecules are also unable to pass through the bilayer, unless
they have enough energy to disrupt the forces that hold the bilayer together.
Being large, they have to push aside more lipid molecules in order to pass
through than a smaller molecule would have to do. So, they also have to have
enough energy to disrupt more of the forces holding the lipid molecules
together. On the other hand, small, uncharged, nonpolar (or only slightly polar)
molecules can pass through the bilayer with relative ease.
The forces that hold the bilayer together are dispersion forces, the hydropho-
bic effect, and, if cations are present, an additional stabilization by the cations
acting as counterions to reduce the repulsive force between the phosphate head
groups. Obviously since cations stabilize the bilayer, their presence has a
strengthening effect on the bilayer. This makes it more difficult for large mol-
ecules to enter, since large molecules have to somehow push the lipids aside.
Pushing the lipids aside requires disrupting the dispersion forces that attract the
hydrocarbon tails to one another. This takes energy, and the larger the molecule,
the more lipid molecules it has to push aside (and the more energy required).
On the other hand, at lower concentrations of cations, the repulsive force of the
head groups may assist the large molecules getting through, by making it easier
to push the lipids aside.
The strength of the dispersion forces depends largely on the amount of close
contact between the hydrocarbon chains. This in turn depends on how close
together the chains can pack and how long the chains are. Longer chains have
more contact over which the dispersion forces can attract adjacent molecules. So,
longer chains make stronger dispersion forces and more stable bilayers. (Longer
chains also mean a thicker bilayer and thus a greater distance over which a mol-
ecule passing through the bilayer has to travel.) Another significant factor is
whether the hydrocarbon chains are saturated, unsaturated, or polyunsaturated.
Unsaturated chains (recall from Chap. 7) have one or more double bonds between
some of the carbon atoms. Double bonds restrict free rotation, so each chain will
be stiff at the location of the double bond, with a particular angle or kink in the
chain at that location. The end result, due to these kinks, is that unsaturated and
polyunsaturated chains prevent the lipid molecules from packing as closely
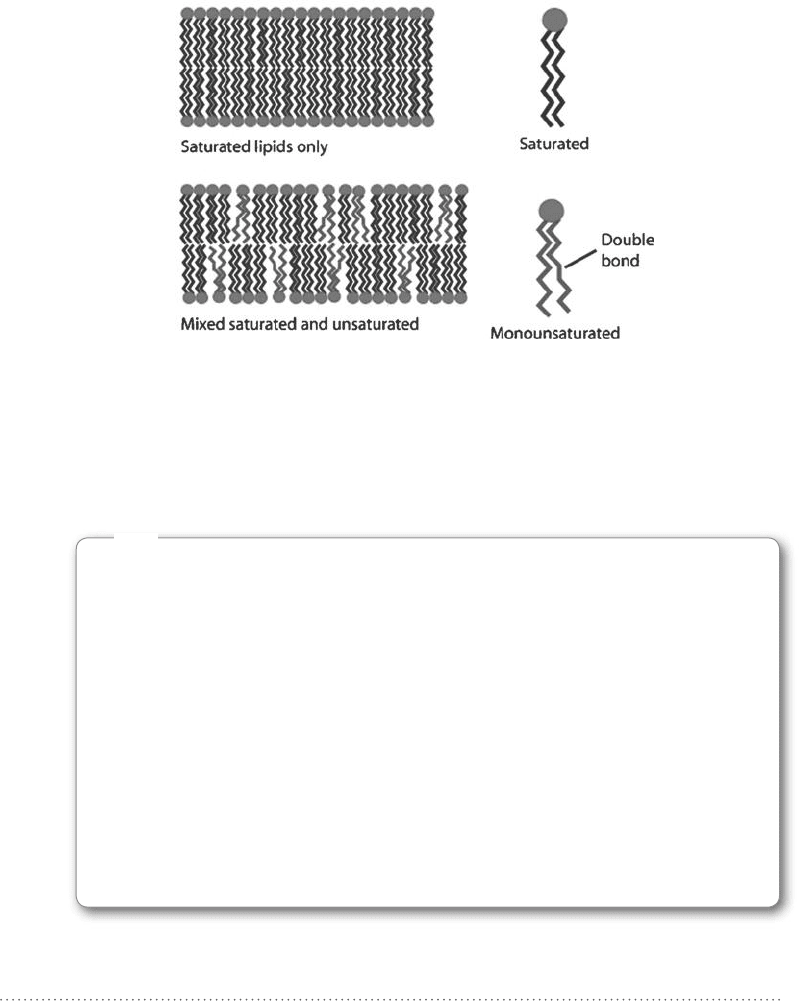
together as saturated lipids can. This is illustrated in Fig. 11-6. Dispersion forces
are proportional to 1/r
6
;
that is, they decrease in proportion to the sixth power of
the distance. Less tightly packed chains mean that on average the chains are fur-
ther apart from each other; this leads to weaker dispersion forces.
Chapter 11 MEMBRANE BIOPHySICS 273
Fluid Mosaic Model
Our current working model of biological membranes is the fluid mosaic model,
which was first proposed by Singer and Nicolson in 1972. The model states that
biological membranes are composed primarily of a phospholipid bilayer. Other
Figure 11-6 • Lipid bilayers that contain only saturated lipids are
able to pack the lipids much closer together than bilayers that
contain unsaturated lipids or a mixture of saturated and
unsaturated lipids.
still struggling
The main forces that hold the bilayer together are the hydrophobic effect and dis-
persion forces. The hydrophobic effect is driven by entropy: water has more freedom
of movement when it stays away from hydrophobic portions of the lipid molecules.
Bilayer formation is favorable because it keeps the hydrophobic lipid tails away from
the water, and the polar lipid head groups facing the water. Dispersion forces are
due to synchronous fluctuations in the electron density of the atoms within the
hydrophobic tails. The electron densities fluctuate rapidly and in synch with each
other. At any given moment, in a place where one tail has a slightly positive charge
an adjacent tail has a slightly negative charge. This causes the hydrophobic tails to
attract one another giving the membrane strength and stability.
?
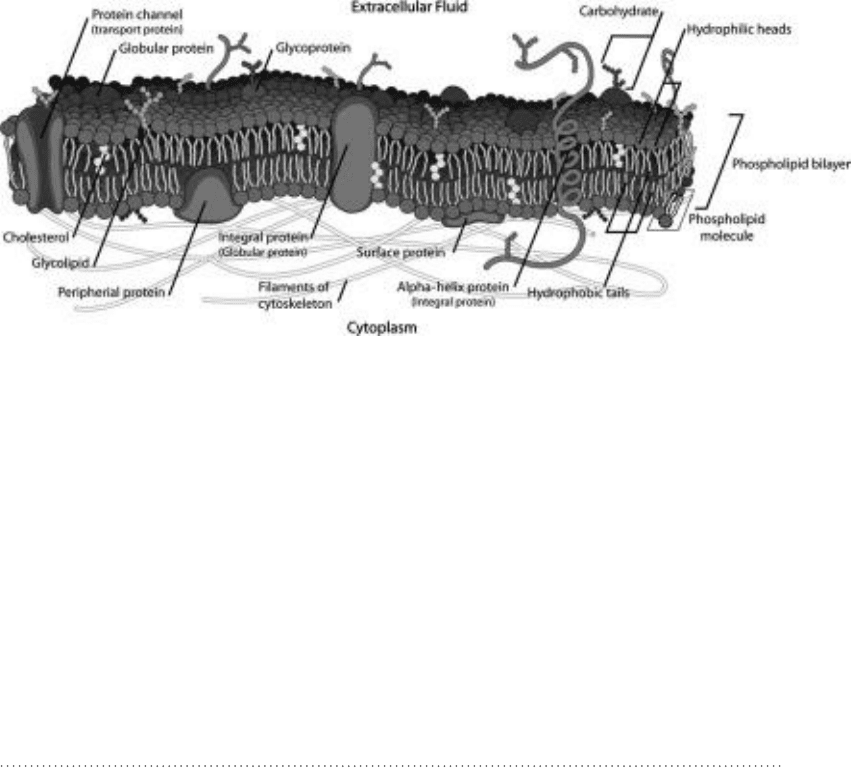
274 Biophysics DemystifieD
molecules that are part of the membrane structure, such as proteins, glycopro-
teins, cholesterol, and carbohydrates, form a mosaic with the lipid molecules. See
Fig. 11-7. This is in contrast to earlier biomembrane models in which the lipids
were thought to form a monolayer or in which proteins were also thought to
form one or more layers within the membrane. In the fluid mosaic model, the
lipids and other molecules are free to move about within the two-dimensional
mosaic.
Phase Transitions in Phospholipid Bilayers
Phospholipids in bilayers and micelles can exist in a solid or fluid phase. We will
focus our discussion on bilayers, since that is what we find in cell membranes,
but the basic concepts apply also to micelles. The solid phase is called the gel
phase. In the gel phase the bilayer is still somewhat flexible and permeable, but
lipid molecules (and other molecules within the mosaic) are fixed to a particu-
lar position within the two-dimensional mosaic. In simple terms, they don’t
move around within the membrane.
The fluid phase is called the liquid crystalline phase. In the liquid crystalline
phase phospholipids next to each other are able to change positions, sometimes
as often as millions of times per second. In this way, any given lipid molecule is
able to gradually diffuse and move about in two dimensions within the plane
Figure 11-7 • Fluid mosaic model of biological Membranes. The phospholipids in the bilayer form a mosaic with other
molecules making up the membrane. These molecules and the phospholipids are free to move about in two dimensions,
that is, in parallel with the bilayer.
chapter 11 MeMBrane Biophysics 275
of the bilayer. However, in both the gel and the liquid crystalline phases, lipid
molecules generally do not move from one layer to the other, since this would
involve a large, positive Gibbs energy change. Proteins and other molecules in
the mosaic also move about within the plane of the bilayer.
The Melting Transition
Phase transitions in lipid bilayers can be temperature induced. Therefore (as we
saw with DNA unwinding) the transition is sometimes referred to as melting.
The melting transition in phospholipid bilayers is highly cooperative, meaning
that it tends to happen in an all-or-none, two-state manner. Figure 11-8 shows
a typical melting curve for a sample of liposomes. The melting temperature T
m
is the halfway point of the transition. Below the melting temperature the lipids
are in the gel state; above the melting temperature they are in the liquid crystal-
line state. The transition results from the disruption of the dispersion forces
among the hydrocarbon tails. The dispersion forces otherwise hold adjacent
phospholipids together. The lipid bilayer itself remains intact due to hydropho-
bic forces, which can be further strengthened by counterions along the phos-
phate surfaces of the bilayer.
Biological Significance
Biological membranes are most commonly found to be in the fluid (liquid
crystalline) state; this allows the free movement of molecules within the two-
dimensional mosaic. However, the melting temperature is usually close to
5
0.1
0.2
15 25
Temperature (°C)
Heat capacity
35 45 55
Figure 11-8 • Typical heat capacity melting profile for a highly
cooperative gel to liquid crystal phase transition in a sample of
liposomes (phospholipid bilayer vesicles).
276 Biophysics DemystifieD
physiological temperatures. This means that the cell can somewhat regulate the
extent of gel versus liquid crystalline state of the membrane. In the liquid crys-
talline state, membranes are more permeable; this makes it easier for various
molecules to pass into or out of the cell. Cells can decrease their overall perme-
ability and increase the rigidity of their boundary by having some of the cell
membrane in the gel state. However, if the entire cell membrane is in the gel
state for a significant period of time, this typically results in the cell “freezing”
to death. The ability of organisms to regulate the fluidity of their own mem-
branes is called homeoviscous adaptation.
In the fluid state, the mobility of molecules within the mosaic plane enhances
a cell’s ability to move membrane proteins and receptors around to where they
are needed. It also gives the cell the ability to reseal small holes in the bilayer,
by shifting molecules around to fill in the hole. This important feature is lacking
in gel phase bilayers. In some cases it may be necessary to keep some molecules
on one particular side of the cell, for example, cilia (hairlike filaments) that
extend from the surface of cells in the lung. These cilia could not do their job
of sweeping dust out of the lungs if they were allowed to freely diffuse to the
opposite side of the cell. On the other hand, keeping the cell membrane in the
gel state would reduce permeability too much and prevent important mole-
cules (e.g., oxygen) from getting through the cell membrane. The cell solves this
problem by maintaining the bilayer in a liquid crystalline state, while anchoring
some molecules in place with the cytoskeleton.
Factors Affecting Membrane Fluidity
The same factors that affect membrane permeability also affect membrane
fluidity. Longer hydrocarbon tails increase dispersion forces making it more
difficult to melt the bilayer. Experimentally we observe a higher melting tem-
perature for bilayers made from lipids with longer hydrocarbon tails. Con-
versely, unsaturated lipids and lipids with shorter tails have lower melting
temperatures due to weaker dispersion forces. In general, anything that weak-
ens dispersion forces will reduce the melting temperature, increase fluidity, and
increase permeability. Anything that strengthens dispersion forces will increase
the melting temperature, decrease fluidity, and decrease permeability.
Cholesterol
Cholesterol is a significant component of many biological membranes, and it
has a mixed effect on membrane fluidity depending on whether the bilayer is
in the gel or liquid crystalline phase. Cholesterol tends to weaken dispersion
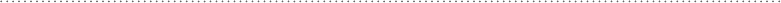
chapter 11 MeMBrane Biophysics 277
forces by positioning itself between the phospholipid molecules. It also disrupts
the orderly arrangement of the phospholipids in the gel phase, increasing
entropy. Both of these effects lower melting temperature and favor the fluid
phase of the bilayer.
However, in the fluid phase, the flat surface of cholesterol’s interconnected
rings (see Fig. 7-13) is significantly wider than the two chains of a single phos-
pholipid molecule. And the interconnected rings are rigid. So each cholesterol
molecule acts as an unbending barrier that limits some of the motion of the
phospholipids. So, while the cholesterol prevents the formation attractive dis-
persion forces, the steric restriction of phospholipid movement decreases mem-
brane fluidity and increases membrane rigidity. Many organisms are able to
regulate membrane fluidity and rigidity by regulating the amount of cholesterol
incorporated into their membranes. Organisms that live primarily in cold envi-
ronments are typically found to have less cholesterol in their membranes. This
prevents the organism from freezing to death.
The cholesterol molecule is almost entirely hydrophobic. Only the hydroxyl
group at one end of the cholesterol molecule is hydrophilic. The hydroxyl group
hydrogen bonds itself to the base of the phosphate head groups of the bilayer.
Since the hydroxyl group is smaller than the phosphate head groups, the cho-
lesterol is entirely submerged in the bilayer. The vast majority of the cholesterol
molecule is comfortably inside the hydrophobic portion of the bilayer.
Membrane growth
As cells grow, their membranes need to grow also. The cell somehow needs to
add lipid molecules to the bilayer. Lipids are synthesized in the cell interior
through a series of biochemical reactions mediated by enzymes. The resulting
lipids aggregate to form liposomes. The lipids are then added to the membrane
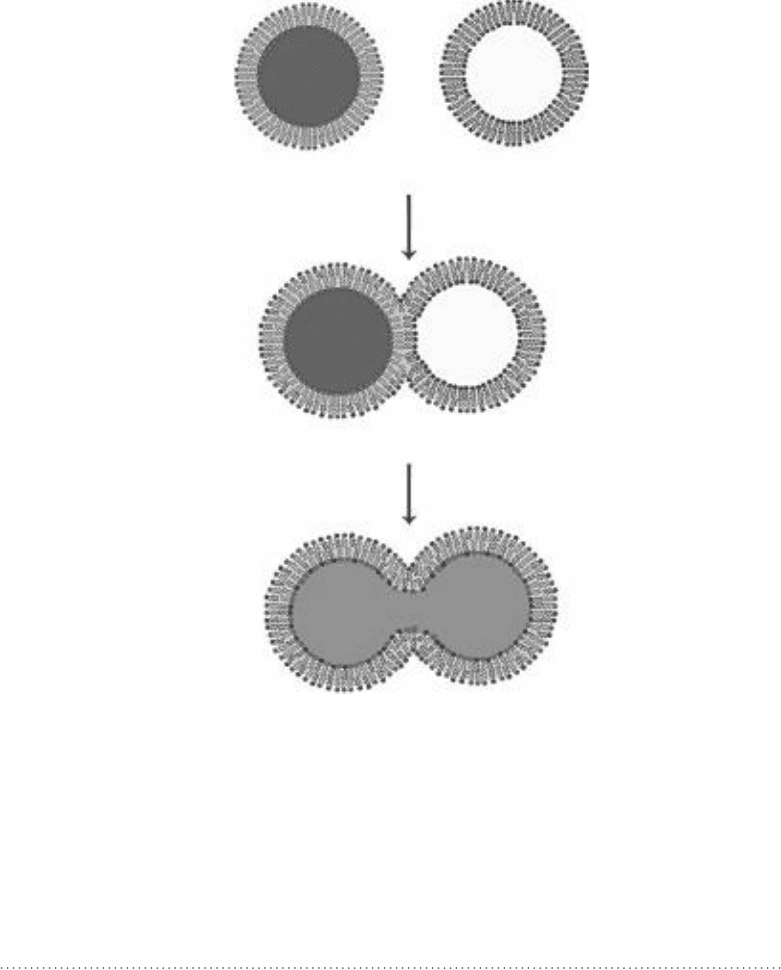
by fusing the liposomes with the existing membrane. See Fig. 11-9.
As their membranes grow, cells need to incorporate additional protein and
other molecules that are part of the membrane structure. This can be done in
a variety of ways. Some relatively hydrophobic proteins and glycolipids are able
to simply penetrate the bilayer due to a favorable Gibbs energy change. Once
inside the bilayer the hydrophobic effect helps to keep them in place. Some-
times proteins are inserted into the cell membrane while still in the form of a
polypeptide chain, before the protein folds into its native state. The fact that
protein synthesis typically takes place just below the surface of the cell mem-
brane facilitates this process. Once the protein is inside the bilayer, the Gibbs
278 Biophysics DemystifieD
energy change of protein folding becomes favorable. At the same time, the fold-
ing of the protein typically further stabilizes its position in the bilayer making
it unfavorable for the protein to exit the bilayer.
Membrane Permeability and Transport
Let’s revisit the idea of bilayer permeability and the need to transport mole-
cules across membranes. As we noted earlier in this chapter, the lipid bilayer is
semipermeable, meaning that some molecules can pass through the bilayer while
others can’t. Sometimes biological membranes are described as selectively
Unfused
Hemifused
Fully fused
Figure 11-9 • Fusion of two liposomes. The process is the same for
a liposome fusing with an existing larger membrane.
chapter 11 MeMBrane Biophysics 279
permeable, meaning that the cell somehow chooses what to let in, what to let
out, what to keep in, and what to keep out. The cell does this with a combina-
tion of active and passive transport. Active transport means that the cell expends
energy to move a molecule or ion across the membrane that would otherwise
not favorably move across the membrane. Passive transport means that the mol-
ecule or ion passes through the membrane by virtue of a favorable Gibbs energy
change without the need for the cell to expend any energy.
Passive Transport
In the case of passive transport there are two important points to keep in mind.
The first is that sometimes passive transport is the result of some earlier active
transport. In other words, the cell regularly expends energy moving molecules
against an otherwise unfavorable Gibbs energy change. Later, when the needs
of the cell require it, the cell allows the molecules to flow in the direction of a
favorable Gibbs energy change via passive transport. This type of passive trans-
port may be for the purpose of simply adjusting the concentration of an ion or
molecule. But that is usually not the case. Rather this type of passive transport
is more commonly used as the driver of a secondary active transport. In second-
ary active transport, the energy released from a passive transport is used to drive
an active transport.
The second point to know regarding passive transport is that it is not always
a simple diffusion or movement through the lipid bilayer (combined with a
favorable Gibbs energy change). Instead, passive transport can occur as the
result of a tunnel, or channel, created through the membrane by the presence
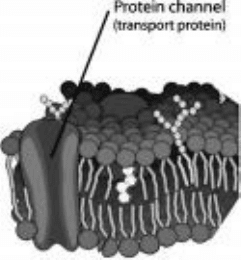
of transport protein. An example is shown in Fig. 11-10. Transport proteins can
be involved in either active or passive transport. Transport proteins involved in
passive transport contain a combination of hydrophobic and hydrophilic amino
acid residues. The sequence of resides and the forces involved in protein folding
cause the protein to fold in a way that creates a tunnel or channel through the
center of the folded protein. The hydrophobic residues are in the outside of the
protein exposed to the hydrophobic portion of the bilayer. The hydrophilic
residues are on the inside of the protein, away from the hydrophobic lipid
chains. These hydrophilic residues line the walls of the channel through the
center of the protein. There are also usually some hydrophilic residues near the
openings of the channel at each end of the transport protein. These may form
hydrogen bonds with the phosphate head groups and help to keep the protein
oriented so that the transport channel remains perpendicular to the plane of
the bilayer (see Fig. 11-10).
280 Biophysics De mystifieD
Gated Ion Channels
Earlier in this chapter we pointed out that lipid bilayers are very much not
permeable to ions. In almost all cases, transport of ions across the membrane
requires the existence of an ion channel, which is a transport protein as
we described above with a hollow channel lined with hydrophilic residues.
(One exception is vesicle transport discussed later in this chapter.) In some
cases the native state of the membrane transport protein involves two or more
native conformations. In at least one of these conformations the transport chan-
nel is blocked, preventing the movement of ions into the channel through
either steric or hydrophobic forces. Such a channel is said to be gated because
the conformation of the protein acts as a gate that can open and close. Depend-
ing on the particular transport protein and its specific purpose, various factors
can act to trigger the conformational change to open or close the gate. For
example, binding of a protein or other molecule to a specific receptor on the
surface of the membrane may trigger the gate to open or close. Or the presence
of a certain voltage difference across the membrane may trigger the conforma-
tional change that opens or closes the ion channel. This latter situation is
referred to as a voltage gated ion channel. Voltage gated ion channels are com-
mon in nerve cells (neurons) and other excitable tissue (muscle cells). Typically
there is a very specific voltage at which the gated ion channels of a cell open in
a highly cooperative manner, so that once one channel is open, the other chan-
nels along the length of the neuron open rapidly in succession, creating an
impulse that travels down the length of the neuron.
Figure 11-10 • A channel is a type of
transport protein that folds in a way so as to
form a hydrophilic channel or tunnel through
the phospholipid bilayer.
chapter 11 MeMBrane Biophysics 281
Active Transport
Active transport always involves the use of energy to drive the transport of a
molecule or ion across the membrane. There are two reasons why energy may
be needed to drive the transport of a molecule or ion across the membrane. The
first reason is that there may be an otherwise unfavorable Gibbs energy change
just to get the molecule into the hydrophobic interior of the bilayer. This is the
case with ions and other charged or large and highly polar molecules. The over-
all Gibbs energy change to get the ion from one side of the membrane to the
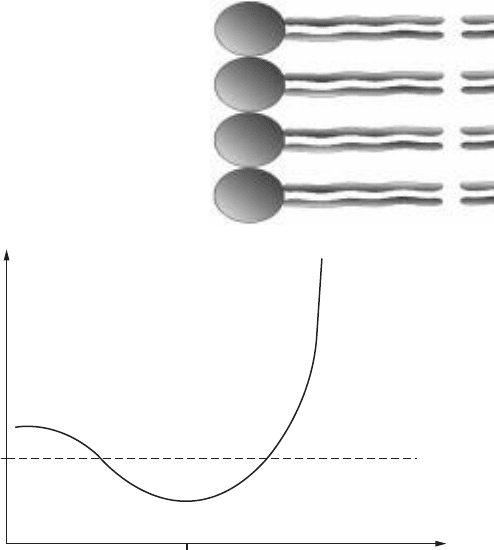
other may be small or even negative (favorable), but there is an energy hump,
or barrier, to get over in order to get the ion into the bilayer. This energy barrier
is illustrated in Fig. 11-11. In most cases, if the overall Gibbs energy change is
0
Edge of bilayer Distance into bilayer
∆G
Figure 11-11 • Gibbs energy change for passing a positive ion into a lipid
bilayer, as a function of distance into the bilayer. The Gibbs energy change is
initially negative as the ion approaches the negatively charged phosphate
head groups.
if the concentration of the ion is smaller on the other side of the
bilayer, then the overall Gibbs energy change will be negative (favorable). But
as the positive ion passes the lipid head groups and into the hydrophobic
interior of the bilayer the Gibbs energy rises sharply.
