Goldfarb D. Biophysics DeMYSTiFied
Подождите немного. Документ загружается.


262 Biophysics D e mystifieD
Quiz
Refer to the text in this chapter if necessary. Answers are in the back of the
book.
1. What do A-, B-, C-, and Z-DNA describe?
A.
The four nucleotide bases found in dNA.
B.
Four DNA secondary structures: hairpins, loops, cruciforms, and tails.
C. Double-helical structures that differ in pitch and width of the helix.
D. The four types of single-stranded DNA.
2. Which statements are true?
S1. The DNA double helix must unwind for transcription and replication.
S2. Whether the DNA will unwind depends on the Gibbs energy change.
S3. Unwinding circular DNA will change the writhe of the helix.
A.
S1 and S2
B. S2 and S3
C.
S1 and S3
D. S1, S2, and S3
3. What is a nucleic acid palindrome?
A.
A nucleotide sequence that can form a stem-and-loop or cruciform structure.
B.
A nucleotide sequence that reads the same forward or backward.
c. A protein that binds to certain dNA sequences.
D. A curved, dome-shaped secondary structure in nucleic acids.
4. What is homogenous DNA?
A.
dNA that has been blended together.
B.
dNA from only one species.
c. dNA with a sequence made from only a single nucleotide.
d. dNA with a simple sequence that repeats throughout the molecule.
5. The root mean square end-to-end distance of a freely jointed chain is propor-
tional to what?
A. The length of a segment and the square root of the number of segments.
B. The square root of the length of a chain segment.
c. The number of segments and the square root of the length of each segment.
d. The difference between the length of one end of the chain and the other.
6. What is the primary driving force that causes DNA to take a helical shape?
A. hydrogen bonds between base pairs
B. Aromatic base stacking
c. steric interactions
D.
Ionic charges on the phosphate backbone
chapter 10 Nucleic Acid Biophysics 263
7. What is the nearest-neighbor approximation?
A. Approximating the DNA sequence of one organism with the organisms that live
nearby.
B. Assuming that the dNA sequence of one gene can only affect the genes that are imme-
diately next to it.
C.
Ignoring interactions between nucleotides that are not immediately next to one another
in the dNA sequence.
D. Approximating the DNA sequence by looking at which nucleotides are next to one
another.
8. Which of the following statements is false?
S1. Superhelical DNA requires a force to hold the superhelix in place.
S2. Circular DNA is always superhelical.
S3. A superhelix is a quaternary structure.
S4. Energy stored in the superhelix can be used to unwind the DNA.
A.
S1 and S2
B.
S1 and S3
c. s1 and s4
D. S2 and S3
E.
S2 and S4
9. What is a topological invariant?
A. A property that does not change under smooth deformations of shape
B. The writhe
C. The linking number
d. A and B
e. B and c
F. C and A
10. A 5000-base-pair circular DNA has a linking number of 500, and its most stable
amount of twist is 10 base pairs per turn. What is the writhe?
A. 10
B. –10
c. 0
d. –0.1
This page intentionally left blank

Quar
k
w
B
E
De Broglie’s photon
sin
sin
S
D
ec
ec
2
B
B
Electr yclosity
E
lec
ty closit
y
Relativist
Ong
in
ca
a
a
e
a
e
ev
m
m
k
e
265
In Chaps. 7 and 8 we learned some basics of lipids and cell membranes. In this
chapter we explore membrane biophysics. Biological membranes do more than
just providing a barrier between the cell and the outside world, and they are
made up of more than just lipids.
CHAPTer OBJeCTiVeS
In this chapter, you will
review the basic structure of biological membranes.
•
Learn the principles that govern self-assembly in lipid bilayers.
•
Learn about the fluid mosaic model.
•
study phase transitions in lipids and the forces that affect them.
•
Gain an understanding of the energetics of membrane permeability.
•
Learn about active and passive transport across membranes.
•
chapter
11
Membrane Biophysics
266 Biophysics DemystifieD
Membrane Functions
Membranes play important biological functions. They provide a barrier between
the cell and the outside world, and regulate what can enter the cell and what
leaves it. Many organelles within the cell are themselves membrane bound,
providing an isolated biophysical environment even within the cell. In eukary-
otes a membrane surrounds the nucleus, keeping the activities of DNA (tran-
scription and replication) separate from the rest of the cell. Membranes also
provide surfaces on which biochemical reactions take place. One such example
is the endoplasmic reticulum, a network of membranes and tubules on which
synthesis of proteins and lipids occurs. Membranes also provide anchorage
points for the cytoskeleton which gives shape and rigidity to cells and plays a
role in intracellular transport of biomolecules.
Membrane Structure
The main structural components of biological membranes are amphipathic lip-
ids (see Chap. 7). Although biological membranes also contain carbohydrates
and proteins (some membranes contain as much as 50% protein), the primary
character of biological membranes is derived from its amphipathic lipids. The
most common lipids found in biological membranes are two-chain phospholip-
ids. These are molecules with a phosphate head group, attached to a two-chain
fatty acid. The amphipathic character comes from the combination of the
hydrophobic, hydrocarbon tails, along with the hydrophilic phosphate head
group. In biological membranes, the phospholipids arrange themselves into a
bilayer, in which the hydrocarbon tails face each other and are isolated from the
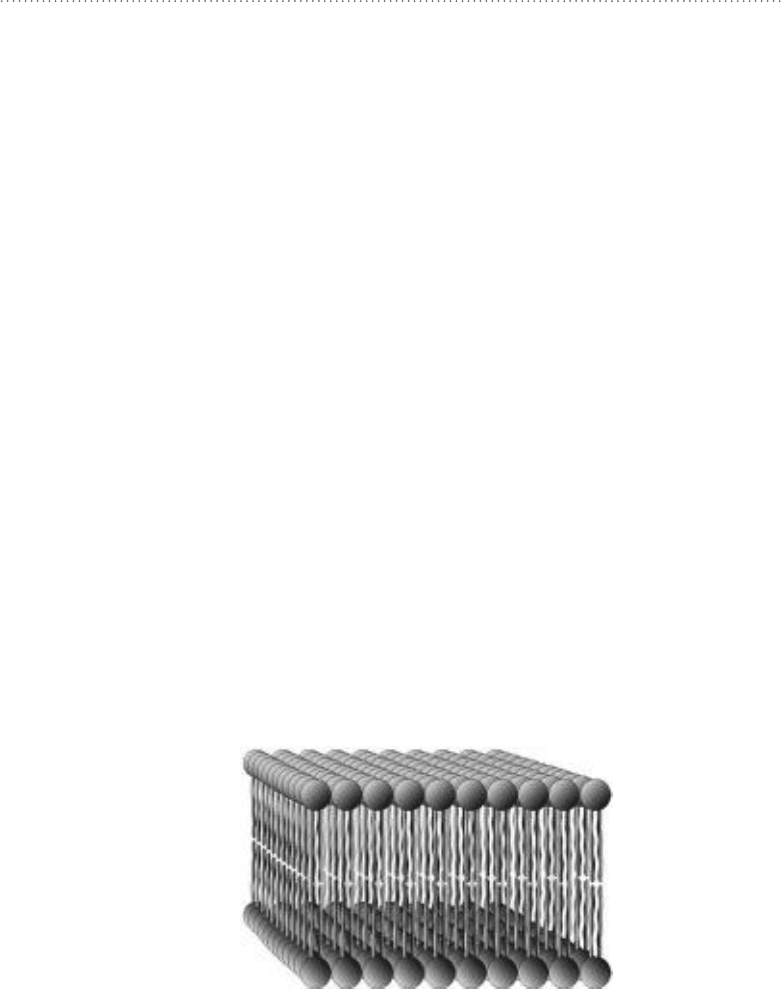
surrounding environment by the phosphate head groups. See Fig. 11-1.
Figure 11-1 • The basic structure of a biological membrane is a phospholipid bilayer with
hydrophobic tails in the center of the bilayer and hydrophilic heads at the surfaces. Biological
membranes also typically contain proteins, nonpolymeric lipids such as cholesterol, and
carbohydrates typically in the form of glycolipids (a carbohydrate attached to a lipid).
chapter 11 MeMBrane Biophysics 267
Phospholipid Behavior and Self-Assembly
Phospholipids exhibit a physical behavior that makes them ideal for the forma-
tion of membranes. Biophysicists call this behavior self-assembly. Self-assembly
means that the molecules will aggregate together to form various structures
without need for energy input, catalysts, or other helper molecules. Let’s first
look at self-assembly for single-chain phospholipids and then for double-chain
phospholipids.
Single-Chain Phospholipids and Micelle Formation
If we slowly add a single-chain phospholipid to an aqueous solution, at first the
lipid molecules are dispersed among the water molecules. Later, as the concentra-
tion of lipids is increased, a point is reached where the lipid molecules merge
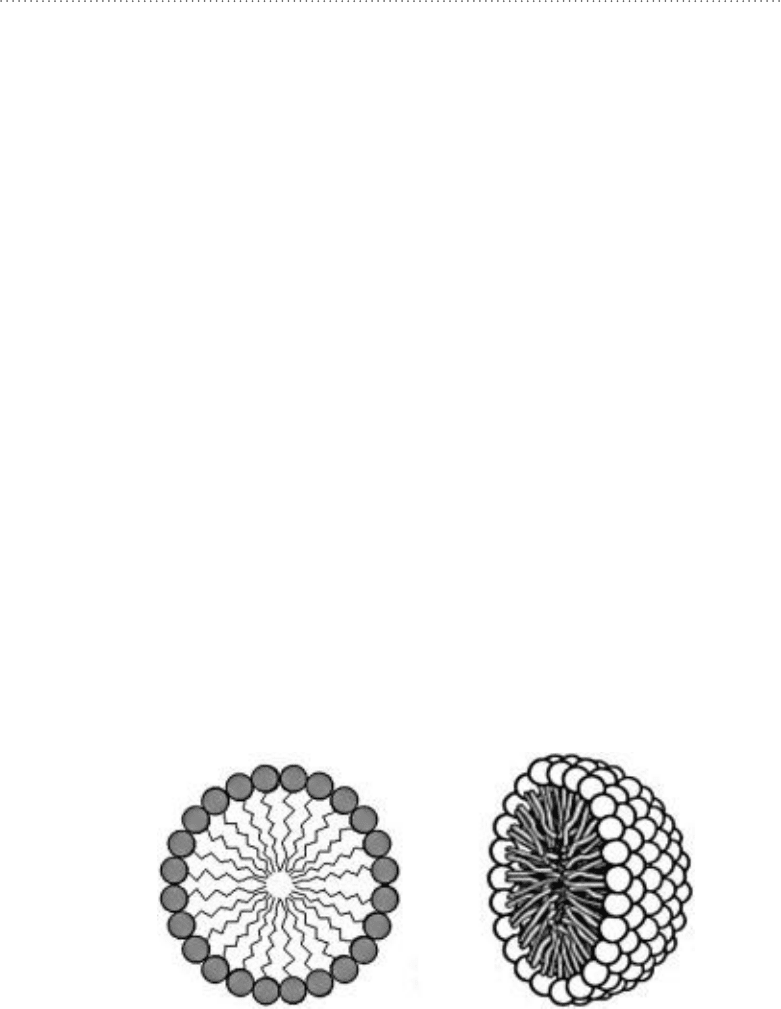
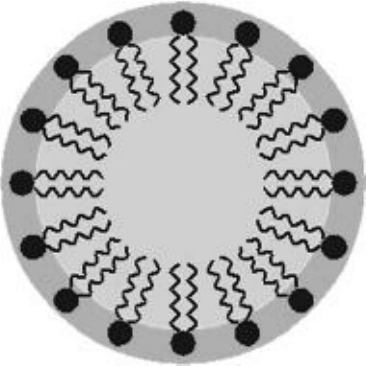
together forming aggregates called micelles. A micelle is simply a lipid ball with the
hydrocarbon chains pointed in toward the center, as illustrated in Fig. 11-2. The
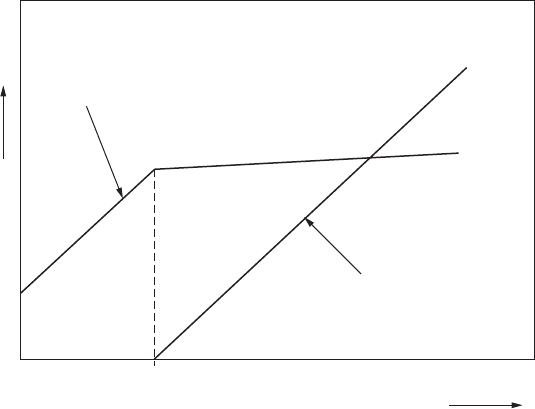
concentration, at which the lipids self-assemble into micelles, is called the critical
micelle concentration (CMC). The aggregation process is highly cooperative. Once
the critical micelle concentration is reached, any further lipid added to the solution
either associates with existing micelles or forms new micelles. This causes the con-
centration of micelles to increase, while the concentration of free lipid molecules
remains relatively constant, as shown in Fig. 11-3.
There are a number of forces that contribute to self-assembly in micelle
formation. As the hydrocarbon tails approach one another, dispersion forces
(see Chap. 6) provide a strong attractive force pulling them together. Close
association of the tails is also favored by the hydrophobic effect. As the tails are
Figure 11-2 • Single-chain phospholipids self-assemble into micelle
structures.
268 Biophysics DemystifieD
drawn together, water molecules along the length of the hydrophobic tails are
disrupted (disorganized) and excluded (pushed away). Recall from Chap. 6 that
water along the edge of a hydrophobic molecule has its motion restricted; this
in turn limits its ability to hydrogen-bond with other water molecules. As the
hydrocarbon tails come together, the water along their surface is pushed aside.
This increases the entropy of the water by allowing it to rotate freely. The free
rotation of the water molecules also increases the possibilities for hydrogen
bond formation with other water molecules in solution. The increased entropy
and the formation of water-water hydrogen bonds both contribute favorably to
the Gibbs energy of micelle formation.
In addition to these forces there is a mixed effect from the negatively charged
phosphate head groups. On the one hand, the charge on the head groups creates
a repulsive force between them. This is, in part, what gives the micelle its spheri-
cal shape. As the hydrocarbon tails come together by virtue of the dispersion
forces and the hydrophobic effect, we need to somehow reduce the repulsive
force of the head groups. To do this, nature puts the head groups on the outside
of the micelle and shapes the micelle like a sphere. This configuration maximizes
the distance between the head groups; this reduces their repulsive interaction. It
CMC
Concentration of
micelles
Concentration of
free phospholipid
(not micelles)
Concentration
Amount of single-chain phospholipid added to solution
Figure 11-3 • As single-chain phospholipid is added to an aqueous
solution, the concentration of free phospholipid increases, until the critical
micelle concentration is reached.
above the cMc, micelle formation is highly
cooperative, and additional lipid added to the solution increases the micelle
concentration.
chapter 11 MeMBrane Biophysics 269
also allows space between the head groups for water molecules and counterions,
both of which act to reduce the repulsive force. The water molecules do so by
aligning their dipole moment opposite the electric field (as was explained in
Chap. 6). If cations such as calcium, magnesium, or sodium are present, they
associate as counterions with the negative charge on the phosphate. This effec-
tively neutralizes the negative charge. Neutralizing the negative charges elimi-
nates the need for energy (enthalpy) to overcome the repulsive force, so the
Gibbs energy of micelle formation becomes even more negative, making it easier
to form micelles. The critical micelle concentration is the concentration of lipid
necessary for all of these forces combined to produce a negative Gibbs energy of
micelle formation. At that point formation of micelles is highly cooperative, pro-
ceeding in an almost all-or-none fashion. The fact that counterions act to elimi-
nate the repulsive force between head groups means that fewer lipid molecules
are required to provide the energy (from dispersion forces and the hydrophobic
effect) needed to achieve micelle formation. Fewer lipid molecules required
means the critical micelle concentration is lower. This explains why, as the con-
centration of positive ions increases, the CMC decreases.
Two-Chain Phospholipids and Liposome Formation
Although micelles can be formed from two-chain phospholipids, this is usually
not the preferred configuration. Compare Fig. 11-4 with Fig. 11-2. In the case
of two-chain phospholipids, the hydrocarbon chains from adjacent lipid mol-
ecules are not able to pack as closely together as they can when all of the lipids
have only a single hydrocarbon chain. The single-chain phospholipids get closer
together both in terms of aligning side by side, as well as in terms of reaching
deep into the center of the micelle. The extra width of the two-chain phospho-
lipids causes steric interactions that increase the distance between hydrocarbon
chains of adjacent molecules. This reduces the strength of dispersion forces. The
extra width also blocks the hydrocarbon chains from reaching as deep into the
micelle. This creates a void in the center of the micelle and increases the size of
micelle, compared to a micelle made from the same number of single-chain
lipid molecules. Both the void and the increased size (per number of lipid mol-
ecules) increase the energy cost of micelle formation. The void does so either
because it will contain water molecules, thus putting them right next to the
ends of the hydrophobic tails, or because somehow the water must be excluded
from the center of the micelle without filling that space with something else
(e.g., hydrocarbon, as with the single-chain lipids). Both of these are energeti-
cally unfavorable. The increased size of the micelle decreases its curvature.
270 Biophysics DemystifieD
Given the same number of lipid molecules, a decreased curvature puts the
repulsive phosphate head groups closer together. This is also unfavorable. So
altogether it’s a lot more expensive to make two-chain lipid micelles than it is
to make single-chain lipid micelles.
Nature solves this problem by bringing a second layer of lipids opposite the
ends of the hydrocarbon chains. It’s as if the sides of the micelle split open,
allowing the ends of the phospholipid chains across from each other to collapse
into the void of the micelle and line up to form a lipid bilayer. This process is
also highly cooperative, and it continues aggregating phospholipids in this way
until a liposome has formed. A liposome is a lipid bilayer sphere with a void in
the middle, as illustrated in Fig. 11-5. In this case, however, the void in the
middle is favorable. It can be filled with water molecules and counterions which
stabilize the liposome (contribute to a favorable Gibbs energy change) by inter-
acting with the phosphate head groups. The interaction between water,
Figure 11-4 • A micelle made of two-chain
phospholipids. in the micelle configuration, the
lipid molecules are not able to pack as closely
together as is the case with single-chain
phospholipids. The lipid chains are also not able to
reach as deep into the center of the micelle. This
reduces the amount of contact between the
hydrocarbon chains; this reduces the favorable
energy from dispersion forces. These factors make
micelle formation more difficult to achieve with
two-chain phospholipids.
instead two-chain
phospholipids prefer a bilayer configuration.
(Courtesy of Wikimedia Commons.)
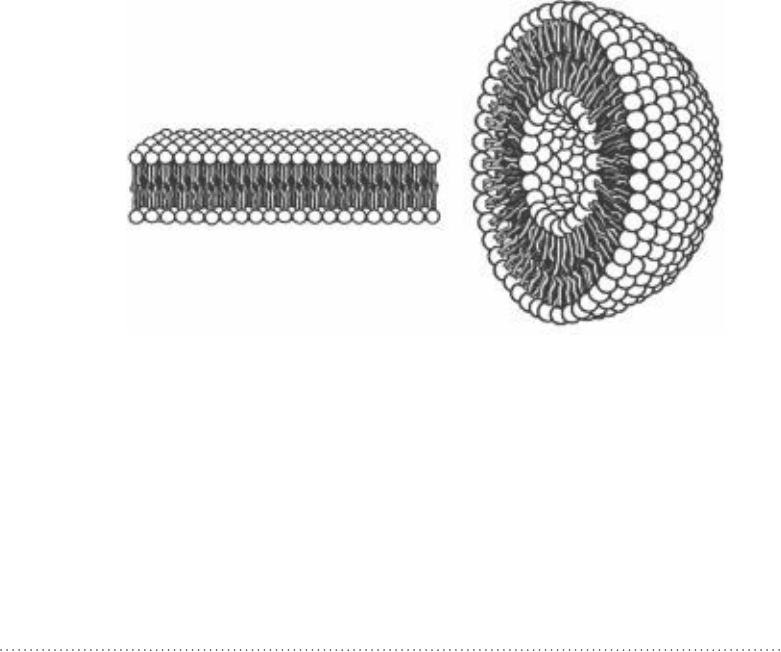
chapter 11 MeMBrane Biophysics 271
counterions, and the negatively charged phosphates is the same as was described
for micelles earlier. But in the case of a liposome, there are two surfaces of
phosphate head groups on which this favorable interaction can take place.
Lipid Bilayer energetics and Permeability
The lipid bilayer is semipermeable meaning that some molecules can pass
through the bilayer while others can’t. The ease with which any molecule can
pass through the lipid bilayer depends on the Gibbs energy change of getting
the molecule past the charged phosphate head groups and into the hydropho-
bic interior of the bilayer. After that we have the Gibbs energy change for
removing the molecule from the hydrophobic interior and getting past the
charged phosphate head groups on the other side of the bilayer. Both of these
Gibbs energy changes depend largely on the strength of the forces that hold
the bilayer together. They also depend on the interaction of these forces with
the particular molecule attempting to pass through the bilayer. We’ll look at the
factors that affect the strength of these forces in a minute, but first let’s take
some general examples of molecules and how they interact with the bilayer.
Charged and polar molecules experience a significant increase in Gibbs
energy just to penetrate the hydrophobic interior of the bilayer. As a result, they
are typically not able to pass through the bilayer. (If a charged or polar molecule
Lipid bilayerLiposome
Figure 11-5 • Liposome formation. Two-chain phospholipids prefer liposomes to
micelles because the energy cost is less.
