Goldfarb D. Biophysics DeMYSTiFied
Подождите немного. Документ загружается.

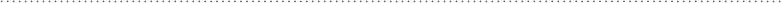
132 Biophysics DemystifieD
quickly becomes so large that the molecules do not have enough energy to move
any closer together. The steric interaction (repulsion) keeps them apart.
This illustrates the general principle that attractions occur where the poten-
tial energy is minimal or decreasing, and repulsions occur when the potential
energy increases. This is an important concept to remember. The potential
energy function depicted in Fig. 6-13 is called the Lennard-Jones potential,
named for John Lennard-Jones who first proposed it in 1924 as a mathematical
model to describe two neutral atoms approaching each other.
Hydrophobic Interactions
The highly polar character of water makes for interesting interactions between
water and nonpolar substances. The statement “oil and water don’t mix” comes to
mind. The interaction between water and a nonpolar substance like oil is called
the hydrophobic effect or hydrophobic interaction. The interaction does not involve
a single, physical force. Nonetheless hydrophobic interactions are sometimes also
called hydrophobic forces, because the hydrophobic effect forces molecules to
arrange themselves in a way that minimizes the contact between water and the
nonpolar substance. Thus, water and oil separate, forming two separate phases; this
minimizes the amount of contact between oil molecules and water molecules.
The driving force behind hydrophobic interactions is the fact that water “likes”
to form hydrogen bonds. Hydrogen bonding between water and itself or between
water and other polar molecules lowers the potential energy. It is thus energetically
favorable. When a nonpolar or hydrophobic molecule is placed into water, it takes
up space between the water molecules restricting their ability to hydrogen bond
with each other. There is a price to pay for this in terms of free energy.
First, there is the enthalpy needed to break some of the hydrogen bonds
between the water molecules. Second, although hydrogen bonding in water rep-
resents a highly ordered, low-entropy state for the molecules (since they must
line up with particular orientations to bond with each other), the presence of a
nonpolar (hydrophobic) molecule actually decreases the entropy of the water.
Remember order in this context is inversely related to the number of ways
of doing something. More order means fewer ways of doing something (less
entropy). Less order means more ways of doing the same thing (more entropy).
Water molecules by themselves are free to rotate and form hydrogen bonds in
many orientations. But, at the surface of a nonpolar or hydrophobic molecule,
where the water comes in contact with the hydrophobic molecule, steric inter-
actions restrict the water from rotating freely.
chapter 6 Forces AFFecting conFormAtion in BioLogicAL moLecULes 133
Figure 6-14 illustrates water molecules at the surface of a hydrophobic mol-
ecule. The water molecules orient themselves with the hydrogen atoms toward
the hydrophobic surface. This orientation allows the water to come as close as
possible to the hydrophobic molecule, while at the same time exposing only
the most neutral side of the water molecule toward the hydrophobic surface.
In this orientation, the surface water is restricted from rotating freely due to
steric interactions. This restricted rotation then limits the possible angles for
hydrogen bonds to form between the surface water molecules and other water
molecules in solution, thus decreasing the entropy of water that is closest to the
surface water. This close-to-the-surface water, however, is free to rotate and will
do so if it can favorably break the hydrogen bonds with the surface water and
make them with other water molecules in solution.
(As a side note, we can explain why this is the most neutral side of the water
molecule. First, the partially positive charge on this side of the molecule is split
between the two hydrogen atoms, and separated by the large angle between
them. This makes the charge effectively weaker in this orientation. Second, the
large angle between the hydrogen atoms exposes some of the electronegative
oxygen on that side of the molecule as well. This further neutralizes the amount
of charge that is exposed on that side of the water molecule.)
Recall Eq. (4-13), shown here as Eq. (6-14).
DG = DH – T DS (6-14)
Hydrophobic molecule
FIguRE 6-14•Watermoleculesatthesurfaceofa
hydrophobic molecule have their movement limited by
steric interactions; this in turn limits the possibilities for
hydrogen bonding with other water molecules.
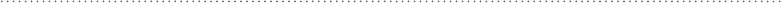
134 Biophysics DemystifieD
Both an increase in enthalpy (needed to break some of the water-water
hydrogen bonds) and a decrease in entropy (the restricted movement of water
molecules at the surface of the hydrophobic molecule) contribute to an increase
in the Gibbs energy. Thus mixing of water and hydrophobic substances is ener-
getically unfavorable. This energetic cost can be minimized by minimizing con-
tact between the water and the hydrophobic molecules. In the case of oil and
water mixed together, the oil droplets begin to coalesce into larger drops, even-
tually separating into two phases. The result is a decrease in the Gibbs energy
by minimizing contact between the oil and water. So the process of oil and
water separating is spontaneous, that is, energetically favored.
The hydrophobic molecules that concern us in biological systems are mostly
hydrocarbons, molecules made up of only hydrogen and carbon. Hydrocarbons
are found abundantly in living systems. Fats and oils are mostly hydrocarbons.
Many biomolecules are not purely hydrophobic, but have a portion of the
molecule that is hydrophobic and a portion of the molecule that is hydrophilic.
Hydrophilic means that the molecule interacts favorably with water (lowers the
Gibbs energy). Typically a hydrophilic molecule (or the hydrophilic part of a
molecule) is highly polar, and easily forms hydrogen bonds with water.
Molecules that are both hydrophobic and hydrophilic are called amphipathic
or amphiphilic. These mean the same thing. (You may occasionally see the
incorrect word amphiphatic which is a common mistake made by incorrectly
combining amphipathic and amphiphilic.) Phospholipids, which make up the
membranes of cells, are amphipathic. Phospholipids have a highly polar (nega-
tively charged) phosphate “head” that is covalently bonded to a long, hydropho-
bic hydrocarbon “tail.” See Fig. 6-15. Many protein molecules also have parts
that are hydrophobic and other parts that are hydrophilic.
Hydrophilic Interactions
Hydrophilic structures are those that easily hydrogen bond with water. This cre-
ates an attractive force between the hydrophilic structure and water. As men-
tioned, protein molecules often contain hydrophilic structures. The backbone of
DNA and the head groups of phospholipids, because they contain ionized charges,
also are also highly hydrophilic. (In general any ion or ionized portion of a mol-
ecule is hydrophilic since ions easily form hydrogen bonds with water.)
There are situations, however, in biological systems where two hydrophilic
surfaces have to come together, for example, during membrane fusion, in which
the membranes of two cells touch and then fuse together, connecting as a single
chapter 6 Forces AFFecting conFormAtion in BioLogicAL moLecULes 135
membrane. The membrane surface is hydrophilic. Fusing of two membranes
must therefore begin with bringing two hydrophilic surfaces together. When a
protein folds into a globular shape, sometimes different hydrophilic parts of
the protein must also come together. Or when a hydrophilic protein binds to
the hydrophilic DNA backbone, again, two hydrophilic surfaces must
come together. The problem in all of these situations is that water molecules
are tightly bound to the hydrophilic surfaces. This blocks the surfaces from
coming closer than about 25 angstroms (Å) unless the water is somehow
removed and the hydrogen bonds broken. This can be done, but there is an
energetic cost. The presence of the water effectively acts as a close-range repul-
sive force keeping the two hydrophilic surfaces apart. We call this the hydro-
philic force even though it is not a single force but the combined effect of
multiple hydrogen bonds, steric interactions, and entropy considerations.
Overcoming the hydrophilic force to bring two polar surfaces together can
be done through dispersion forces (as is the case with membranes), through
displacement of the water with ions, or through stronger ionic and hydrogen
bonding between the two hydrophilic molecules (e.g., when a protein binds to
DNA). The release of water from a hydrophilic surface increases the entropy
of the water which also contributes to overcoming the hydrophilic force.
Neutral hydrophobic tails
Negatively charged
phosphate head group
FIguRE 6-15•Phospholipidsareamphipathic;they
have a hydrophilic (highly polar) head phosphate
group and a hydrophobic (neutral) tail.

136 Biophysics DemystifieD
Quiz
Refer to the text in this chapter if necessary. Answers are in the back of the
book.
1. Which of the following statements about covalent bonds are true?
S1. Covalent bonds result from sharing of electrons between atoms.
S2. Covalent bonds are polar if electrons are shared unevenly.
S3. Single covalent bonds allow free rotation of the atoms or groups of atoms
on each side of the bond.
S4. Double covalent bonds allow free rotation of the atoms or groups of atoms
on each side of the bond.
S5. Covalent bonds may be single, double, or triple, depending on the number
of electrons that are shared.
S6. Covalent bonds are stronger than hydrogen bonds.
A. s1, s2, s3, s4 and s5
B. s1, s3, s5 and s6
c. s1, s2, s3, s5 and s6
D. s1, s2, s4, s5 and s6
2. The internal energy that results from a charge-charge interaction is proportional
to what?
A. the distance between the charges.
B. the square of the distance between the charges.
c. the reciprocal of the distance between the charges.
D.
the square of the reciprocal of the distance between the charges.
3. The internal energy that results from a charge-dipole interaction
A. Depends on the angle between the dipole axis and a straight line between the dipole
and the charge.
B. Decreases faster than a charge-charge interaction, as the distance between the charge
and the dipole increases.
c. increases faster than a charge-charge interaction, as the distance between the charge
and the dipole increases.
D.
A and c.
e. A and B.
4. If a dipole is allowed to rotate freely in solution, how does this affect the internal
energy of an interaction between the dipole and a charge?
A. the internal energy becomes stronger, due to thermal averaging.
B. the internal energy drops off must faster with distance, due to thermal averaging.
c. increased temperature decreases the effect of thermal averaging.
D.
A and B.
e. B and c.
chapter 6 Forces AFFecting conFormAtion in BioLogicAL moLecULes 137
5. What does the term cation-pi interactions refer to?
A. cations interact with aromatic ring electrons, causing them to fluctuate in a pattern that
resembles slices of pie within the aromatic ring.
B. the internal energy due to interactions between cations and aromatic ring electrons can
only occur in multiples of pi, or 3.14 J.
c. the layer of positive charge along the edges of an aromatic ring behaves like a ring-
shaped cation.
D. Delocalized aromatic ring electrons form a layer of negative charge that can interact with
nearby cations.
6. Forces that arise from synchronized fluctuating charge distributions are called
what?
A. stacking interactions
B. synchronization forces
c. charge-flux interactions
D. Dispersion forces
7. What are stacking interactions?
A. A specific type of charge-flux interaction unique to aromatic ring structures.
B. repulsive forces that prevent molecules from stacking up too high before they fall
over.
c. A type of dispersion force that results in an attraction between stacked aromatic ring
structures.
D.
synchronized charged distributions in platelets.
8. The hydrophobic effect causes water and oil to separate. Why?
A. Direct contact between water and hydrophobic substances, such as oil, greatly decreases
the entropy of the water. When the oil and water separate, this minimizes their con-
tact, thus minimizing the gibbs energy cost of restricting the movement of the water
molecules.
B.
Direct contact between water and hydrophobic substances, such as oil, is encouraged by
the hydrophobic effect. When the oil and water separate, it is easier to see where they are
directly in contact with each other.
c. oil has a physical property called hydrophobia.
D. the gibbs energy of mixing oil and water decreases the entropy of the water more than
it does of the oil.
separating the oil and water allows nature to reduce the entropy of the
water without affecting the oil.
9. Which of the following statements are true?
S1. Hydrophilic molecules easily form hydrogen bonds with water.
S2. Hydrophobic bonds occur between hydrophobic molecules.
138 Biophysics DemystifieD
S3. The hydrophilic force accounts for the difficulty in bringing two hydrophilic
molecules so close together that water cannot fit in between them.
S4. The hydrophilic force accounts for the attraction between hydrophilic mol-
ecules and water.
A.
s1, s2, s3, and s4
B.
s1, s2, and s4
c. s2 and s3
D. s1 and s4
e. s1 and s3
10. Steric interactions describe what?
A. repulsive forces between atoms at very close range.
B. repulsive forces between steroids and similar aromatic compounds.
c. Attractive forces between proteins.
D.
sharing of electrons.

139
In this chapter we review the most commonly studied biomolecules: what they
are, what elements they are made from, the basics of their structure, and the
roles that they play. In the next chapter, we will review the environment in
which biomolecules are most commonly found: the cell. These two chapters
together will prepare us for a more detailed study of the physics of these
amazing molecules.
CHAPTer OBJeCTiVeS
In this chapter, you will
Review the most commonly studied biomolecules.
•
learn the basics about the composition and structure of carbohydrates, lipids,
•
proteins, and nucleic acids.
learn the various roles that biomolecules play.
•
Quar
k
w
B
E
De Broglie’s photon
sin
sin
S
D
ec
ec
2
B
B
Electr yclosity
E
lec
ty closit
y
Relativist
Ong
in
ca
a
a
e
a
e
ev
m
m
k
e
chapter
7
Biomolecules 101

140 BiophysiCs DemystifieD
Classes of Biomolecules
It makes sense that the most studied biomolecules are the ones that play the
most important roles in life. Following is a table of common classes of biomol-
ecules and some of the roles they play.
CHNOPS
CHNOPS is an acronym for the elements most commonly found in living cells
(pronounced chi-nops, with a short i as in “chip” and a short o as in “top”).
CHNOPS stands for Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and
TABLE 7-1 Common classes of biomolecules and their roles.
Class of Molecule Some Major Roles
Carbohydrates
Energy storage
Structural material
Recognition (receptors)
Components of nucleic acids and some coenzymes
Lipids
Membrane structure
Energy storage
Insulation and protection
Proteins
Catalysis (speeding up) of biochemical reactions
Proteins that catalyze biochemical reactions are
called enzymes
Control or regulation of biophysical processes
Protection–immunization
Transport of other molecules across membranes
and around the body
Structural protection for nucleic acids
Muscle contraction and intracellular movement
Nucleic acids
Storage of genetic information
Replication of genetic information (i.e., passing to
the next generation)
Expression of genetic information
Protein manufacture
Chapter 7 BiomoleCules 101 141
Sulfur. Living things do contain small amounts of other elements, for example
fluorine (Fl) in tooth enamel, iron (Fe) in blood, chlorine (Cl) to help with
digesting food, calcium (Ca) for bones and muscle and nerve function, and
sodium (Na) also involved in muscle and nerve function. Together CHNOPS
accounts for about 98% of the atoms in most living things. For organisms with
bones, calcium adds another 1.5% meaning that for those organisms, CHNOPS
plus calcium accounts for about 99.5% of all the atoms in the organism.
Biopolymers
As was briefly discussed in Chap. 2, many biomolecules are polymers, macro-
molecules that are formed by covalently chaining together a series of smaller
molecules. The individual smaller molecules that are chained together are called
residues. When the residues are all identical, the polymer is called a homopoly-
mer. When the residues are different, it is called a heteropolymer. Typically bio-
polymers are heteropolymers. However, although the residues are different, the
individual residues making up a given polymer always have something in com-
mon, something that makes them all similar to one another. Nearly all of the
molecules discussed in this chapter are biopolymers or form biopolymers. So in
this chapter we will get to see some specific examples of biopolymers.
Hydrocarbons and Hydrocarbon Chains
A hydrocarbon is a molecule made of only hydrogen and carbon. Many biomol-
ecules either are hydrocarbons or large portions of the molecule are hydrocar-
bons. A hydrocarbon chain is a hydrocarbon in which the carbon atoms are
covalently connected to one another in a line, like links in a chain. Each carbon
atom has anywhere from zero to three hydrogen atoms attached to it.
The covalent bonds between any two carbon atoms may be single, double, or
even triple bonds, indicating one, two, or three electron pairs shared between the
atoms. Figure 7-1 shows some example hydrocarbons. Two of the examples in
Fig. 7-1 are hydrocarbon chains. One is an aromatic hydrocarbon (see the previ-
ous chapter for the definition of aromatic molecules). Many biomolecules consist
of hydrocarbon chains with various other atoms attached to the hydrocarbon.
Carbon Covalent Bonds
While we are on the topic of hydrocarbons, this is a convenient time to talk
about the nature of chemical bonding with carbon atoms and some convenient
and important rules to remember.
