Ghasem D. Najafpour. Biochemical Engineering and Biotechnology
Подождите немного. Документ загружается.


394 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
and the hydrophobic groups of the adsorbent phase. The principal application of affinity
chromatography, which facilitates the selective adsorption of target proteins to biological
specific ligands (for example protein A and antibodies) immoblised on and within the adsor-
bent phase, makes it well suited for the final stages of purification of protein products.
20
However, recent developments
21
have suggested that the adoption of this high-resolution
chromatography system in the earlier stage of downstream processing can facilitate the
overall recovery performance (for example reduce working and processing time). The robust
and inexpensive synthetic ligands (for example triazine dyes) are well suited to such appli-
cations (see the following section).
17.4.2 Dye-Ligand Pseudo-Affinity Adsorption
Historically, chlorotriazine dyes such as Cibacron Blue 3GA, Procion Red H-E7B, Procion
Green H-4G and Yellow H-E3G were designed as cheap chemicals for use in the textile and
printing industries. The chlorotriazine dyes are known to show affinities for several classes
of proteins such as dehydrogenase, phosphatransferases and plasma proteins by virtue of an
approximate mimic of the structure of various cofactor nicotinamide adenine dinucleotide
(NAD) and flavin adenine dinucleotide (FAD).
22
As a result, the chlorotriazine dyes have been
widely exploited as ligands in affinity chromatography for the purification of protein prod-
ucts. In general, dye ligands that tend to show affinities for specific classes of protein (i.e.
dehydrogenases, phosphotransferases, plasma proteins, etc.) include reactive dyes belong-
ing to the chlorotriazine group.
23
The dyes provide interactions with enzymes that mimic
interactions provided by natural ligands or their chemical analogues and, because these
dyes have no biological relationship with the macromolecules, the terms ‘pseudo-ligand’ or
‘pseudo-affinity’ are commonly used to describe them or their interactions.
24
Dissociation
constants, K
d
, of dye ligands are commonly in the range 10
⫺6
to 10
⫺7
M , which lies interme-
diate between ion exchange, 10
⫺4
to 10
⫺6
M and truly biospecific ligands, 10
⫺6
to 10
⫺8
M.
25
The dyes are particularly promising pseudo-affinity ligands because they offer several advan-
tages over biospecific ligands including ease of coupling to support matrices, low cost, wide
availability and high stability operational and sanitisation operations.
24
17.5 GENERAL PROBLEMS ASSOCIATED WITH
CONVENTIONAL TECHNIQUES
From an economic point of view, the number of sequential operations necessary to achieve
the desired purity of a protein product contributes significantly to the overall cost of the
downstream process. This is due to the capital investment and amount of consumables needed
for each step as well as the individual time required for each operation. Additionally, the
overall yield of the purification is reduced with each additional process step as a result of
inherent handling losses of product and/or product activity. It has been estimated that the
overall cost of the downstream process is closely correlated with the number of purification
steps involved and that cost may account for up to 80% of the final process investment.
26
Ch017.qxd 10/27/2006 10:52 AM Page 394

ADVANCED DOWNSTREAM PROCESSING IN BIOTECHNOLOGY 395
Traditional techniques employed both for harvesting biomass and feedstock clarification
are centrifugation and filtration.
27
Centrifugation might need to be undertaken twice, while
an additional depth or microfiltration step is commonly included to ensure a particle-free
(99–99.9% in terms of cell clearance) solution which can be fractionated by traditional
packed-bed chromatography. Although filtration has been applied successfully in numerous
solid–liquid operations, performance is usually diminished as a result of membrane fouling
(for example by cells, cell debris, lipids and nucleic acids) during operation.
28
In addition,
combined centrifugation and filtration operations often result in long processing times.
Furthermore, it has been noted that the presence of large amounts of insoluble and highly vis-
cous materials (for example cells, cells debris and long chain genomic DNA) in the process
feedstock can further restrict the clarification performance. This problem is especially critical
in the case of a cell disruptate, which results in the generation of cell debris, colloidal materi-
als and the release of large amounts of intracellular products.
29
A rapid method of product
capture of the target protein is therefore preferred because the time taken to remove partic-
ulates can promote denaturation due to process conditions that are detrimental to structural
integrity, for example the action of proteases, carbohydrates or oxidising conditions. Thus, it
is obvious that the development of fast and cost-effective primary recovery steps form the basis
for a successful downstream process, especially in the production of intracellular proteins.
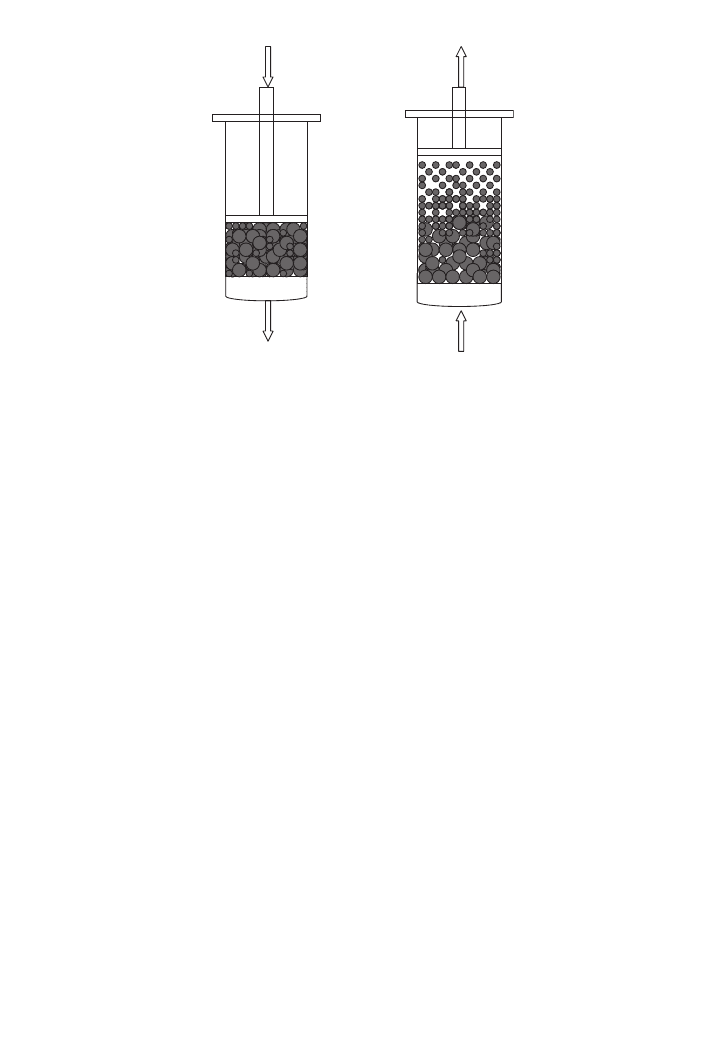
17.6 FLUIDISED BED ADSORPTION
Fluidised bed adsorption (FBA) has emerged as an efficient recovery method proven to
have significant advantages over conventional procedural sequences, for example discrete
feedstock clarification followed by fixed-bed adsorption of the product. In fluidised beds,
liquid is pumped upwards through a bed of adsorbent beads which, in contrast to a packed
bed, are not constrained by an upper flow adapter. Thus, the bed can expand and spaces
open up between the adsorbent beads. The increased voidage of the bed allows particulates
in the feed to pass freely through the spaces without entrapment (see Figure 17.3). Thus,
the need for prior removal of cells and/or debris is eliminated. After the adsorption stage,
the remaining feedstock and particulates are washed from the adsorbent bed and the product
is subsequently eluted either in fluidised or packed-bed mode. As a consequence, clarification,
concentration and initial fractionation are combined in one unit operation and thus fluidised
beds exhibit great potential for simplifying downstream processes with concomitant savings
in capital and operating costs.
Fluidised beds have been used previously for the industrial-scale recovery of the antibio-
tics streptomycin and novobiocin.
30
However, more recently, considerable interest has been
shown in the use of fluidised beds for the direct extraction of proteins from whole fermen-
tation broths.
31
In a packed bed, the adsorbent particles are packed within the contactor.
The voidage, that is, the inter-particle space, is minimal and thus feedstock clarification is
mandatory to avoid clogging of the bed. In a fluidised/expanded bed, the adsorbent bed is
allowed to expand by irrigation with feedstock. Bed voidage is increased, allowing the pas-
sage of particulates in the feed. The diameters of the adsorbent beads are exaggerated for
illustrative clarity.
Ch017.qxd 10/27/2006 10:52 AM Page 395
396 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
17.6.1 Mixing Behaviour in Fluidised/Expanded Beds
The conventional chemical engineering view of a fluidised bed is one in which there is a
significant degree of mixing, in both the solid and fluid phases, for example in gas-fluidised
systems.
32
In many applications, mixing of the solid phase is desirable, for example to obtain
high rates of heat transfer and a uniform temperature within the bed. Gas fluidised beds are
characterised by an ‘aggregative’ behaviour in which bubbles of gas pass through a bed of
particles which are just fluidised resulting in considerable mixing of the solid phase as well
as distinct bypassing of the gas phase. In general, mixing in liquid fluidised systems is not as
severe as in gas fluidised systems. Here, the density differences between the solid and the
fluid phase are comparatively small, and thus the bed shows a ‘particulate’behaviour in which
the bed retains a uniform character.
31
In a packed bed, the adsorbent beads are stationary and liquid flow through the bed approx-
imates plug flow. Thus, the number of theoretical equilibrium stages (referred to as plates) is
maximised, which results in good adsorption and chromatographic performance. As a con-
sequence of the absence of plug flow in the liquid phase, compounded by the mixing of the
adsorbent, a fluidised bed would be expected to show an inferior adsorption performance
compared with that of a packed bed. Thus, for protein recovery in liquid fluidised beds, it
is highly desirable to minimise the degree of mixing so as to mimic the adsorption charac-
teristics found in a packed bed contactor with respect to capacity and resolution.
Several strategies have been reported to limit the mixing of adsorbent particles within liq-
uid fluidised beds. One approach is to divide the bed into sections by the introduction of baf-
fles into the contactor. Other approaches seek to keep the adsorbent beads in a fixed position
or at least localise their movement to achieve a stable fluidised bed which subsequently
behaves like a packed bed, but with a greater voidage. For example, by using magnetically
susceptible adsorbent particles, a fluidised bed can be stabilised by subjecting it to a magnetic
Packed bed
Fluidised/
expanded bed
FIG. 17.3. Adsorbent particles in a packed and a fluidised bed.
Ch017.qxd 10/27/2006 10:52 AM Page 396

ADVANCED DOWNSTREAM PROCESSING IN BIOTECHNOLOGY 397
field. Such beds are claimed to exhibit little or no back-mixing and can be operated continu-
ously.
33
The practical benefit of this approach, i.e. restricted movement of adsorbent particles
and associated uncoupling of the bed expansion from fluidisation velocity has subsequently
been demonstrated by Zhang.
34
In this work, magnetically stabilised fluidised beds (MSFBs)
were exploited for: (i) the direct recovery of the intracellular enzyme glyceraldehydes-
3-phosphate dehydrogenase (G3PDH) from unclarified yeast disruptates; and (ii) for the recov-
ery of antibody fragments from Escherichia coli fermentation broths. However, this technique
requires complicated and relatively expensive equipment, particularly at large scale.
A simpler approach has been designed for the physical properties of the solid phases in
such a way that they generate an inherently stable fluidised bed. If the adsorbent beads have
an appropriate distribution of sizes and/or densities, grading or classification of the adsor-
bent occurs within the bed, with the larger/denser particles being located near the bottom of
the bed and the smaller/lighter particles nearer the top. The segregation behaviour restricts the
local mobility of the fluidised particles. Such a bed exhibits dispersion characteristics sim-
ilar to a packed bed.
36
Thus, the hydrodynamic properties of a fluidised bed are combined with
the chromatographic properties of a packed bed. The degree of classification is dependent
on the ratio of the size of the largest and smallest particle within the bed. This ratio has been
claimed to be at least 2.2.
37
To account for the difference in the dispersion characteristics of the classified, stable flu-
idised bed and the conventional, well-mixed fluidised bed, the term ‘expanded bed’ has been
used by several authors and the leading manufacture of chromatography media and equip-
ment.
38
In the work presented here, the term ‘fluidised bed’ will be used synonymously with
‘expanded bed’ to refer to adsorbents fluidised under conditions that seek to minimise particle
mixing.
17.7 DESIGN AND OPERATION OF LIQUID FLUIDISED BEDS
17.7.1 Hydrodynamic Characterisation of Flow in
Fluidised/Expanded Beds and Bed Voidage
The voidage () of a bed of particles is the fraction of the bed volume occupied by the inter-
stitial space between the particles. Its value depends upon the geometrical configuration of
the beads, the pattern in which they are arranged within bed, the size distribution of the par-
ticles and the ratio of mean particle and contactor diameter. The bed voidage can be calcu-
lated using the following equation:
(17.7.1.1)
where V
p
is the volume of the particles and V
b
is the volume of the bed. The voidage of a
packed bed is related to the sphericity of the particles, with a sphericity value of 1 for fully
spherical particles. The bed voidage values can typically range from 0.32 for a densely
packed adsorbent to 0.43 for a loosely packed adsorbent.
39
However, the voidage of a bed
containing a range of particle sizes and geometrical shapes cannot be predicted accurately.
Voidage ( )
P
b
⫽⫺1
V
V
Ch017.qxd 10/27/2006 10:52 AM Page 397

398 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Therefore, an assumed value for
o
of 0.40 for a perfectly packed adsorbent bed is com-
monly found in chromatographic studies.
40
This value has also been applied throughout this
study for all the materials used.
17.7.2 Minimum Fluidisation Velocity of Particles
The minimum fluidisation velocity of the particles is achieved when the adsorbent becomes
suspended in the liquid. This occurs when the drag forces exerted by the upward flow of
the liquid phase are equal to the weight of particles in the liquid. Therefore, at minimum
fluidising conditions, it can be described by the following expression:
drag by upward liquid flow ⫽ weight of the particles ⫺ buoyancy of the particles
This expression can be also presented as:
pressure drop across the bed ⫻ cross section area of the bed ⫽ volume of the bed ⫻
fraction of the particles ⫻ specific weight of the particles
or
(17.7.2.1)
where ⌬P is the pressure drop, A
c
is the cross-sectional of the column area, H
mf
and
mf
are
the bed height and bed voidage at the minimum fluidisation velocity (U
mf
), respectively, r
p
is the density of the particles, r is the density of liquid phase and g is acceleration due to
gravity. Rearranging (17.7.2.1) gives:
(17.7.2.2)
However, on the basis of the relation between pressure drop and the minimum fluidisation
velocity of particles, the point of transition between a packed bed and a fluidised bed has
been correlated by Ergun
41
using (17.7.2.3). This is obtained by summing the pressure drop
terms for laminar and turbulent flow regions.
(17.7.2.3)
where m is the viscosity of the liquid and d
p
is the diameter of the particle. The first term
of the Ergun equation is linear with respect of velocity and this will be dominant when the
flow in the voids is laminar. Hence, (17.7.2.3) can be simplified to:
(17.7.2.4)
⌬P
H
U
d
Re
dU
mf
mf
mf
3
mf
P
2
P
Pmf
when⫽⫻
⫺
⫻
⫻
⫽
⫻⫻
⬍150
1
20
2
()mr
m
⌬P
H
U
d
U
d
mf
mf
mf
3
mf
P
2
mf
mf
3
P
2
⫽⫻
⫺
⫻
⫻
⫹⫻
⫺
⫻
⫻
150
1
175
1
2
2
()
.
()m
r
⌬P
H
g
mf
mf P
⫽⫺ ⫻ ⫺⫻()()1 rr
⌬PA A H g⫻⫽⫻ ⫻ ⫻ ⫺⫻
ccmf mf P
()[()]1- rr
Ch017.qxd 10/27/2006 10:52 AM Page 398

ADVANCED DOWNSTREAM PROCESSING IN BIOTECHNOLOGY 399
The second term relates to turbulence. Therefore, (17.7.2.3) can be simplified to:
(17.7.2.5)
In the intermediate region both terms have to be used. Therefore, the superficial velocity at
minimum fluidising conditions can be found by combining (17.7.2.2) and (17.7.2.3) and mul-
tiplying both sides by rd
3
p
/m
2
(1 ⫺
mf
) to yield:
(17.7.2.6)
If
mf
is unknown, the following equation suggested by Wen and Yu
41
can be used to deter-
mine the minimum fluidisation velocity for the whole range of Reynolds numbers by
assuming:
(17.7.2.7)
Hence, solving explicitly for U
mf
(17.7.2.8)
Thus, at low particle Reynolds numbers (Re
p
), (17.7.2.8) can be simplified to:
(17.7.2.9)
Equation (17.7.2.9) was originally used to correlate the minimum fluidisation velocity for
gas–solid fluidisation beds but has been successfully employed by Lan and his co-workers
42
for adsorbents in the field of direct recovery using liquid–solid systems (Figure 17.4).
17.7.3 Terminal Settling Velocity of Particles
If a single particle is falling freely under gravity in an infinitely dilute suspension, it will
accelerate until it reaches a steady-state velocity. This final velocity is known as the terminal
settling velocity (U
t
) and represents the maximum useful superficial velocity achievable in
a fluidised bed. Thus, the contained particles will be elutriated from the column if the super-
ficial velocity is above U
t
, the value of which can be predicted using the Stokes equation
U
dg
mf
PP
⫽
⫻⫺⫻
⫻
2
1650
()rr
m
U
d
gd
mf
P
PP
2
⫽
⫻
⫻⫹⫻
⫻⫺⫻⫻
⫺
m
rrr
m
r
(.) .
()
.
/
33 7 0 0408 33 7
2
2
12
È
Î
Í
Í
˘
˚
˙
˙
()1
11
1
14
⫺
mf
mf
3
mf
3
and
150
1
175
2
()
.
()
⫺
⫻
⫻⫻
⫹
⫻
⫻
⫽
⫻⫺
mf
mf
3
Pmf
mf
3
mf P
P
dU Udr
m
r
m
rr r
Ê
Ë
Á
ˆ
¯
˜
⫻⫻⫻g
P
2
d
m
2
⌬P
H
U
d
Re
dU
mf
mf
mf
3
P
2
P
Pmf
when⫽⫻
⫺
⫻
⫻
⫽
⫻⫻
⬎175
1
1000
2
.
()
r
r
m
Ch017.qxd 10/27/2006 10:52 AM Page 399
400 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
(17.7.3.1). A wide range of particle terminal velocities for various Reynolds numbers have
been investigated by Kunii and Levenspiel.
43
They suggested that if the particles were
assumed to be spherical and operated at low particle Reynolds number (Re
p
⬍ 0.4), the
Stokes equation was found to be acceptable (see Figure 17.4). Therefore, the terminal
velocity U
t
can be expressed as:
(17.7.3.1)
where d
p
is the diameter of the particle, r
p
and r are the density of the particle and liquid
phase respectively, m is the velocity of liquid phase and g is acceleration due to gravity. The
Stokes equation has been reported in the literature as being successfully used to predict ter-
minal settling velocities of fluidised/expanded bed adsorbents.
44,45
Alternatively, the model of Shiller and Naumann is commonly used for the prediction of
terminal velocity of a spherical particle:
(17.7.3.2)
where
(17.7.3.3)
Ga
gd
⫽
⫻⫺⫻⫻rr r
m
()
PP
3
2
Ga Re Re Ga⫽⫹ ⬍⬍18 27 36 10
1 687
5
tt
..
.
U
dg
t
P
2
P
⫽
⫻⫺⫻
⫻
()rr
m18
Flow
U
mf
H
0
H
U
U
t
particle
entrainment
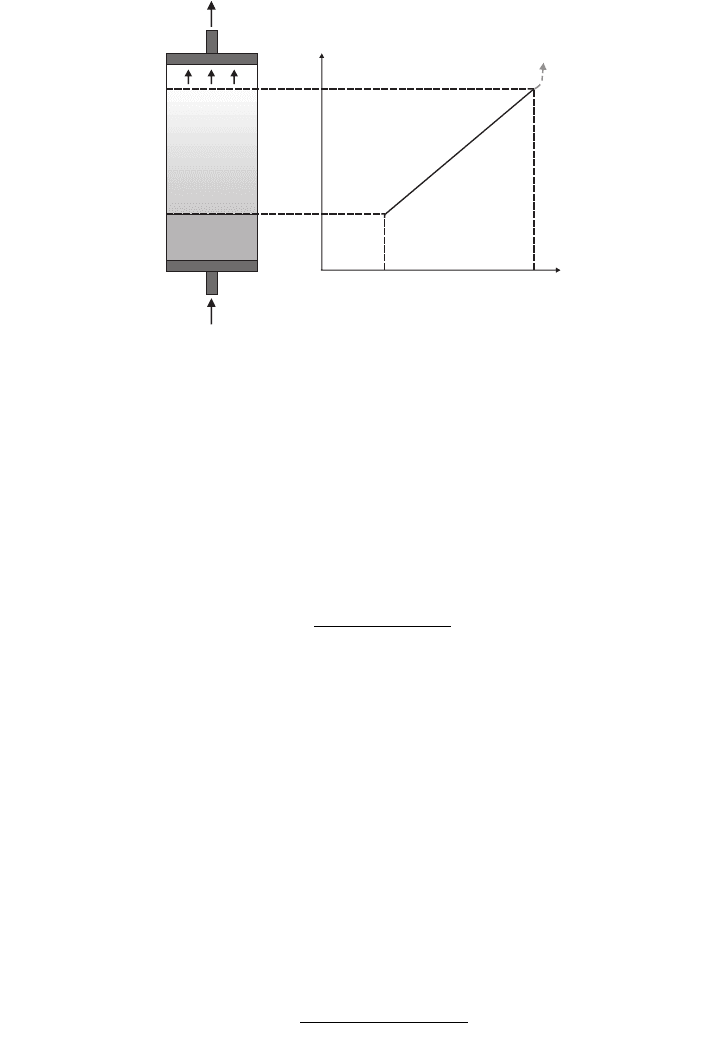
FIG. 17.4. Operational window of fluidisation velocities.
Ch017.qxd 10/27/2006 10:52 AM Page 400

ADVANCED DOWNSTREAM PROCESSING IN BIOTECHNOLOGY 401
and
(17.7.3.4)
where Ga is the Galileo number, Re
t
represents the terminal Reynolds number and d is the
particle diameter. The model of Shiller and Naumann has been successfully used to estimate
the particle terminal velocity by Thomas and Yates,
46
and Jahanshahi and his co-workers.
47
17.7.4 Degree of Bed Expansion
The degree of bed expansion contributes to the efficiency of fluidised bed/expanded bed
adsorption as a composite function of liquid distribution, liquid and particle properties (size,
shape and density) and process conditions. Besides being an important design feature, the
degree of bed expansion may be used as a quick and simple measure of bed stability.
48
The relation between superficial velocity (U) and bed voidage () in a fluidised bed can
be described by the classical correlation first postulated by Richardson and Zaki:
49
(17.7.4.1)
where is the fluidised voidage and n is the Richardson–Zaki coefficient. It allows the cal-
culation of the particle terminal velocity of any given suspension of uniformly dispersed
spheres together with the liquid velocity required to perform a given expansion of a flu-
idised bed by the logarithmic plot of linear velocity against fluidised voidage. Thus:
(17.7.4.2)
The void fraction () is estimated from the measured height of the expanded bed (H) and
is a function of superficial liquid velocity. Thus:
(17.7.4.3)
where M is the mass of particles. Also, the fluidised voidage can be determined as the total
volume of particles (for a given bed) is constant in both packed bed and fluidised bed con-
figurations. Therefore:
(17.7.4.4)
where H
o
and
o
are the packed bed height and voidage respectively. Hence, by rearranging
(17.7.4.4), the bed voidage at any fluidised bed height can be estimated using the equation:
(17.7.4.5)
⫽ ⫺
⫺ ⫻
1
1()
oo
H
H
AH AH
co o c
(1 )⫺ ⫽ ⫺()1
⫽ ⫺
⫻⫻
1
M
AHr
Pc
log logUUn⫽⫹⫻
t
log
UU
n
⫽
t
Re
dU
t
Pt
⫽
⫻⫻r
m
Ch017.qxd 10/27/2006 10:52 AM Page 401

402 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
The Richardson–Zaki coefficient (n) can be calculated from the following correlations:
(17.7.4.6)
(17.7.4.7)
(17.7.4.8)
(17.7.4.9)
(17.7.4.10)
where D is the column diameter and Re
t
is the particle Reynolds number based on the ter-
minal velocity. The popularity of the Richardson–Zaki correlation in this field of study
arises from its simplicity and good agreement with experimental data.
48,50
The operational window of a fluidised bed process is defined by the minimum fluidisa-
tion velocity, U
mf
, at which a settled bed of adsorbent beads starts to fluidise and the termi-
nal velocity (U
t
) at which the bed stabilises and adsorbent beads are entrained from the bed.
17.7.5 Matrices for Fluidised Bed Adsorption
Early work exploiting extensively cross-linked agarose adsorbents originally designed for
conventional, packed bed processes demonstrated the principal of operation and the potential
of fluidised bed adsorption for processing particulate feedstocks.
31
However, it was found
that these materials were not optimally suited because the combination of particle diame-
ter and density allowed only at low flow rates (for example 10–30 cm.h
⫺1
) which resulted
in low overall productivities. Denser particles such as silica were more appropriate in this
respect.
51
However, a drawback of silica-containing material is the limited stability at high
pH values, which makes it less suitable for biopharmaceutical production where alkaline
conditions are commonly used for cleaning-in-place and sanitisation-in-place procedures.
The development of denser adsorbents enabled the use of higher flow rates and improved
the stability of operation of expanded beds. Tailor-made adsorbents were produced using
hydrophilic natural polymers such as cellulose, agarose or synthetic trisacrylate-based mate-
rials. To enhance the particle density, heavy, inert filler materials have been incorporated
during assembly. The resulting composite materials included cellulose-titanium dioxide
and dextran-silica.
52
Other materials reported for the fabrication of denser adsorbents were
glass and zirconia. For example, Thömmes and his co-workers
53
exploited custom-derived
controlled pore glass particles for the purification of monoclonal antibodies. Zirconia-based
nRe⫽⬎2 4 500.
t
nRe Re⫽⬍⬍
⫺
4 4 200 500.
t
0.1
t
n
d
D
Re Re⫽⫹ ⬍⬍
⫺
44 18
01
.
.
Ê
Ë
Á
ˆ
¯
˜
tt
1 200
n
d
D
Re Re⫽⫹ ⬍⬍
⫺
44 18
003
.
.
Ê
Ë
Á
ˆ
¯
˜
tt
0.2 1
n
d
D
Re⫽⫹ ⬍465 20.
t
0.2
Ch017.qxd 10/27/2006 10:52 AM Page 402

materials exhibit a significantly higher density than silica. It has been demonstrated that
even small particles (for example less than 50 µm in diameter) may be fluidised at linear
flow rates similar to those used for silica or density-enhanced agarose particles having a
greater diameter.
54
In another approach, McCreath and colleagues developed perfluo-
ropolymer particles which were derived with dye ligands for the affinity purification of
dehydrogenases from disrupted baker’s yeast.
55
The increased density of the support (2.20
g⭈ml
⫺1
) also allowed the use of comparatively small particles (50–80 m) at an acceptable
linear flow rate of 120 cm⭈h
⫺1
.
Agarose-based materials have been commercialised specifically for fluidised bed
adsorption by increasing their specific weight with incorporated quartz or steel particles
(STREAMLINE
TM
).
37
The densities so achieved have been exploited at 1.15 g⭈ml
⫺1
for
agarose-quartz and 1.3 g⭈ml
⫺1
for agarose-steel composites. These materials are available with
a range of ligand functionalities such as anion exchange (DEAE, Q), cation exchange (SP),
chelating ligand (iminodiacetic acid for immobilised metal affinity chromatography, IMAC),
protein A (affinity purification of antibodies) and phenyl groups (hydrophobic interaction
chromatography, HIC). More recently, so-called pellicular adsorbents were defined as suit-
able for fluidised bed adsorption. These adsorbents are characterised by a dense core such
as glass or stainless steel
47,56
coated with a layer of porous material, such as agarose. Such
matrices promise high rates of adsorption/desorption owing to the absence of deep convec-
tive pores and the short diffusion distances within the thin porous layer that comprises the
pellicle.
17.7.6 Column Design for Fluidised Bed Adsorption
To achieve a stable fluidised bed, the column has to fulfil some simple but important demands.
A suitable liquid distribution is crucial to accomplish plug flow conditions within the bed
and thus minimise particle dispersion. A prerequisite for the generation of an even velocity
profile across the cross section of a column is an evenly distributed pressure drop across the
distributor at the column inlet. Pressure drop fluctuations lead to the development of chan-
nels which are the most important influence upon homogeneity in an adsorption process.
Flow distribution can be achieved by using sieve plates, meshes or a bed of glass ballotini.
43
Bascoul and his colleagues
57
have investigated bed stability as a function of the distributor
design, showing that channelling in the lower part of a fluidised bed due to uneven flow dis-
tribution is reduced with increasing column length. This has led to the conclusion that the
fluidised bed itself serves as an effective flow distribution system. More recently, a novel
distributor design was introduced which uses a stirrer in the bottom of the contactor to dis-
tribute the incoming feedstock. This configuration divides the fluidised bed in a limited,
well-mixed zone at the bottom and a stable fluidised bed above it. Such contactors were
included in the study presented here. Besides the demand for an even flow distribution, the
distributor has to enable the unhindered passage of particulates without becoming blocked
or damaging shear sensitive cells. Partial blockage of a distributor will cause channelling in
the fluidised bed. Cell breakage in the flow distributor can lead to the unwanted release of
intracellular compounds which may impair the purification process of an extracellular
product for whole broths.
ADVANCED DOWNSTREAM PROCESSING IN BIOTECHNOLOGY 403
Ch017.qxd 10/27/2006 10:52 AM Page 403
