Ghasem D. Najafpour. Biochemical Engineering and Biotechnology
Подождите немного. Документ загружается.


16.3 MEMBRANE PROCESSES
Industrial membrane processes may be classified according to the size range of materials
that they are to separate and the driving force used in separation. There is always a degree
of arbitrariness about such classifications, and the distinctions that are typically drawn.
Table 16.1 presents classification of membrane separation processes for liquid systems.
The four developed industrial membrane separation processes are microfiltration (MF),
ultrafiltration (UF), reverse osmosis (RO) and electrodialysis. These processes are well-
established large-scale industrial processes. The range of application of the three pressure
driven membrane water separation processes, reverse osmosis, ultrafiltration and microfil-
tration is illustrated in Figure 16.2. Microfiltration membranes filter colloidal particles and
bacteria from 0.1 to 10 m in diameter. Ultrafiltration membranes can be used to filter dis-
solved macromolecules, such as proteins, from solutions. The mechanism of separation by
reverse osmosis membranes is quite different. In reverse osmosis membranes, the membrane
pores are so small, from 3 to 5 angstroms (1 angstrom ⫽ 10
⫺10
m) in diameter that they are
within the range of thermal motion of the polymer chains that form the membrane.
4,10
The
accepted mechanism of transport through these membranes is called the solution-diffusion
model. According to this model, solutes permeate the membrane by dissolving in the mem-
brane material and diffusing down to concentration gradient. Separation occurs because of
the difference in solubilities and mobilities of different solutes in the membrane. The prin-
cipal application of reverse osmosis is the desalination of brackish groundwater or seawater.
Figure 16.2 show the reverse osmosis, ultrafiltration, microfiltration and conventional fil-
tration processes, which are related but differ principally in the average pore diameter of the
membrane filter. Reverse osmosis membranes are so dense that discrete pores do not exist;
transport occurs by statistically distributed free volume areas. The relative size of different
solutes removed by each class of membrane is illustrated in the schematic.
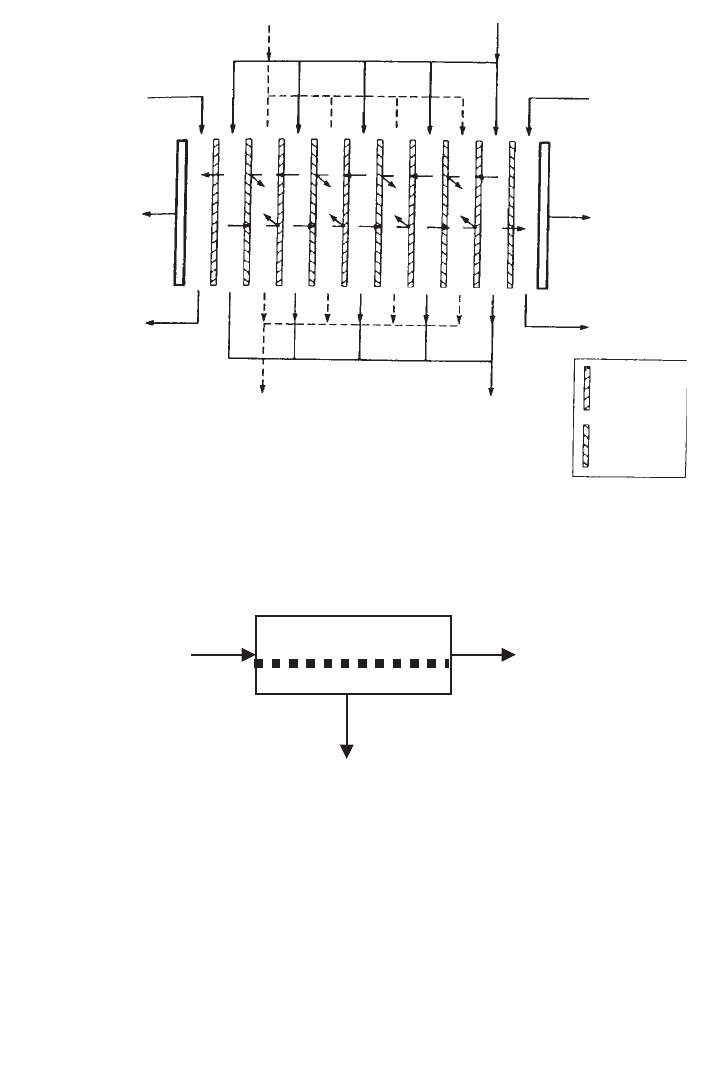
The fourth fully developed membrane process is electrodialysis, in which charged mem-
branes are used to separate ions from aqueous solutions under the driving force of an elec-
trical potential difference. The process utilises an electrodialysis stack, built on the filter press
principle and containing several hundred individual cells, each formed by a pair of anion and
354 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
TABLE 16.1. Classification of membrane separation processes for liquid systems
Process Driving force Separation Examples of materials
size range separated
Microfiltration Pressure gradient 0.1–10 m Small particles, large colloids,
microbial cells
Ultrafiltration Pressure gradient ⬍ 0.1 m–5 nm Emulsions, colloids,
macromolecules, proteins
Reverse osmosis Pressure gradient ⬍ 5 nm Dissolved salts, small organics
Electrodialysis Electric field ⬍ 5 nm Dissolved salts
gradient
Dialysis Concentration ⬍ 5 nm Treatment of renal failure
gradient
Ch016.qxd 10/27/2006 10:52 AM Page 354
cation exchange membranes. The principal application of electrodialysis is the de-salting of
brackish groundwater. However, industrial use of the process in the food industry, e.g., to
deionise cheese whey, is growing, as is its use in pollution control applications. A schematic
of the process is shown in Figure 16.3.
In gas separation with membranes, a gas mixture at an elevated pressure is passed across
the surface of a membrane that is selectively permeable to one component of the mixture.
The basic process is illustrated in Figure 16.4. Major current applications of gas separation
membranes include the separation of hydrogen from nitrogen, argon and methane in ammo-
nia plants; the production of nitrogen from air; and the separation of carbon dioxide from
methane in natural gas operations. Membrane gas separation is an area of considerable
research interest and the number of applications is expanding rapidly.
Pervaporation is a relatively new process that has elements in common with reverse osmo-
sis and gas separation. In pervaporation, a liquid mixture contacts one side of a membrane, and
the driving force for the process is low vapour pressure on the permeate side of the membrane
generated by cooling and condensing the permeate vapour. The attraction of pervaporation is
that the separation obtained is proportional to the rate of permeation of the components of the
liquid mixture through the selective membrane. Therefore, pervaporation offers the possibility
of separating closely boiling mixtures or azeotropes that are difficult to separate by distillation
MEMBRANE SEPARATION PROCESSES 355
FIG. 16.2. Reverse osmosis, ultrafiltration, microfiltration and conventional filtration with distinct pore size.
H
2
O
(2 Å)
1 Å
10 Å
100 Å
1000 Å
1 μm
10 μm
100 μm
Na
+
(3.7 Å)
Sucrose
(10 Å)
Haemoglobin
(70 Å)
Influonza
virus
(1000 Å)
Pseudomonas
diminuta
(0.28 μm)
Staphylococcu
s
becteria
(1 μm)
Starch
(10 μm)
Pore diameter
Reverse
osmosis
Ultrafiltration
Microfiltration
Conventional
filtration
Ch016.qxd 10/27/2006 10:52 AM Page 355
or other means. A schematic of a simple pervaporation process using a condenser to generate
the permeate vacuum is shown in Figure 16.5. Currently, the main industrial application of per-
vaporation is in the dehydration of organic solvents, in particular, the dehydration of 90–95%
ethanol solutions, a difficult separation problem because of the ethanol–water azeotrope at
95% ethanol. Pervaporation membranes can produce more than 99% ethanol from a 90%
ethanol feed solution. Pervaporation processes are also being developed for the removal of
dissolved organics from water and for the separation of organic mixtures.
356 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Self solution
SPick-up solution
Cathode
feed
Anode
feed
Anode
(+)
Anode
efficent
c
cation charge
membrane
A
Anion-charge
membrane
Cathode
efficent
Concentrated efficent
deconcentrated product
To positive pole
of rectifier
Cathode
(-)
To negative pole
of rectifier
C
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
AAAACCCC
FIG. 16.3. Schematic diagram of an electrodialysis.
Residue
Feed
Permeate
Membrane
module
FIG. 16.4. Schematic diagram of the basic membrane gas separation process.
Ch016.qxd 10/27/2006 10:52 AM Page 356
16.4 NATURE OF SYNTHETIC MEMBRANES
Membranes used for the pressure driven separation processes, microfiltration (MF), ultrafil-
tration (UF) and reverse osmosis (RO), as well as those used for dialysis, are most commonly
made of polymeric materials. Initially most such membranes were cellulosic in nature.
These are now being replaced by polyamide, polysulphone, polycarbonate and several
other advanced polymers. These synthetic polymers have improved chemical stability and
better resistance to microbial degradation. Membranes have most commonly been pro-
duced by a form of phase inversion known as immersion precipitation.
11
This process has
four main steps:
(1) the polymer is dissolved in a solvent to 10–30% by weight;
(2) the resulting solution is cast on a suitable support as a film of thickness of about 100m;
(3) the film is quenched by immersion in a non-solvent bath, typically water or an aqueous
solution;
(4) the resulting membrane is annealed by heating.
The third step gives a polymer-rich phase forming the membrane, and a polymer-depleted
phase forming the pores. The ultimate membrane structure results as a combination of phase
separation and mass transfer, variation of the production conditions giving membranes with
different separation characteristics. Most MF membranes have a systematic pore structure,
and they can have porosity as high as 80%.
11,12
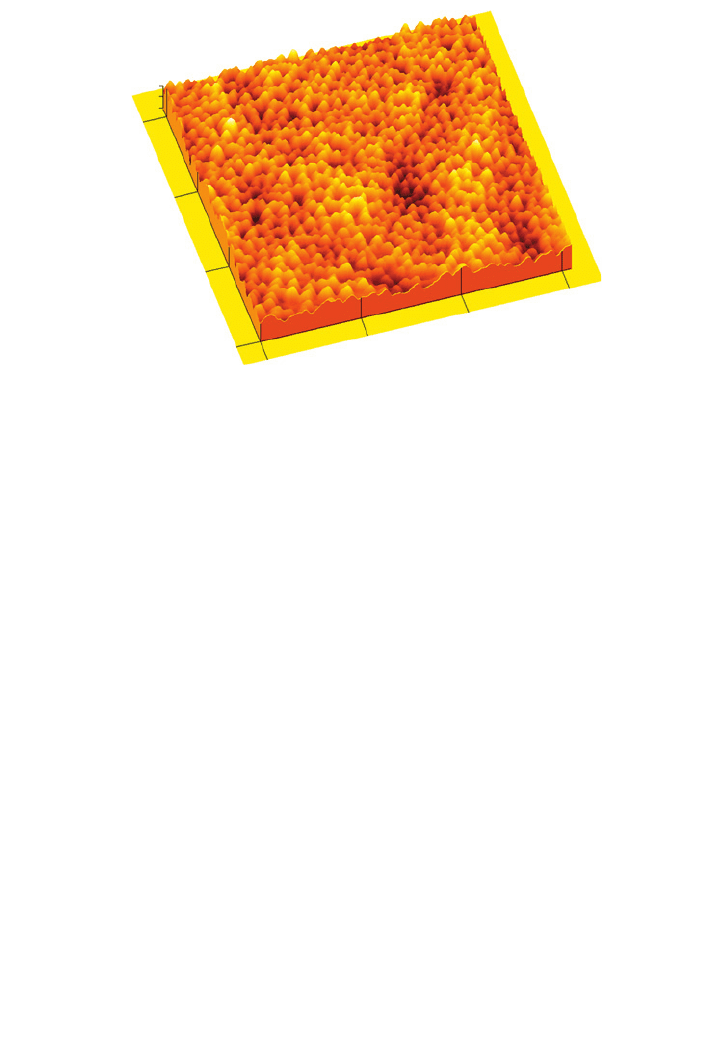
Figure 16.6 shows an atomic force microscope
MEMBRANE SEPARATION PROCESSES 357
Condenser
Feed liquid
Condensed
permeate
liquid
Purified
liquid
FIG. 16.5. Schematic diagram of the basic pervaporation process.
Ch016.qxd 10/27/2006 10:52 AM Page 357

image of polycarbonate MF membrane (0.2 m mean pore size). UF and RO membranes
have an asymmetric structure comprising a 1–2 m thick top layer of finest pore size sup-
ported by a approximately 100 m thick more openly porous matrix (Figure 16.7). Such an
asymmetric structure is essential if reasonable membrane permeation rates are to be obtained.
Another important type of polymeric membrane is the thin-film composite membrane. This
consists of an extremely thin layer, typically about 1m, of finest pore structure deposited
358 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Cyclopore membrane
0.20
0.10
0.00
3
3
2
2
1
1
0
0
μm
μm
μm
FIG
. 16.6. Atomic force microscope image of a polycarbonate microfiltration membrane (cyclopore), 0.2 m
pore size.
FIG. 16.7. Scanning electron micrograph of a section of an asymmetric polyamine ultrafiltration membrane showing
finely porous ‘skin’ layer on more openly porous supporting matrix.
Ch016.qxd 10/27/2006 10:52 AM Page 358
on a more openly porous matrix.
13,14
The main layer is formed by phase inversion or inter-
facial polymerisation on to an existing microporous structure.
Polymeric membranes are most commonly produced in the form of flat sheets, but they
are also widely produced as tubes of diameter 10–25 mm and in the form of hollow fibres
of diameter 0.1–2 mm.
A significant recent advance has been the development of MF and UF membranes com-
posed of inorganic oxides.
11,13
These are currently produced by two main techniques:
(1) deposition of colloidal metal oxide on to a supporting material such as carbon;
(2) as purely ceramic materials by high temperature sintering of spray-dried oxide micro-
spheres.
Other innovative production techniques lead to the formation of membranes with very reg-
ular pore structures, as shown in Figure 16.8. The main advantages of inorganic membranes
compared with the polymeric types are their higher temperature stability, allowing steam
sterilisation in biotechnological and food applications, increased resistance to fouling, and
a narrower pore size distribution.
14
The physical characterisation of membrane structure is important if the correct membrane
is to be selected for a given application. The pore structure of microfiltration membranes is
relatively easy to characterise, SEM and AFM being the most convenient method and allow-
ing three-dimensional structure of the membrane to be determined. Other techniques such as
the bubble point, mercury intrusion or permeability methods use measurements of the perme-
ability of membranes to fluids. Both the maximum pore size and the pore size distribution
may be determined.
13,15
A parameter often quoted in manufacturer’s literature is the nominal
MEMBRANE SEPARATION PROCESSES 359
Anopore membrane, 0.2 micron
1000
500
0
Å
3
3
2
2
1
1
0
0
μm
μm
FIG. 16.8. Atomic force microscope image of anodisc microfiltration membrane, 0.2 m pore size.
Ch016.qxd 10/27/2006 10:52 AM Page 359
molecular weight cut-off (MWCO) of a membrane.
13,14
This is based on studies of how
solute molecules are rejected by membranes. A solute will pass through a membrane if it is
sufficiently small to pass through a pore, if it does not significantly interact with the mem-
brane and if it does not interact with other (larger) solutes. It is possible to define a solute
rejection coefficient R by:
(16.4.1)
where C
f
is the concentration of solute in the feed stream and C
p
is the concentration of
solute in the permeate. For a given UF membrane with a distribution of pore sizes there is
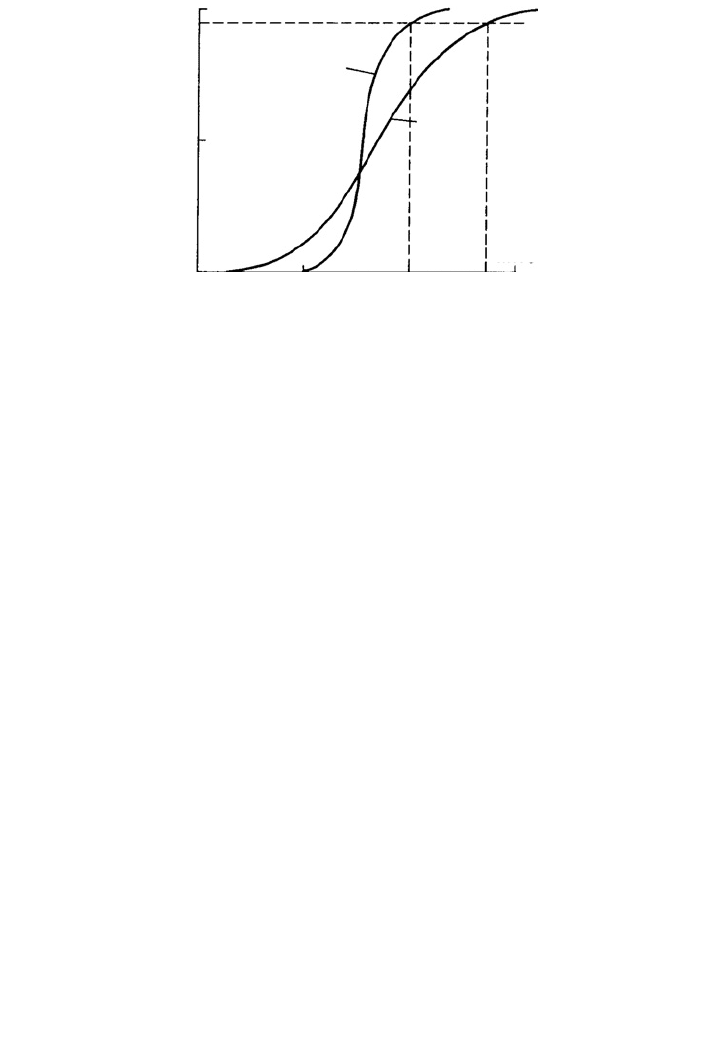
a relation between R and the solute molecular weight, as shown in Figure 16.9.
The nominal molecular weight cut-off is normally defined as the molecular weight of a
solute for which R ⫽ 0.95. Values of MWCO typically lie in the range 2000–100,000, with
values of the order of 10,000 being most common. Figure 16.10 shows an AFM scan of
30,000 MWCO membrane.
16.5 GENERAL MEMBRANE EQUATION
It is not possible at present to provide an equation, or set of equations, that allows the pre-
diction from first principles of the membrane permeation rate and solute rejection for a given
real separation. Research attempting such prediction for model systems is underway, but the
physical properties of real systems, both the membrane and the solute, are too complex for
such analysis. An analogous situation exists for conventional filtration processes. The general
RCC⫽⫺1( / )
pf
360 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
1.0
0.5
Rejection coefficient (R)
0
1000 10,000 100,000
MWCO
MWCO
Molecular weight
Diffuse
cutoff
Sharp cutoff
R = 0.95
FIG. 16.9. Dependence of rejection coefficient on molecular weight for ultrafiltration membranes.
Ch016.qxd 10/27/2006 10:52 AM Page 360

membrane equation is an attempt to state the factors that may be important in determining
the membrane permeation rate for pressure driven processes.
1,4
This takes the form:
(16.5.1)
where J is the membrane permeation rate (flux expressed as volumetric ate per unit area),
| ⌬P | is the pressure difference applied across the membrane (transmembrane pressure), ⌬P
is the difference in osmotic pressure across the membrane, R
m
is the resistance of the mem-
brane, R
c
is the resistance of layers deposited on the membrane (filter cake, gel solution)
and R⬘
f
is the ‘resistance’ of the film layer. If the membrane is only exposed to pure solvent,
say water, then (16.5.1) reduces to J ⫽ | ⌬P |/R
m
m. Knowledge of such water fluxes is
useful for characterising new membranes and for assessing the effectiveness of membrane
cleaning procedures. In the processing of solutes, (16.5.1) shows that the transmembrane
pressure must exceed osmotic pressure for flow to occur. It is generally assumed that
the osmotic pressure of most retained solutes is likely to be negligible in the formation
of a gel when the concentration of macromolecules at the membrane surface exceeds
their solubility giving rise to a precipitation, or due to materials in the process feed that
adsorb on the membrane surface producing an additional barrier to solvent flow. The
separation of a solute by a membrane gives rise to an increased concentration of that
solute at the membrane surface, an effect known as ‘concentration polarisation’. This
may be described in terms of an additional “resistance,” R⬘
f
. The limitation of (16.5.1) is
that the resistances are not readily calculable. However, it is within the framework of
this equation that the factors influencing membrane permeation rate will be discussed in the
following section.
J
P
RRR
⫽
⫺
⫹⫹
⬘
||| |
()
⌬⌬P
m
mcf
MEMBRANE SEPARATION PROCESSES 361
Å
Å
Nadir membrane of 30,000 MWCO
Å
10.00
5.00
0.00
40
20
0
0
10
20
30.00
40
FIG. 16.10. Atomic force microscope image of 30,000 MWCO ultrafiltration membrane.
Ch016.qxd 10/27/2006 10:52 AM Page 361

16.6 CROSS-FLOW MICROFILTRATION
The solid–liquid separation of slurries containing particles below 10 m is difficult by con-
ventional filtration techniques. A conventional approach would be to use a slurry thickener
in which the formation of a filter cake is restricted and the product is discharged continu-
ously as concentrated slurry. Such filters use filter cloths as the filtration medium and are
limited to concentrating particles above 5 m in size. Dead end membrane microfiltration,
in which the particle-containing fluid is pumped directly through a polymeric membrane,
is used for the industrial clarification and sterilisation of liquids. Such process allows
the removal of particles down to 0.1m or less, but is only suitable for feeds containing
very low concentrations of particles as otherwise the membrane becomes too rapidly
clogged.
2,4,8
The concept of cross-flow microfiltration is shown in Figure 16.11, which represents a
cross-section through a rectangular or tubular membrane module. The particle-containing
fluid to be filtered is pumped at a velocity in the range 1–8 m/s parallel to the face of the
membrane and with a pressure difference of 0.1–0.5MN/m
2
(MPa) across the membrane.
The liquid permeates through the membrane and the feed emerges in a more concentrated
form at the exit of the module.
16,17
All of the membrane processes are listed in Table 16.2.
Membrane processes are operated with such a cross-flow of the process feed.
The advantages of cross-flow filtration over conventional filtration are:
(1) A higher overall liquid removal rate is achieved by prevention of the formation of an
extensive filter cake.
(2) The process feed remains in the form of mobile slurry suitable for further processing.
(3) The solids content of the product slurry may be varied over a wide range.
(4) It may be possible to fractionate particles of different sizes.
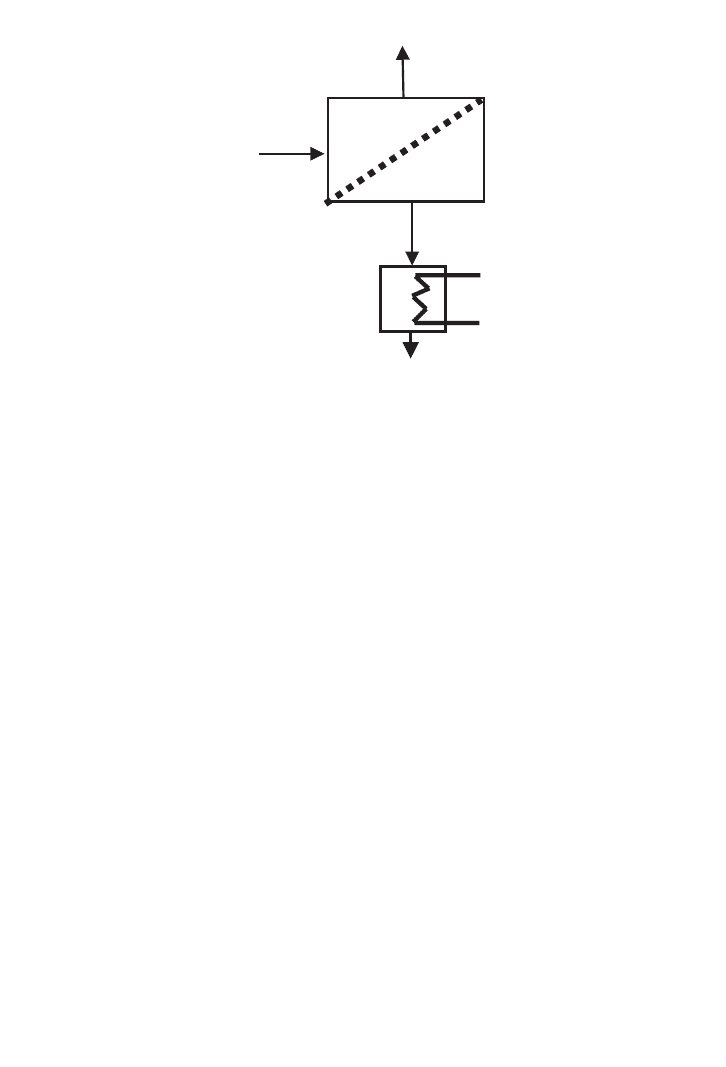
A flow diagram of a simple cross-flow system is shown in Figure 16.12. This is the system
likely to be used for batch processing or development rigs; it is in essence a basic pump
recirculation loop. The process feed is concentrated by pumping it from the tank and across
the membrane in the module at an appropriate velocity. The partly concentrated retentate is
recycled into the tank for further processing while the permeate is stored or discarded as
required. In cross-flow filtration applications, product washing is frequently necessary and
362 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Permeate
Membrane
Process feed
cross-flow
permeate
Retentate
FIG. 16.11. The concept of cross-flow filtration.
Ch016.qxd 10/27/2006 10:52 AM Page 362
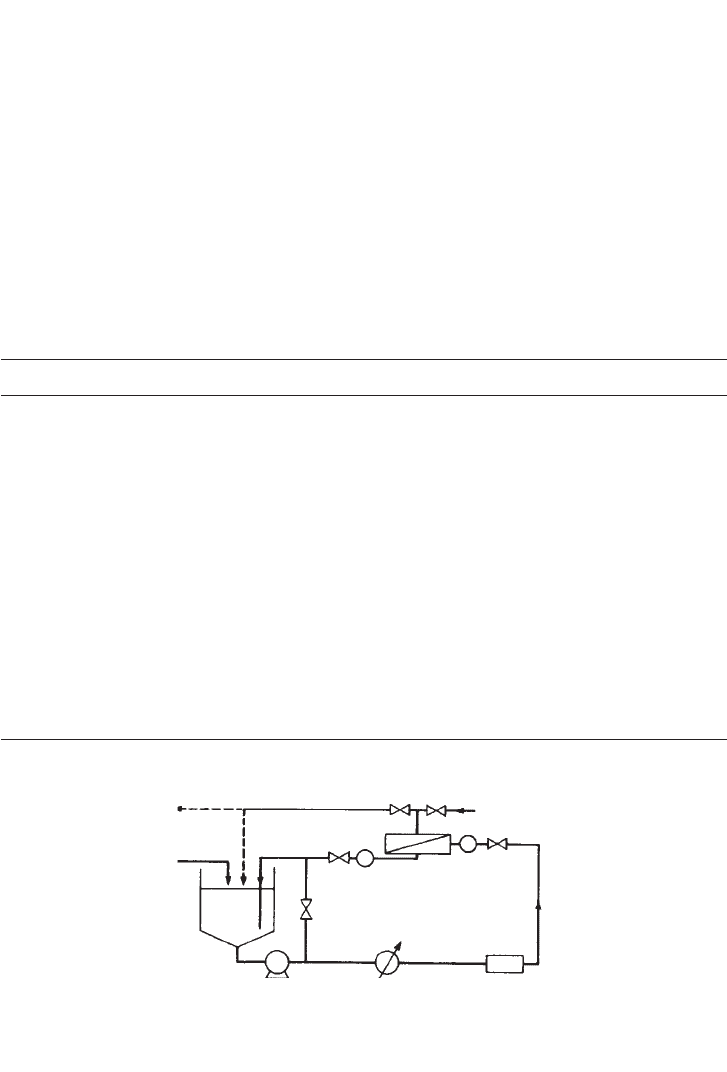
is achieved by a process known as diafiltration in which wash water is added to the tank at
a rate equal to the permeation rate.
In practice, the membrane permeation rate falls with time owing to membrane fouling;
that is, blocking of the membrane surface and pores by particulate materials (Figure 16.13).
The rate of fouling depends on the nature of the materials being processed, the nature
of the membrane, the cross-flow velocity and the applied pressure. For example, increasing
the cross-flow velocity results in a decreased rate of fouling. Backflushing the membrane
using permeate is often used to control fouling (Figure 16.13c). Further means of control-
ling membrane fouling are discussed later.
Ideally, cross-flow microfiltration would be the pressure-driven removal of the process
liquid through a porous medium without the deposition of particulate material. The flux
decrease occurring during cross-flow microfiltration shows that this is not the case. If the
MEMBRANE SEPARATION PROCESSES 363
TABLE 16.2. Module designs most commonly used in major separation processes
Application Module type
Reverse osmosis: seawater Both hollow-fibres and spiral-wound modules
Reverse osmosis: industrial Spiral-wound modules used almost exclusively; fine fibres too
and brackish water susceptible to scaling and fouling
Ultrafiltration Tubular, capillary and spiral-wound modules all used. Tubular
generally limited to highly fouling feeds (automotive paint),
spiral-wound to clean feeds (ultrapure water).
Gas separation Hollow-fibre for high-volume applications with low-flux, low-selectivity
membranes in which concentration polarisation is
easily controlled (nitrogen from air)
Spiral-wound when fluxes are higher, feed gases more contaminated,
and concentration polarisation a problem (natural gas separations,
vapour permeation).
Pervaporation Most pervaporation systems are small so plate-and-frame systems
were used in the first systems. Spiral-wound and capillary
modules are being introduced.
To drain or
store tank
Permeate
Concentrate
Cross-flow
microfiltration
module
Wash liquid
Compressed air
foor backflush
Flow meter
Heat
exchan
g
er
Variable speed
pump
P
P
FIG. 16.12. Flow diagram for a simple cross-flow system.
Ch016.qxd 10/27/2006 10:52 AM Page 363
