Ghasem D. Najafpour. Biochemical Engineering and Biotechnology
Подождите немного. Документ загружается.

344 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
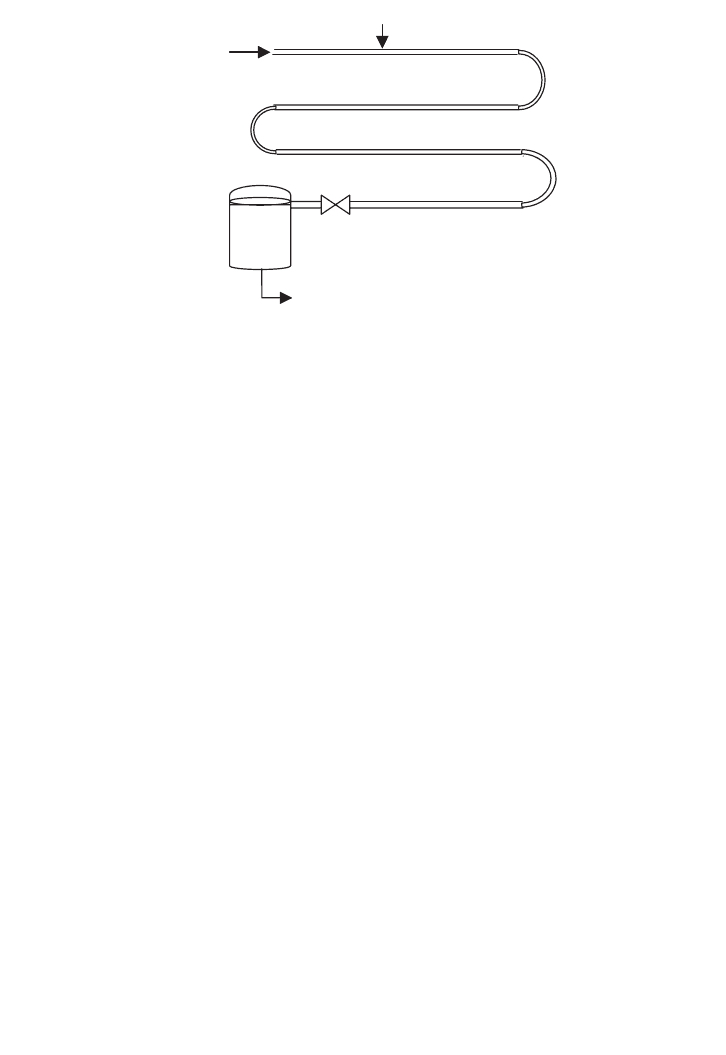
microorganisms. The medium from a make-up vessel flowing through the exchanger is held
in the coil, and then goes through the heat exchanger, heating more unsterile medium while
become cool itself, as it is collected in a sterile fermenter. Figure 15.2 presents the sequence
of piping media goes through, along with steam. Finally, the media is collected in a vacuum
flash drum.
Heat economisers are important for large-scale plant unit in continuous sterilisation. For
a small-scale bioreactor use of direct steam injection is much simpler to operate. A heat
exchanger is then needed with cooling water to bring the medium back to ambient tempera-
ture. Therefore jacketed vessels are commonly used with cooling water. There are advan-
tages and disadvantages to batch and continuous sterilisation. The energy savings are
related to the use of an economiser and direct stream or indirect sterilisation. In a large-
scale system economy and role decide which one is the most suitable process to be imple-
mented. For each case the specific design needs to be evaluated in terms of the thermal
efficiency and fixed costs involved.
15.4 HOT PLATES
In directing steam heating, continuous sterilisation is preferable. The medium passed
through a preheater may reach about 90 ⬚C; then quite fast sterilisation may take at 140 ⬚C.
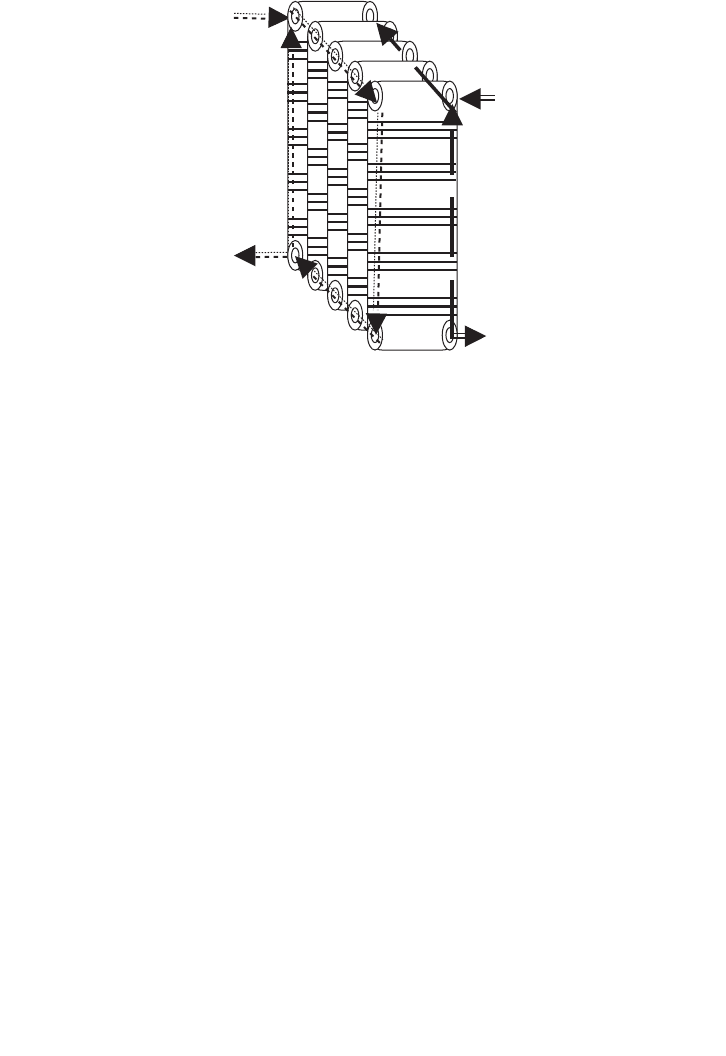
Figure 15.3 shows a counter-current hot-plate heat exchanger is normally used for contin-
uous sterilisation. Hot-plate heat exchangers are extremely efficient and easily maintained
if any fouling or scale deposition takes place. Normally fouling is serious problem, occur-
ing by deposition of proteins on the hot surface of the exchanger, which causes reduction
in the overall heat transfer coefficient. Plate exchangers are easily separated and cleaned.
Special care must be taken when the media contains any agglomerated particles. Correla-
tion is required to calculate the suitable residence time for sterilisation. The choice of a
Medium
Steam injector
Holding section
Expansion valve
Sterile medium
Vacuum
flash
chamber
FIG. 15.2. Continuous sterilisation.
Ch015.qxd 10/27/2006 10:52 AM Page 344
STERILISATION 345
suitable sterilisation process is based on economics and cost of the heat exchangers to
reduce energy consumption. In a suitable exchanger, the larger the heat transfer coefficient,
the greater the energy recovery.
15.5 HIGH TEMPERATURE STERILISATION
Fast sterilisation is performed at high temperature. High-temperature sterilisation requires
short holding times. This technique is used in the fast preparation of nutrient media for
industrial bioprocesses and in pasteurising milk. A short retention time may favour media
with heat-sensitive proteins and cause less damage to the biochemical composition of the
media than more prolonged times at lower temperatures. The ability of high temperatures
to perform rapid sterilisation is related to activation energies. That is affected by how fast
bacteria are killed in an elevated temperature. On the other hand, long sterilisation at high
temperatures may destroy the protein and biochemical composition of the media. Short-
duration sterilisation at high-temperatures are more lethal to organisms and less chemically
damaging than are longer sterilisation processes at lower temperatures. Sterilisation at high
temperature is recommended for 3 minutes at 134 ⬚C. It is preferable to 20 minutes at 115 ⬚C
in conventional operation.
15.6 STERILISED MEDIA FOR MICROBIOLOGY
Sterile media are used for pure culture. The media used to culture microorganisms depend
on the living conditions of the microorganisms. Media compositions must be identified
Hot liquid out
Cold liquid in
Cold liquid out
Hot liquid in
FIG. 15.3. Counter current hot plate heat exchanger.
Ch015.qxd 10/27/2006 10:53 AM Page 345

346 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
based on the needs of the organisms for carbon, nitrogen and phosphorus. Nutrients such
as protein and sugars are added at wide ranges of pH. The acidic, neutral and alkaline con-
ditions are defined based on differentiation of microbes in their biochemical reactions. Colour
indicators are used to observe any transition of pH. Buffer is used in the media to maintain
constant pH. Growth factors and growth stimulants are also used to accelerate microbial
growth and to maintain exponential growth. The most common media in the liquid phase
is nutrient broth; in the solid phase, nutrient agar is used to propagate microorganisms.
When microbiological media is prepared, it has to be sterilised before any inoculation of
organisms. Sterilisation is used to eliminate any microbial contamination that may originate
from air, glassware or hands. Microbes propagate quite quickely: within a few hours there
will be thousands of organisms reproduced in the media. To control the growth of unwanted
organisms, the media has to be sterilised quickly before any microbes start to utilise the
nutrients. The sterilisation process makes the media absolutely free of contaminants and
guarantees that the medium will stay sterile, so that is used only for the desired organisms.
The kinetics of culture media sterilisation describe the rate of destruction of microor-
ganisms by steam using a first-order chemical reaction rate model. As the population of
microorganisms (N) decreases with time, the rate is defined by the following equation:
(15.6.1)
where N is the number of viable organisms present in the culture media, t is the retention
time or sterilisation time, and k
d
is the reaction rate constant as it is known for a specific
death rate. Using separation of variables and integrating with initial condition, the follow-
ing useful expression is obtained:
(15.6.2)
where N
o
is the number of viable organisms present in the media at the starting point before
sterilisation. Now take the natural log of (15.6.2); it is reduced to a linear model:
(15.6.3)
The graphical presentation of the equation shows a straight line with a negative slope for
k
d
. As the death rate constant follows Arrhenius’ law,
1
the death rate constant is tempera-
ture dependent. The value of k
d
is about 0.02 min
⫺1
at 100 ⬚C, the death rate constant
increases by 10-fold at 110 ⬚C and 100-fold at 120 ⬚C.
2
where k
o
is the death rate constant at a reference temperature also known as Arrhenius’
constant, R is the gas constant, T is the absolute temperature, E is the activation energy,
kk
ERT
do
e⫽
⫺ /
In
o
d
Nt
N
kt
()
=-
Nt N
kt
()⫽
⫺
o
e
d
⫺
⫽
d
d
d
N
t
kN
Ch015.qxd 10/27/2006 10:53 AM Page 346

STERILISATION 347
which is 60–70 kcal per mole for microorganisms, 100–150 kcal per mole for spores, and
30–40 kcal per mole for media with vitamins and protein solution.
2
Figure 15.4 shows the linear model for (15.6.3), the loss of cell viability at various tem-
peratures. As the temperature increases from 105 to 121 ⬚C, the value for the slope of the
line increases. This means that the number of viable cells at a fixed time of sterilisation will
drastically decrease as the temperature increases by 16 ⬚C.
15.6.1 Sterilisation of Media for Stoke Cultures
To clear slant tubes, culture agar solution is prepared. To have clear agar solution, it has to
boil. Agar is a polysaccharide. It is not soluble in cold water, so heating helps to dissolve it
in water. Once the solution is dispensed in the tube, it goes for sterilisation. Agar is in the
solid state at room temperature, about 35 ⬚C.
15.6.2 Sterilisation of Bacterial Media
Often, sterilisation of culture media is done in an autoclave at temperatures between 121 and
134 ⬚C. It is good to know the damage caused to the medium by heating it. Heating the cul-
ture media, which contains peptides, sugars, vitamins, minerals and metals, results in nutrient
destruction, either thermal degradation or reaction between the components of the media.
Protein in the media is denatured by high temperature, or sugars are easily caramelised.
During the heat treatment, toxic compounds are formed, which may retard or inhibit the
microbial growth. For minimal damage to the ingredients of the media, it is important to opti-
mise and minimise the heating and holding time of the sterilisation process, respectively.
15.6.3 Sterilise Petri Dishes
There are several ways to handle Petri dishes. The standard dishes are 15–20 mm deep and
12–15 cm in diameter. They are normally available in glass and also transparent plastic.
Petri dishes are individually wrapped. The media is separately sterilised while we add the
sterilised media to the Petri dishes in front of a flame. It is recommended to use 20–25 ml
Time, t
N
o
tN )(
ln
increasing
-k
d
T
1
= 105 °C
T
4
=121 °C
FIG. 15.4. The loss of cell viability at various temperatures.
Ch015.qxd 10/27/2006 10:53 AM Page 347

348 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
of medium in each Petri dish. These are all called culture dishes. The media contains about
1.5–2% agar. After the media cools off it solidifies. The Petri dishes with the nutrient agar
should be sterilised and stored in a refridgerator.
It is common to sterilise the media and Petri dishes separately. When the medium is
cooled to about 55 ⬚C, in front of a flame or in a laminar flow chamber, lift the lid of the dish
enough to pour about 25 ml of the medium to the desired depth and lower the lid in place.
It is best to gently move the Petri dish in way that spreads a thin layer of agar uniformly
without any air bubbles. Distribution of media in the Petri dishes should be done in front
of a flame. Most plastic Petri dishes are made of polystyrene and are not autoclaveable.
Plastic Petri dishes are easily deformed during sterilisation at high temperature. Some plastic
dishes can be autoclaved, but they are more expensive. Please follow the instructions given
by the manufacturer or obtain information from catalogues.
It is recommended to autoclave glass dishes and medium separately. If your dishes are
autoclaveable, you may dispense the agar into the dish and then autoclave it. In this case it
is best to cool the dishes in the autoclave or in the pressure cooker to reduce the amount of
water that will condense on the cover of the dish.
15.7 DRY HEAT STERILISATION
Dry heat sterilisation is used for equipment that can withstand high temperature and dry heat
but cannot withstand wet or steam autoclave. This method is often used for glassware as it
dries and sterilises in one operation. The pipets must be wrapped in dustproof aluminum
foil or placed in metal pipette cans. The can lids are removed during heating and replaced
after sterilisation, that is before any dust can get in the can. Disposable items are not recom-
mended for dry heat sterilisation. This method may only be good for permanent reusable glass
pipettes.
Normal laboratory glassware must first be washed and cleaned. It has to be rinsed with
deionised water. The clean glassware is sterilised in an oven set at 200 ⬚C for 1–4 hours. It is
suitable to cover glassware with aluminum foil to maintain aseptic conditions after removing
the glassware from the oven. If aluminum foil is not available, special heat-resistant wrap paper
can be used. The sterile glassware must be protected from the air, which has micro-flora,
or any contaminants. Avoid the use of any plastic caps and papers. Detach any labelling tape
or other flammable materials, as they are fire hazards.
15.8 STERILISATION WITH FILTRATION
Certain media components are susceptible to heat; it is going to be denatured if it is heated.
Therefore they must be added to the media after autoclaving. To do so, it is necessary to carry
out filtration, the components using a 0.22 m pore size filter that is appropriate to the sol-
vent used. Filters are normally available from Whatman, or Fisher Scientific. It is recom-
mended you consult filter experts or suppliers about the solvent and the special application
you are intending to implement for the desired filters for the process. Normally filtration of
Ch015.qxd 10/27/2006 10:53 AM Page 348

STERILISATION 349
media is used instead of sterilised media with an autoclave. To filter sterilised media, filter
the water using a 0.45 m pore size filter, before using a 0.22 m pore size filter for final
sterilisation.
15.9 MICROWAVE STERILISATION
Rapid sterilisation of media is achieved by using microwave ovens. Most plant tissue culture
media can be sterilised using a microwave, although it may not be suitable with a medium
containing complex additives like oatmeal.
15.10 ELECTRON BEAM STERILISATION
Electron beam sterilisation is a high-voltage potential established between a cathode and an
anode in an evacuated tube. The cathode emits electrons, as a cathodic ray or electron beam.
A high intensity of electrons is produced. These electrons are accelerated to extremely high
velocities. These accelerated electron intensities have great potential as a bacteriocide. Most
electron beams operate in a vacuum. As a result the unwanted organisms in the media vanish
and the media is sterilised.
The electron accelerator equipment producing the high-voltage beam also has various
applications in the medical field and surgical supplies. Electron beam sterilisation is a suc-
cessful technology used for sterilisation of disposable medical appliances and devices with
a wide range of densities. The electron beam inactivates microorganisms that cause destruc-
tion of biomolecules, and results in death of microbes by an indirect chemical reaction.
Irradiation by gamma rays is a similar mechanism. Advanced electronics precisely control
the use of electron beams in the sterilisation of medical devices. Electron beam sterilisation
results in less material degradation than with gamma irradiation. Medical products are ster-
ilised in the original shipping containers, saving lots of time and maintaining integrity of
the original package.
15.11 CHEMICAL STERILISATION
Chemical agents are used to sterilise heat-sensitive equipment. Chemical solutions are used
as a suitable method for sterilising long pipettes and glassware. Normally at pilot scale in
the absence of life, steam and chemical agents are often used. Application of an oxidising
agent such as 10% chlorox for 20 minutes or longer proves the system operates without any
contamination. Excess amounts of chemical agent have to be removed; otherwise organisms
are able to grow in a toxic environment. The bleach (sodium hypochlorite) then needs to
be removed by rinsing with sterile water or rubbing with ethyl alcohol, 70% ethanol–water
(pH ⫽ 2) or an alcohol solution of 70% iso-propanol without recontaminating the glassware
or tools. Ethanol is commonly used for cleaning surfaces. Use of bleach on metal devices is
not suitable as it corrodes metals rapidly. Ethylene oxide in the gas phase is commonly used.
Ch015.qxd 10/27/2006 10:53 AM Page 349

350 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
This is a special chemical effectively used for column bioreactors. It is a volatile compound
and strong oxidising agent. It boils at ambient temperature, therefore the solution of ethyl-
ene oxide (liquid phase) must be stored in a refrigerator (4 ⬚C). An excellent oxidising agent
such as a 3% sodium hypochlorite is used for chemical sterilisation of equipment.
REFERENCES
1. Pelczar, M.J., Chan, E.C.S. and Krieg, N.R., “Microbiology”. McGraw-Hill, New York, 1986.
2. Scragg, A.H., “Bioreactors in Biotechnology, A Practical Approach”. Ellis Horwood Series in Biochemistry and
Biotechnology, New York, 1991.
3. Doran, P.M., “Bioprocess Engineering Principles”. Academic Press, New York, 1995.
4. Shuler, M.L. and Kargi, F., “Bioprocess Engineering, Basic Concepts”. Prentice-Hall, New Jersey, 1992.
5. Baily, J.E. and Ollis, D.F., “Biochemical Engineering Fundamentals”, 2nd edn. McGraw-Hill, New York, 1986.
6. Matthews, I.P., Gibson, C. and Samuel, A.H., J Biomed. Mat. Res. 23, 143 (1989).
Ch015.qxd 10/27/2006 10:53 AM Page 350

CHAPTER 16
Membrane Separation Processes
16.1 INTRODUCTION
Membranes have gained an important place in chemical technology and are used in a broad
range of applications ranging from pharmaceuticals to water treatment. Industrial applications
are divided into six main subgroups: reverse osmosis, nanofiltration, ultrafiltration, microfil-
tration, gas separation, pervaporation and electrodialysis.
1
The key property that is exploited
is the ability of a membrane to control the permeation rate of a chemical species through the
membrane.
2
In controlled drug delivery, the goal is to moderate the permeation rate of a drug
from a reservoir to the body. In separation applications, the goal is to allow one component
of a mixture to permeate the membrane freely, while hindering permeation of other com-
ponents.
3
This chapter provides a general introduction to membrane science and technology.
16.2 TYPES OF MEMBRANE
This chapter is limited to synthetic membranes, excluding all biological structures, but the
topic is still large enough to include a wide variety of membranes that differ in chemical and
physical composition and in the way they operate. In essence, a membrane is nothing more
than a discrete, thin interface that moderates the permeation of chemical species in contact
with it. This interface may be molecularly homogeneous, i.e., completely uniform in compo-
sition and structure; or the interface may be chemically or physically heterogeneous, e.g., con-
taining holes or pores of finite dimensions or consisting of some form of layered structure.
A normal filter meets this definition of a membrane, but, by convention, the term ‘filter’ is
usually limited to structures that separate particulate suspensions larger than 1–10m.
3,4
The
351
This case study was contributed by:
Ghasem Najafpour,
1
Nidal Hilal,
2
and Abdul Latif Ahmad
3
1
Biochemical Engineering, Program Chairman of Biotechnology, Faculty of Chemical Engineering,
Noshirvani Institute of Technology, University of Mazandaran, Babol, Iran.
2
Reader in Chemical Engineering, Director of Centre for Clean Water Technologies, University of
Nottingham, Deputy Director of Research/School of Chemical, Environmental and Mining Engineering,
University Park, Nottingham, NG7 2NR, United Kingdom.
3
Membrane Technology, School of Chemical Engineering, Engineering Campus, Universiti Sains
Malaysia, Seri Ampangan, 14300 Nibong Tebal, S.P.S., Pulau Pinang, Malaysia.
Ch016.qxd 10/27/2006 10:52 AM Page 351
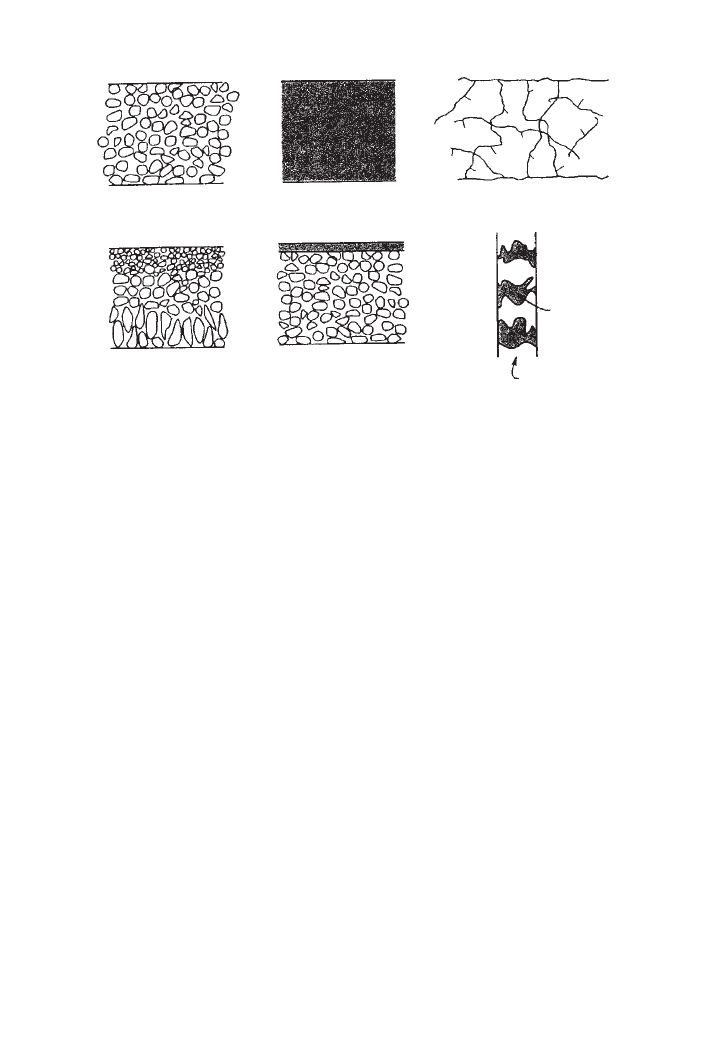
principal types of membrane are shown schematically in Figure 16.1 and are described
briefly in the following pages.
16.2.1 Isotropic Membranes
16.2.1.1 Microporous Membranes
A microporous membrane is very similar in structure and function to a conventional filter. It
has a grid, highly voided structure with randomly distributed, interconnected pores. However,
these pores differ from those in a conventional filter by being extremely small, of the order of
0.01–10 m in diameter. All particles larger than the largest pores are completely rejected by
the membrane. According to the pore size distribution of the membrane, the particles smaller
than the largest pores and the particles larger than the smallest pores are partly rejected.
Particles much smaller than the smallest pores are absolutely passed through the membrane.
Thus, separation of solutes by microporous membranes is mainly a function of molecular
size and pore size distribution. In general, only molecules that differ considerably in size can
be separated effectively by microporous membranes, e.g., in ultrafiltration and microfiltra-
tion.
5,6
Isotropic membranes are shown in Figure 16.1a.
16.2.1.2 Non-porous, Dense Membranes
Non-porous, dense membranes consist of a dense film through which permeats are trans-
ported by diffusion under the driving force of a pressure, concentration or electrical potential
gradient. The separation of various components of a mixture is related directly to their relative
transport rates within the membrane, which are determined by their diffusivity and solubility in
the membrane material. Thus, non-porous, dense membranes can separate permeats of similar
352 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
(a)
(b)
Isotropic membranes
Anisotropic membranes
Isotropic microprous
membrane
Non-porous dense
membrane
Supported liquid
membrane
Liquid-filled
pores
Polymer
matrix
Thin-film composite
anisotropic membranes
Loeb−Sourirajan
anisotropic membranes
Electrically charged
membrane
coo
-
coo
-
coo
-
coo
-
coo
-
coo
-
coo
-
coo
-
coo
-
coo
-
coo
-
coo
-
FIG. 16.1. Schematic diagrams of the principal types of membrane.
Ch016.qxd 10/27/2006 10:52 AM Page 352

size if their concentration in the membrane material (i.e. their solubility) differs significantly.
Most gas separation, pervaporation and reverse osmosis membranes use dense membranes
to perform the separation. Usually these membranes have an anisotropic structure to
improve the flux.
16.2.1.3 Electrically Charged Membranes
Electrically charged membranes are dense or microporous. Most commonly these mem-
branes are very fine microporous, with the pore walls carrying fixed positively or negatively
charged ions. A membrane with fixed positively charged ions is referred to as an anion-
membrane because it binds anions in the surrounding fluid. Similarly, a membrane contain-
ing fixed negatively charged ions is called a cation-exchange membrane. Separation with
charged membranes is achieved mainly by exclusion of ions of the same charge as the fixed
ions of the membrane structure, and to a much lesser extent by the pore size. The separa-
tion is affected by the charge and concentration of the ions in solution. For example, mono-
valent ions are excluded less effectively than divalent ions, and in solutions of high ionic
strength, selectivity decreases. Electrically charged membranes are used for processing
electrolyte solutions in electrodialysis.
7,8
16.2.2 Anisotropic Membranes
The transport rate of a species through a membrane is inversely proportional to the membrane
thickness. High transport rates are desirable in membrane separation processes for economic
reasons; therefore, the membrane should be as thin as possible. Conventional film fabrica-
tion technology limits manufacture of mechanically strong, defect-free films to about 20 m
thickness.
2,9
The development of novel membrane fabrication techniques to produce
anisotropic membrane structures has been one of the major breakthroughs of membrane
technology during the past 30 years. Anisotropic membranes consist of an extremely thin
surface layer supported on a much thicker, porous structure. The surface layer and its struc-
ture may be formed in a single operation or separately. In composite membranes, the layers
are usually made from different polymers. The separation properties of permeation rates of
the membrane are determined exclusively by the surface layer; the substructure functions as
a mechanical support. The advantages of the higher fluxes provided by anisotropic mem-
branes are so great that almost all commercial processes use such membranes.
16.2.3 Ceramic, Metal and Liquid Membranes
The discussion so far implies that membrane materials are organic polymers, and in fact
most membranes used commercially are polymer-based. However, in recent years, interest
in membranes made of less conventional materials has increased. Ceramic membranes, a
special class of microporous membranes, are being used in ultrafiltration and microfiltra-
tion applications for which solvent resistance and thermal stability are required. Dense, metal
membranes, particularly palladium membranes, are being considered for the separation of
hydrogen from gas mixtures, and supported liquid films are being developed for carrier-
facilitated transport processes.
MEMBRANE SEPARATION PROCESSES 353
Ch016.qxd 10/27/2006 10:52 AM Page 353
