Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


Fullerene - Rich Nanostructures
Fernando Langa and Jean - Fran ç ois Nierengarten
699
Advanced Nanomaterials. Edited by Kurt E. Geckeler and Hiroyuki Nishide
Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
ISBN: 978-3-527-31794-3
22
22.1
Introduction
In recent years, the rapid advances in dendrimer synthetic chemistry have moved
towards the creation of functional systems with increased attention to potential
applications [1] . Among the large number of molecular subunits used for den-
drimer chemistry, C
60
has proven to be a versatile building block and fullerene -
functionalized dendrimers – that is, fullerodendrimers [2] – have generated signifi cant
research activities in recent years [3, 4] . In particular, the peculiar physical proper-
ties of fullerene derivatives make fullerodendrimers attractive candidates for a
variety of interesting features in supramolecular chemistry and materials science
[5] . C
60
itself is a convenient core for dendrimer chemistry [3] , and the functionali-
zation of C
60
with a controlled number of dendrons dramatically improves the
solubility of the fullerenes [6] . Furthermore, variable degrees of addition about the
fullerene core are possible, and its almost spherical shape leads to globular
systems, even with low - generation dendrons [7] . On the other hand, specifi c advan-
tages are brought about by the encapsulation of a fullerene moiety in the middle
of a dendritic structure [8] . The shielding effect resulting from the presence of the
surrounding shell has been found useful for optimizing the optical limiting prop-
erties of C
60
derivatives [9] , to obtain amphiphilic derivatives with good spreading
characteristics [10] , or to prepare fullerene - containing liquid crystalline materials
[11] . Use of the fullerene sphere as a photoactive core unit has also been reported
[12] . In particular, the special photophysical properties of C
60
have been used to
evidence dendritic shielding effects [13] and to prepare dendrimer - based, light -
harvesting systems [14] . Whereas, the majority of the fullerene - containing den-
drimers reported to date have been prepared with a C
60
core, dendritic structures
with fullerene units at their surface, or with C
60
spheres in the dendritic branches,
have been essentially ignored. This is mainly associated with diffi culties related to
the synthesis of fullerene - rich molecules [4] . Indeed, the two major problems for
the preparation of such dendrimers are the low solubility of C
60
and its chemical
reactivity, which limits the range of reactions that can be used for the synthesis of
700 22 Fullerene-Rich Nanostructures
branched structures bearing multiple C
60
units. The most recent developments
on the molecular engineering of covalent and noncovalent fullerene - rich dendrim-
ers will be presented in the chapter, the aim of which is not to provide an exhaus-
tive review on such systems, but rather to present signifi cant examples that
illustrate the current state of fullerene chemistry for the development of new
nanostructures.
22.2
Fullerene - Rich Dendritic Branches
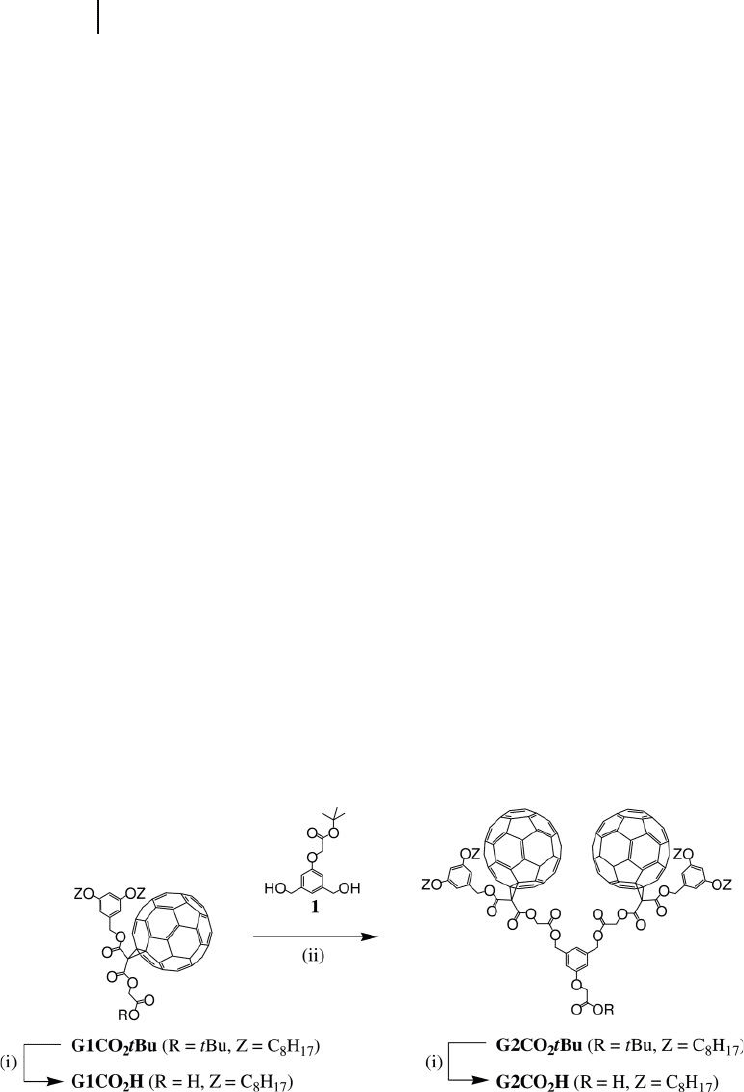
Over the past years, Nierengarten and coworkers have developed synthetic
methodologies allowing for the preparation of dendrons substituted with several
fullerene moieties [15] . As a typical example, the convergent preparation of den-
dritic branches with fullerene subunits at the periphery is depicted in Figures
22.1 – 22.3 . The starting fullerene derivative, G1CO
2
t Bu, is easily obtained on a
multi - gram scale, and is highly soluble in common organic solvents, based on the
presence of two long alkyl chains [16] . The iterative reaction sequence used in
the preparation of the subsequent dendrimer generations relies upon successive
cleavage of a t - butyl ester moiety under acidic conditions, followed by a N , N ’ -
dicyclohexylcarbodiimide ( DCC ) - mediated esterifi cation reaction with the A
2
B
building block 1 possessing two benzylic alcohol functions and a protected car-
boxylic acid function [16] .
Selective cleavage of the t - butyl ester group in G1CO
2
t Bu was achieved by
treatment with an excess of CF
3
COOH (TFA) in CH
2
Cl
2
to afford G1CO
2
H in a
quantitative yield. Reaction of the diol 1 with carboxylic acid G1CO
2
H under
esterifi cation conditions using DCC, 4 - 4 - dimethylaminopyridine ( DMAP ), and
1 - hydroxybenzotriazole ( HOBt ) in CH
2
Cl
2
gave the protected dendron of second -
generation G2CO
2
t Bu in 90% yield. Hydrolysis of the t - butyl ester moiety under
acidic conditions then afforded the corresponding carboxylic acid G2CO
2
H in a
Figure 22.1 Reagents and conditions: (i) TFA, CH
2
Cl
2
; (ii) DCC, DMAP, HOBt, CH
2
Cl
2
.
22.2 Fullerene-Rich Dendritic Branches 701
Figure 22.2 Reagents and conditions: (i) 1 , DCC, DMAP, HOBt, CH
2
Cl
2
; (ii) TFA, CH
2
Cl
2
.
702 22 Fullerene-Rich Nanostructures
Figure 22.3 Reagents and conditions: (i) 1 , DCC, DMAP, HOBt, CH
2
Cl
2
; (ii) TFA, CH
2
Cl
2
.
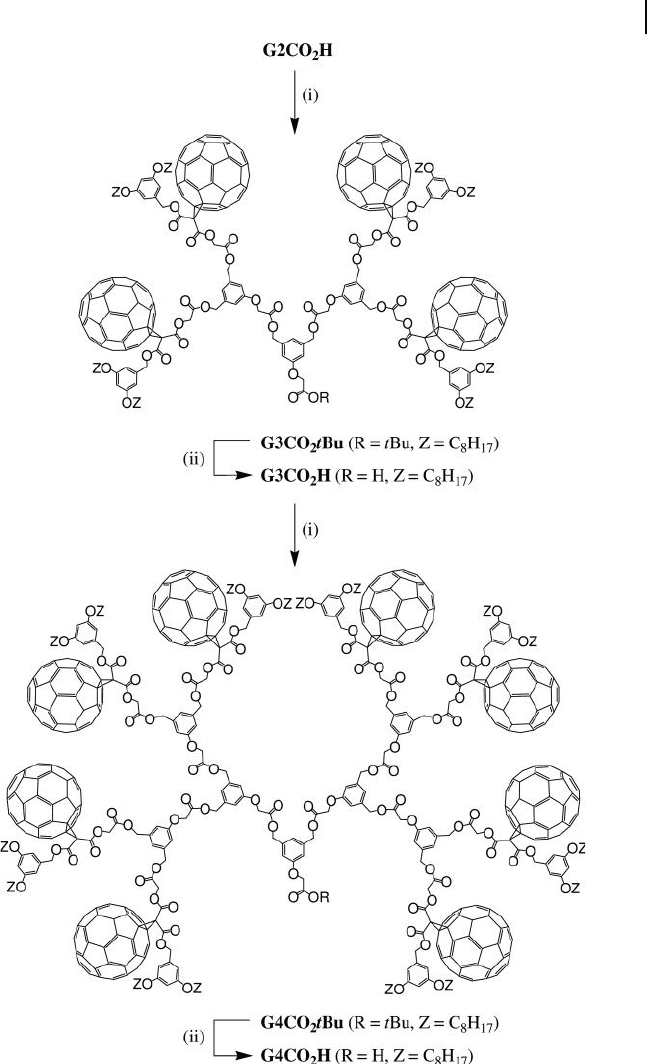
quantitative yield. Esterifi cation of G2CO
2
H with diol 1 (DCC, HOBt, DMAP)
afforded the t - butyl - protected fullerodendron G3CO
2
t Bu in 87% yield (Figure
22.2 ). Selective hydrolysis of the t - butyl ester under acidic conditions afforded acid
G3CO
2
H in 99% yield. Subsequent reaction of G3CO
2
H with the branching unit

22.2 Fullerene-Rich Dendritic Branches 703
1 in the presence of DCC, HOBt and DMAP afforded fullerodendron G4CO
2
t Bu
(95%), which after treatment with CF
3
CO
2
H gave G4CO
2
H (97%).
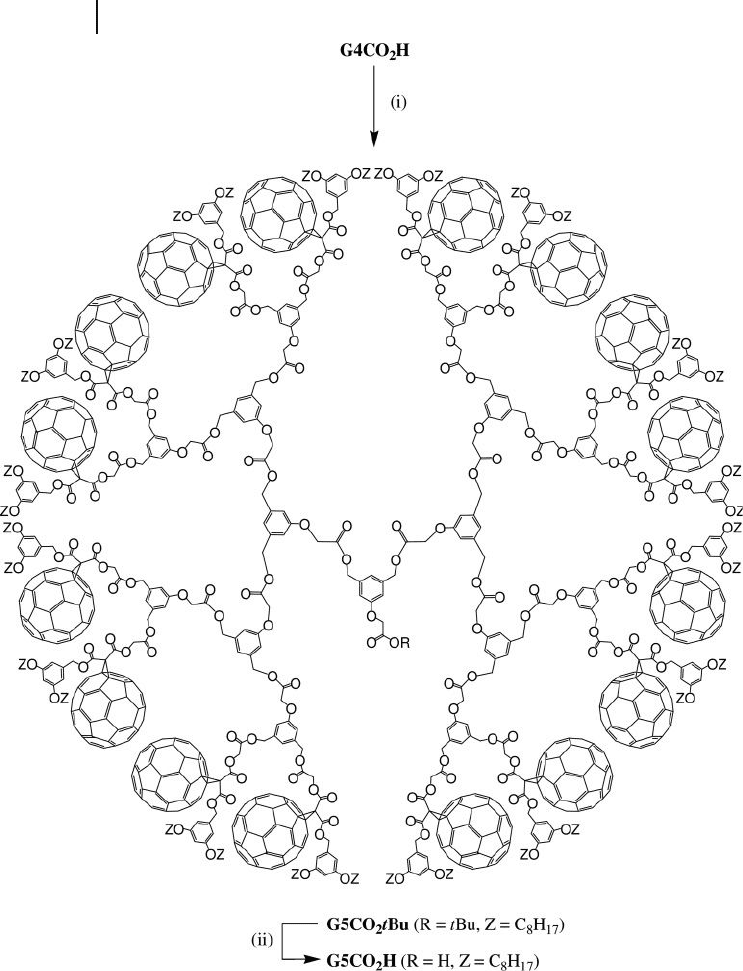
By repeating the same reaction sequence from G4CO
2
H, the fi fth generation
derivatives G5CO
2
t Bu and G5CO
2
H were also prepared (Figure 22.3 ). Compounds
G1 - 5CO
2
t Bu and G1 - 5CO
2
H are highly soluble in common organic solvents such
as CH
2
Cl
2
, CHCl
3
, or tetrahydrofuran ( THF ), and complete spectroscopic charac-
terization was easily achieved.
The unequivocal characterization of these compounds also required their mass
spectrometric analysis; this is quite diffi cult, as no structural features allow for
easy protonation of the molecules. Several ionization techniques such as matrix -
assisted laser desorption/ionization ( MALDI ), or fast - atom bombardment ( FAB )
have been applied for the characterization of fullerodendritic species [17] . These
tools are, however, not always well adapted for C
60
derivatives of high molecular
weight, such as G1 - 5CO
2
t Bu and G1 - 5CO
2
H, as they lead to high levels of frag-
mentation. In contrast, electrospray mass spectrometry ( ES - MS ) has the ability to
desolvate ions that preexist in solution, thus offering the possibility of transferring
such ions into the gas phase without notable fragmentation [18] . Indeed, supramo-
lecular cationic dendritic structures resulting from the self - assembly of fulleroden-
drons possessing an ammonium function at the focal point and bis - crown - ether
receptors have been successfully characterized using ES - MS [19] . Unfortunately,
fullerodendrons G1 - 5CO
2
t Bu and G1 - 5CO
2
H are uncharged in solution, and there-
fore are not suitable for ES - MS analysis, at least in principle. However, it has been
shown that an in situ reduction of fullerenes in the injection capillary of the elec-
trospray mass spectrometer offers an interesting possibility to analyze neutral C
60
derivatives [17] . In other words, fullerene radical anions can be generated during
the electrospray process, owing to the ability of the electrospray source to behave
like an electrolysis cell [17] . Indeed, fullerodendrons G1 - 5CO
2
t Bu and G1 - 5CO
2
H
could be characterized by applying this technique. The ES mass spectra of the
second - generation derivative G2CO
2
t Bu is depicted in Figure 22.4 . The mass
spectrum obtained under mild conditions ( V
c
= 150 V) is dominated by the doubly
charged ion peak at m / z 1343.2, which can be assigned to the radical di - anion of
G2CO
2
t Bu (calculated m/z 1343.43). The experimental isotopic pattern of this ion
is in perfect agreement with that calculated for the doubly reduced compound. A
singly charged ion is also observed as minor signal at m / z 2686.3, and corresponds
to the singly reduced G2CO
2
t Bu - structure. Finally, the minor peak observed at m / z
1226.1 is ascribed to the fragment G1CO
2
−
resulting from the cleavage of a benzylic
ester unit in G2CO
2
t Bu. When the ES spectrum of G2CO
2
t Bu was recorded under
more harsher conditions ( V
c
= 300 V), the characteristic peaks corresponding to
the radical mono - and di - anions were still observed, but an additional signal could
also be detected at m / z 1124. This characteristic fragment was the same as that
observed in the spectra of G1CO
2
t Bu.
The analysis of the higher - generation compounds has been more diffi cult. Effec-
tively, the response factor of the anions is reduced as their molecular weight is
increased. In addition, the number of ester functions is higher, thus leading to
more fragmentation. The resulting fragments having a lower molecular weight – and

704 22 Fullerene-Rich Nanostructures
Figure 22.4 ES - MS spectra of G2CO
2
tBu recorded with V
c
values of 150 (a) and 300 V (b).
Figure 22.5 ES - MS spectrum of G4CO
2
tBu recorded with a V
c
value of 150 V.
therefore a higher response factor – gave signals with signifi cant intensities in all
the spectra, regardless of the V
c
value. However, peaks corresponding to nonfrag-
mented ions could be clearly observed. As shown in Figure 22.5 , two characteristic
molecular ion peaks are observed at m / z 1851.1 and 1586.8 in the ES - MS spectrum
of G4CO
2
t Bu. These correspond to the hexa - and hepta - anions of the fulleroden-
dron, in which six or seven C
60
units are reduced, respectively. Several fragment
ions are also present in both cases, the low (100 V) and high (300 V) voltages.
Importantly, their intensity relative to the molecular ion peaks is clearly increased
when the V
c
value was raised, thus showing that these additional signals result
from a fragmentation of the dendrimer. Within this series of compounds, a
general observation is a decrease in the absolute intensity of the signals arising
from the fullerodendrons when the generation number is increased. As men-
tioned above, this is most likely due to the increased number of labile ester func-
tions giving rise to more fragmentations, and to a decrease in the response factor
when the molecular weight is increased.
22.3
Photoelectrochemical Properties of Fullerodendrons and Their Nanoclusters
The absorption spectra of G1 - 5CO
2
t Bu in a toluene/acetonitrile (1/6, v/v) mixed
solvent exhibit structureless broad absorption in the range of 300 to 800 nm. These
22.3 Photoelectrochemical Properties of Fullerodendrons and Their Nanoclusters 705
results suggest that such compounds aggregate and form large clusters in the
mixed solvents [20] . The particle size of these clusters in the mixed solvent was
measured using dynamic light scattering ( DLS ). In a mixture (1/6) of toluene/
acetonitrile at an incubation period of 15 min after injection of the toluene solution
into acetonitrile, the size distribution of G1 - 5CO
2
t Bu was found to be relatively
narrow, with different mean diameters of 790 nm for (G1CO
2
t Bu)
m
, 210 nm for
(G2CO
2
t Bu)
m
, 170 nm for (G3CO
2
t Bu)
m
, 100 nm for (G4CO
2
t Bu)
m
, and 90 nm for
(G5CO
2
t Bu)
m
. The order of the mean diameters was not consistent with that of
their molecular sizes. Both, dendrimers and dendrons are known to become
compact, rigid structured as the generation number is increased [20] . This sug-
gests that, in the process of cluster formation with the higher dendrimer genera-
tion, each dendritic branch is subject to interactions with branches belonging to
the same dendrimer molecule ( intra molecular), rather than to other molecules
( inter molecular). This results in the formation of densely packed dendrimer clus-
ters with a small, compact size (i.e., 90 – 100 nm). In other words, in the lower
dendrimer generations, inter molecular interactions among branches prevail,
leading to the formation of poorly packed dendrimer clusters with a large size. In
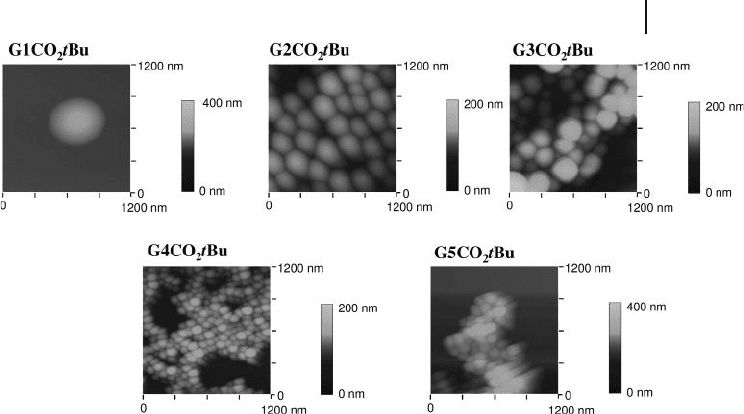
order to assess the shape and morphology of (G1 - 5CO
2
t Bu)
m
clusters, atomic force
microscopy ( AFM ) measurements have been carried out. The AFM images of the
clusters prepared by spin - coating of the (G1 - 5CO
2
t Bu)
m
cluster solutions on mica
surface are depicted in Figure 22.6 . To remove solvent, the resulting substrates
were heated under reduced pressure. The (G1 - 5CO
2
t Bu)
m
clusters were spherical,
with an average horizontal diameter of 900 nm for (G1CO
2
t Bu)
m
, 200 nm for
(G2CO
2
t Bu)
m
, 200 nm for (G3CO
2
t Bu)
m
, 100 nm for (G4CO
2
t Bu)
m
, and 100 nm for
(G5CO
2
t Bu)
m
. The size of the clusters agreed well with the values obtained from
the DLS measurements. The vertical size of the clusters on mica correlated largely
with the horizontal size of the clusters, except in the case of (G1CO
2
t Bu)
m
. The
Figure 22.6 Atomic force microscopy images of (G1 - 5CO
2
tBu)
m
on mica.

706 22 Fullerene-Rich Nanostructures
AFM image of the (G1CO
2
t Bu)
m
cluster revealed a rather disc - like structure with
an average diameter of 900 nm and an average maximum thickness of 170 nm.
Namely, the vertical size of the (G1CO
2
t Bu)
m
cluster is much smaller than the
horizontal size. Although detailed structure at the molecular level is not yet clear,
self - organization of G1CO
2
t Bu in the mixed solvent would lead to the formation
of multilayer vesicle. In such a case, solvent evaporation from the inner space of
the multilayer vesicle may yield the disk - like structure. Similar proposed structures
were reported for amphiphilic fullerene derivatives in water, mixed organic sol-
vents, and cast fi lms [21] .
In order to determine if differences observed in the size of the nanoclusters
obtained from G1 - 5CO
2
t Bu could have any infl uence on their macroscopic proper-
ties, photoelectrochemical cells have been prepared. For this, the clusters
were deposited electrophoretically onto ITO/SnO
2
electrodes by applying a DC
voltage to the electrode [20] . After the electrophoretic deposition, the ITO/SnO
2
electrode turned brown in color, whereas discoloration of the cluster solution took
place. As a result, cluster fi lms with a thickness of 4 – 5 μ m could be obtained. AFM
was used to evaluate the surface morphology of the fi lms deposited on the elec-
trodes. The ITO/SnO
2
/(G1 - 5CO
2
t Bu)
m
fi lms were composed of closely packed
clusters with a size of 800 nm (G1CO
2
t Bu), 200 nm (G2CO
2
t Bu), 200 nm
(G3CO
2
t Bu), 100 nm (G4CO
2
t Bu), and 100 nm (G5CO
2
t Bu). The size of the clus-
ters largely agreed with the diameters of the clusters on the mica obtained from
the cluster solutions. These results also confi rmed that fullerene dendrimer clus-
ters could be successfully transferred onto the nanostructured SnO
2
electrodes.
Photoelectrochemical measurements were performed in deaerated acetonitrile
containing 0.5 M LiI and 0.01 M I
2
with ITO/SnO
2
/(G1 - 5CO
2
t Bu)
m
as a working
electrode, a platinum wire as a counterelectrode, and an I
−
/I
3 −
reference electrode.
The photocurrent responses were prompt, steady, and reproducible during
repeated on/off cycles of visible light illumination. Blank experiments conducted
with a bare ITO/SnO
2
electrode yielded no detectable photocurrent under similar
experimental conditions. A series of photocurrent action spectra was recorded to
evaluate the response of fullerodendron clusters towards photocurrent generation.
The photocurrent action spectra of ITO/SnO
2
/(G1 - 5CO
2
t Bu)
m
devices are shown
in Figure 22.7 . Incident photon - to - photocurrent effi ciency (IPCE) was calculated
by normalizing the photocurrent density for incident light energy and intensity
using the expression:
IPCE W
in
%
()
=× × ×
()
100 1240 i λ
where i is the photocurrent density (A cm
− 2
), W
in
is the incident light intensity
(W cm
− 2
), and λ is the excitation wavelength (nm). The action spectra of ITO/SnO
2
/
(G1 - 5CO
2
t Bu)
m
devices largely agreed with the absorption spectra on ITO/SnO
2
,
supporting the involvement of the C
60
moieties for photocurrent generation. These
results were consistent with the photoelectrochemical properties of the clusters of
fullerene derivatives on SnO
2
electrodes [21] . When the IPCE values of ITO/SnO
2
/
(G1 - 5CO
2
t Bu)
m
devices were compared under the same conditions, the IPCE value
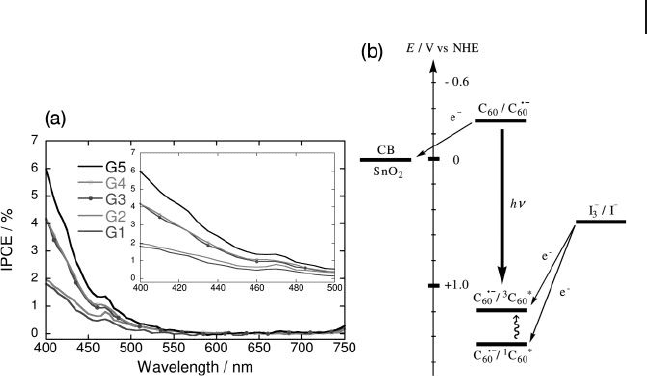
22.3 Photoelectrochemical Properties of Fullerodendrons and Their Nanoclusters 707
at an excitation wavelength of 400 nm were seen to increase with increasing the
generation number: 1.7% for ITO/SnO
2
/(G1CO
2
t Bu)
m
, 1.9% for ITO/SnO
2
/
(G2CO
2
t Bu)
m
, 4.1% for ITO/SnO
2
/(G3CO
2
t Bu)
m
, 4.1% for ITO/SnO
2
/(G4CO
2
t Bu)
m
,
and 6.0% for ITO/SnO
2
/(G5CO
2
t Bu)
m
devices.
Based on previous studies on a similar photoelectrochemical system of C
60
and C
60
derivatives [22] , photocurrent generation diagram is illustrated in Figure
22.7 . The primary step in the photocurrent generation is initiated by photoinduced
electron transfer from I
−
(I
3 −
/I
−
, 0.5 V versus NHE) in the electrolyte solution
to the excited states of fullerodendrimer clusters (C
60
˙
−
/
1
C
60
* = 1.45 V versus
NHE, C
60
˙
−
/
3
C
60
* = 1.2 V versus NHE) [20] . The electron transfer rate is controlled
by the diffusion of I
−
( ∼ 109 s
− 1
) in the electrolyte solution. The resulting reduced
C
60
(C
60
˙
−
/C
60
= − 0.3 V versus NHE) injects an electron directly into the SnO
2
nanocrystallites (ECB = 0 V versus NHE), or the electron is injected into the SnO
2
nanocrystallites through an electron - hopping process between the C
60
molecules
[20] . The electron transferred to the semiconductor is driven to the counterelec-
trode via an external circuit so as to regenerate the redox couple. It is noteworthy
that the IPCE values are dependent on the dendritic generation. With increasing
the dendritic generation the IPCE value increases. The structural investigation on
the fullerodendrimers revealed that the higher dendrimer generation led to the
formation of densely packed clusters with a smaller, compact size ( vide supra ).
Such structures of fullerene dendrimer clusters on ITO/SnO
2
in the higher gen-
eration would make it possible to accelerate the electron injection process from
the reduced C
60
to the conduction band of SnO
2
via the more effi cient electron
hopping through the C
60
moieties, where the average distance between the C
60
moieties is smaller.
Figure 22.7 (a) Photocurrent action spectra of ITO/SnO
2
/
(G1 - 5CO
2
tBu)
m
. Applied potential = +0.11 V versus SCE; 0.5 M
LiI and 0.01 M I
2
in acetonitrile; (b) Photocurrent generation
diagram.

708 22 Fullerene-Rich Nanostructures
22.4
Fullerene - Rich Dendrimers
The results described in the previous section represent a powerful driving force
to develop a new, effi cient synthetic strategy for the preparation of large fullerene -
rich dendritic molecules. In this respect, the self - assembly of dendrons using
noncovalent interactions is particularly well suited to the preparation of fullerene -
rich macromolecules [23, 24] . Indeed, the synthesis itself is restricted to the prepa-
ration of dendrons, and self - aggregation leads to the dendritic superstructure, thus
avoiding tedious fi nal synthetic steps with precursors incorporating potentially
reactive functional groups such as C
60
. For example, Nierengarten and coworkers
have shown that C
60
derivatives bearing a carboxylic acid function undergo self -
assembly with n - butylstannonic acid ( n BuSn(O)OH) to produce fullerene - rich
nanostructures with a stannoxane core in near - quantitative yields [25] . The reac-
tion conditions for the self - assembly of the stannoxane derivatives were fi rst
adjusted with model compound 2 (Figure 22.8 ). Under optimized conditions, a
mixture of 2 (1 equiv.) and n BuSn(O)OH (1 equiv.) in benzene was refl uxed for
12 h using a Dean – Stark trap. After cooling, the solution was fi ltered and evapo-
rated to dryness to afford the hexameric organostannoxane derivative 3 in 99%
yield. The drum - like structure of this compound, composed of a prismatic Sn
6
O
6
core, was confi rmed by its
119
Sn nuclear magnetic resonance ( NMR ) spectrum
recorded in C
6
D
6
, which showed a single resonance at − 479.1 ppm. This chemical
shift is characteristic of a drum - shaped structure with six equivalent Sn atoms
coordinated by fi ve oxygens and one carbon [26] . Crystals suitable for X - ray crystal -
structure analysis were obtained by the slow diffusion of Et
2
O into a C
6
H
6
solution
of 3 . Despite the disorder resulting from one of the fl exible butyl chains, the central
Sn
6
O
6
stannoxane core and the six peripheral 2 - phenoxyacetate units of the struc-
ture were well resolved. As shown in Figure 22.8 , the prismatic tin cage is formed
by two, six - membered (SnO)
3
rings joined together by six Sn – O bonds [27] . The
side faces of the cluster thus comprise six, four - membered (SnO)
2
rings, each of
which is spanned by a carboxylate group that forms a bridge between two Sn
atoms. A detailed observation of the stannoxane framework revealed that the six -
membered rings had a chair - like conformation, with each Sn atom being bonded
to three framework oxygen atoms, while the Sn – O bonds all had comparable
lengths ranging from 208 to 210 pm. The six Sn atoms were seen to be hexacoor-
dinated, with the coordination sphere being completed by a n - butyl group and two
oxygen atoms from two different carboxylate groups. The Sn – O bonds to the bridg-
ing carboxylate atoms were longer than the core bonds, and ranged from 215 to
218 pm.
The reaction conditions used to prepare 3 from 2 - phenoxyacetic acid were
applied to the fullerene building blocks G1 - 3CO
2
H. The organostannoxane deriva-
tives 4 – 6 were thus obtained in almost quantitative yields (Figures 22.9 and 22.10 ).
These compounds are highly soluble in common organic solvents such as CH
2
Cl
2
,
CHCl
3
, C
6
H
6
or toluene, and complete spectroscopic characterization was easily
achieved. The
1
H and
13
C NMR spectra of 4 – 6 clearly revealed the characteristic
