Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

19.4 Carbon Nanotubes as Adsorbents 629
adsorption capacity for Cd(II) than did the pristine, as - grown CNTs. Subsequently,
the authors measured the physico - chemical properties of oxidized CNTs and evalu-
ated their Cd(II) adsorption capacity. The specifi c surface area and pore - size dis-
tributions of the as - grown and oxidized CNTs were measured using nitrogen
adsorption, with the BET (Brunauer – Emmett – Teller) method. The functional
groups on oxidized CNTs were assessed quantitatively using Boehm ’ s titration
method [69] , and the zeta potentials of the as - grown and oxidized CNTs were also
evaluated. Based on these results, it was proposed that the Cd(II) adsorption capaci-
ties for the three types of oxidized CNT were increased due to functional groups
having been introduced by oxidation, compared to the as - grown, pristine CNTs.
The observed Cd(II) adsorption capacity of the as - grown CNTs reached only
1.1 mg g
− 1
, compared to values of 2.6, 5.1, and 11.0 mg g
− 1
for nanotubes treated
with H
2
O
2
, HNO
3
and KMnO
4
, respectively. The authors linked these results to
the increase in surface area observed following each chemical treatment. The data
obtained regarding the particle size distribution and suspensibility of these materi-
als indicated that oxidation with H
2
O
2
and KMnO
4
only partially broke up the
nanotubes, whilst oxidation with HNO
3
cut short completely most of the CNTs.
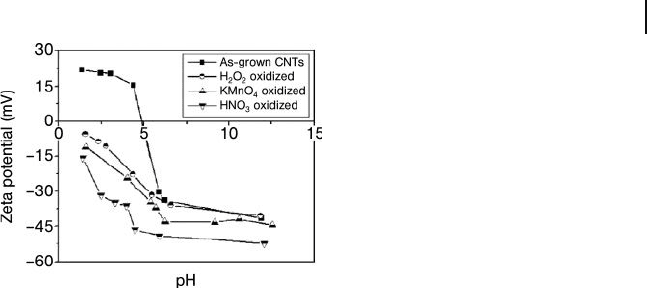
The observed dependence of the zeta potential of the as - grown and oxidized CNTs
on pH is shown graphically in Figure 19.10 . At the same pH value, the zeta potential
for the three types of oxidized CNTs followed the order H
2
O
2
< KMnO
4
< HNO
3
,
and suggests that the amounts of acid - functional groups increase following the
same order. The adsorption isotherms of Cd(II) also indicated that the functional
groups introduced by oxidation improved the ion - exchange capabilities of the CNTs
and thus led to corresponding increases in the Cd(II) adsorption capacities. A
removal effi ciency close to 100% at a CNT dosage of 0.08 g 100 ml
− 1
was observed
for the KMnO
4
- oxidized CNTs, which suggested that this treatment represented an
effective means of improving the Cd(II) adsorption capacity.
19.4.1.4 Adsorption of Copper ( II )
Despite being one of the most widespread environmental contaminants, copper
is essential to human life and health, yet is potentially toxic in larger quantities.
In humans, the ingestion of relative large quantities of copper salts may cause
Figure 19.10 Zeta potential curves versus pH for pristine and
oxidized CNTs. Reproduced from Ref. [68] .
630 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
severe abdominal pain, vomiting, diarrhea, hemolysis, hepatic necrosis, hematuria,
proteinuria, hypotension, tachycardia, convulsions, coma, and death. The major
sources of copper in industrial effl uents are metal cleaning and electroplating.
Wu [70] has evaluated the Cu(II) adsorption effi ciency of pristine and chemically
modifi ed CNTs, the latter being functionalized using HNO
3
and NaOCl. This
chemical treatment caused increases in both the pore volume and average pore
size of the CNTs, while the value of the isoelectric point was shown to decrease.
A comparison of the infrared spectra of the as - produced and modifi ed CNTs
indicated that several functional groups had been generated on the surface of
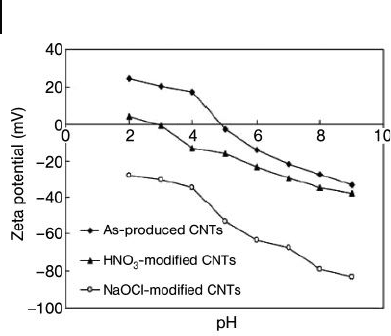
modifi ed CNTs. The zeta potential values of the as - produced and modifi ed CNTs,
as shown in Figure 19.11 , indicated that the surface of the as - produced and HNO
3
-
modifi ed CNTs was positively charged in solution, whereas the zeta potentials of
NaOCl - modifi ed CNTs were all negative. These negatively charged surfaces would
electrostatically favor the adsorption of Cu(II), as was observed by Wu and cowork-
ers. Indeed, the Cu(II) adsorption capacity followed the order NaOCl - modifi ed >
HNO
3
- modifi ed > as - produced CNTs. These fi ndings suggest that modifying the
surface of the CNTs may not only provide a more negatively charged and
hydrophilic surface but also generate a variety of functional groups, markedly
promoting the adsorption of Cu(II) onto the modifi ed CNTs. The maximum
adsorption capacities observed in this study at different temperatures are sum-
marized in Table 19.3 .
19.4.1.5 Adsorption of Zinc ( II )
Whilst zinc (II) is essential for human health, large amounts can be harmful. The
consequences of a relatively large intake of Zn(II) include lethargy, light - headed-
ness, ataxia, oropharyngeal cancer, gastric burns, epigastric tenderness, pharyn-
geal edema, hematemesis, and melena [71] . The suitability of CNTs (both MWNT
and SWNT) to adsorb Zn(II) from water was studied by Lu et al . [72] , whose data
showed the adsorption capacity of CNTs to be greatly improved following a specifi c
Figure 19.11 Zeta potential curves versus pH for pristine
and oxidized CNTs. Reproduced from Ref. [70] .

19.4 Carbon Nanotubes as Adsorbents 631
Table 19.3 Summary of copper adsorption capacities ( q
m
) of
CNT s at different temperatures in terms of the adsorbed
mass of Cu(II) in solution (mg) per mass of nanotube (g).
Reproduced from Ref. [70] .
Temperature ( ° C)
Adsorption capacity ( q
m
) (mg g
− 1
)
As - produced CNTs HNO
3
- modifi ed CNTs NaOCl - modifi ed CNTs
7 6.39 12.46 44.64
17 7.87 13.10 45.87
27 8.25 13.87 47.39
37 9.34 15.11 49.02
47 10.17 16.04 51.81
chemical treatment that renders the CNTs more hydrophilic, and thus more effec-
tive in adsorbing Zn(II).
Lu and coworkers showed that the adsorption capacity of Zn(II) onto CNTs
increased in line with a rising pH of the solution over the range 1 to 8, was
maximal at pH 8 to 11, and then decreased at pH 12. A comparative study on the
adsorption of Zn(II) between CNTs and commercially available, powdered acti-
vated carbon, was also conducted. The maximum adsorption capacities for Zn(II)
observed were 43.66, 32.68, and 13.04 mg g
− 1
with SWNTs, MWNTs, and activated
carbon, respectively. The short contact time required to reach equilibrium, as well
as the high adsorption capacity, suggests that both SWNTs and MWNTs possess
a high potential for the removal of Zn(II) from water. The same group also sug-
gested that the higher adsorption capacity observed for SWNTs over MWNTs
might be due to the higher surface area observed for SWNTs (423 m
2
g
− 1
compared
to 297 m
2
g
− 1
observed for MWNTs), and also to the larger proportion of defects
present on the MWNTs (as observed using Raman spectroscopy).
However, an interesting result was observed for the case of activated carbon.
Although the surface areas of purifi ed SWNTs and MWNTs were much lower than
that of activated carbon, the adsorption capacities of Zn(II) onto purifi ed SWNTs
and MWNTs were much higher than was observed for activated carbon. This
superior adsorption capacity was attributed to a larger number of hydrophilic
groups present in the CNT walls.
Another study which focused on the adsorption kinetics and equilibrium of
Zn(II) adsorbed onto CNTs nanotubes has also been reported [73] . This thermo-
dynamic analysis revealed that the sorption of Zn(II) onto CNTs was endothermic
and spontaneous, and that the Zn(II) ions could easily be removed from the
surface sites of SWNTs and MWNTs by the action of a 0.1 M nitric acid solution.
Moreover, the original adsorption capacity was maintained after 10 cycles of this
sorption – desorption process. Such data suggest that both types of CNT material
could be reused through many cycles of water treatment and regeneration.

632 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
19.4.1.6 Adsorption of Nickel ( II )
Nickel is a toxic metal ion that is present in wastewaters. More than 40% of all
nickel produced is used in steel factories, in nickel batteries, and in the production
of some alloys, which leads to an increase in the Ni(II) burden on the ecosystem
and a deterioration in water quality. If ingested, Ni (II) is harmful, and may cause
vomiting, chest pain, and a shortness of breath [74] .
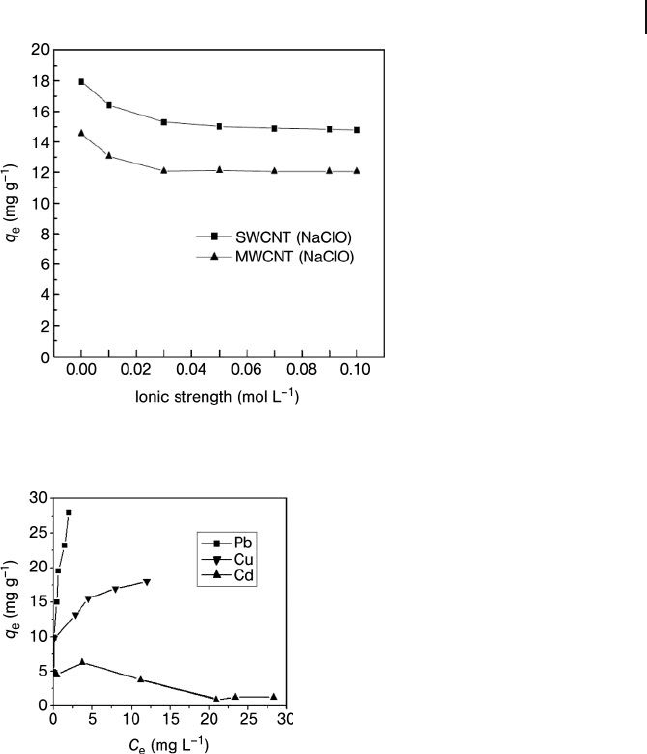
Lu et al. [75] have analyzed the effect of CNT mass, agitation speed, initial Ni(II)
concentration, and solution ionic strength on the Ni(II) adsorption capacity of
CNTs. The effects of agitation speed and solution ionic strength on Ni(II) sorption
by oxidized CNTs are shown in Figures 19.12 and 19.13 . The adsorption capacity
of both SWNTs and MWNTs was shown to increase as the agitation speed
increased, but to decrease when the ionic strength of the solution increased. The
same group also reported that SWNTs showed a better performance for Ni(II)
adsorption than did MWNTs. A similar conclusion was reached by Chen et al . [58] ,
when studying the adsorption of Ni(II) onto oxidized MWNTs as a function of
contact time, pH, ionic strength, MWNT amount, and temperature. The results
showed that Ni(II) adsorption onto MWNTs was heavily dependent on the pH and,
to a lesser extent, the ionic strength. Kinetic data showed the adsorption process
to achieve equilibrium within 40 min, and that the process followed a pseudo
second - order rate equation. The adsorption data fi tted the Langmuir model and,
together with thermodynamic data, indicated the spontaneous and endothermic
nature of the process. The results of a desorption study showed that Ni(II) adsorbed
onto oxidized MWNTs could easily be desorbed at pH < 2. The authors proposed
that ion exchange might be the predominant mechanism for Ni(II) adsorption on
oxidized MWNTs.
Figure 19.12 Effect of agitation speed on Ni(II) adsorption by
oxidized CNTs. Reproduced from Ref. [75] .
19.4 Carbon Nanotubes as Adsorbents 633
19.4.1.7 Competitive Adsorption of Heavy Metals Ions
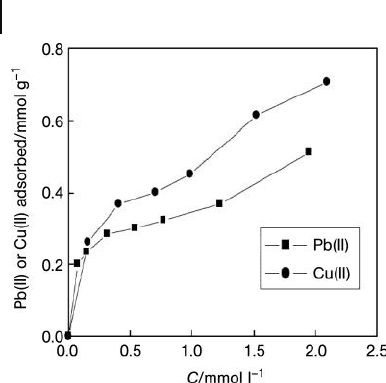
Whilst most reports on heavy metal adsorption using CNTs has focused on a single
metal ion, Li and colleagues [76] were the fi rst to conduct a study on the competitive
adsorption of Pb(II), Cu(II) and Cd(II) onto HNO
3
- treated MWNTs. These studies
showed the affi nity order of these metal ions towards CNTs to vary in the order:
Pb(II) > Cu(II) > Cd(II) (Figure 19.14 ). The Langmuir adsorption model repre-
sented the experimental data for Pb(II) and Cu(II) well, but did not provide a good
fi t for the adsorption data of Cd(II). It was also observed that, at a low pH, the
adsorption percentages were negligible, but that at pH values between 1.8 and 6.0
the proportions of Pb(II) and Cu(II) increased sharply, almost attaining values of
100%, while only a small increase was noted for Cd(II).
Figure 19.13 Effect of solution ionic strength on Ni(II)
adsorption by oxidized CNTs. Reproduced from Ref. [75] .
Figure 19.14 Competitive adsorption data for three ions of
Pb(II), Cu(II) and Cd(II) onto CNTs at room temperature and
pH 5.0. Reproduced from Ref. [76] .
634 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
Another study, conducted by Hsieh et al . [77] , focused on the competitive adsorp-
tion of Pb(II), Cu(II) and Cd(II) by CNTs grown on microsized Al
2
O
3
particles.
The authors noted that the adsorption behavior of these metal ions on CNTs on
Al
2
O
3
particles followed Langmuir ’ s adsorption model, with observed adsorption
capacities on the CNTs for Pb(II), Cu(II) and Cd(II) of 32, 18, and 8 mg g
− 1
, respec-
tively. These results confi rmed that CNTs supported on Al
2
O
3
particles showed
potential for the removal of soluble heavy metals from aqueous solutions.
Peng and collaborators [78] conducted a similar study to determine the adsorp-
tion capacity of Pb(II) and Cu(II) on CNT – iron oxide composites. These ferric
composites offer the advantage of a continuous contaminant adsorption from a
liquid effl uent whilst, when adsorption is complete, the adsorbent can be separated
from the liquid phase simply by using a magnet. Both, X - ray diffraction ( XRD )
and SEM studies indicated the presence of an entangled network of CNTs with
attached iron oxide nanoclusters. The adsorption isotherms obtained for Pb(II)
and Cu(II) adsorbed onto these magnetic composites are shown in Figure 19.15 .
The maximum adsorption capacities for Pb(II) and Cu(II) in the concentration
range studied were 105.67 and 45.12 mg g
− 1
. After adsorption, a magnetic separa-
tion process was carried out using a permanent magnet, providing a 98% recovery
of the Pb(II) and Cu(II) ion mass adsorbed.
19.4.2
Adsorption of Other Inorganic Elements
In addition to heavy metal ions, other common inorganic pollutants in drinking
water include ionic forms of fl uoride, arsenate, and americium - 243(III). Although
fl uoride is added via drinking water, it is often present in surface waters due to
Figure 19.15 Adsorption data for Pb(II) and Cu(II) onto
magnetic CNTs composites (pH = 5.0, T = 20 ° C). Reproduced
from Ref. [78] .
19.4 Carbon Nanotubes as Adsorbents 635
natural erosion or to discharges from fertilizers and aluminum factories. The
ingestion of fl uoride can cause bone disease and mottled teeth in children. Arse-
nate is occasionally present due to erosion of natural deposits, or it may be released
into surface waters via the runoffs from orchards or from glass and electronics
production wastes. Arsenate ingestion causes skin damage and circulatory prob-
lems; it is also a well - known carcinogen [2] . Americium - 243(III) contributes sig-
nifi cantly to the radiotoxicity of nuclear waste, and may be released into the
environment during nuclear waste storage, processing, or disposal. Exposure to
small traces of americium - 243(III) increases the risk of cancer.
19.4.2.1 Adsorption of Fluoride
The acceptable fl uoride concentration in drinking water is generally in the range
of 0.5 to 1.5 mg l
− 1
[79] . Higher concentrations will affect the metabolism of
calcium and phosphorus in the human body, and lead to dental and bone fl uorosis
[80] . Many methods have been adopted to remove excess fl uoride from drinking
water, the most common approach being adsorption with activated alumina, which
has a good adsorption capacity and selectivity for fl uoride. Unfortunately, the
optimum capacity for fl uoride removal in alumina occurs only at pH values below
6.0, which strongly limits the practical applications of this material [81] .
Li and collaborators [52] have reported that amorphous Al
2
O
3
supported on CNTs
represents a major candidate for fl uoride adsorption from water. These authors
used CNTs as a supports for Al
2
O
3
, and showed the composite to have a high poten-
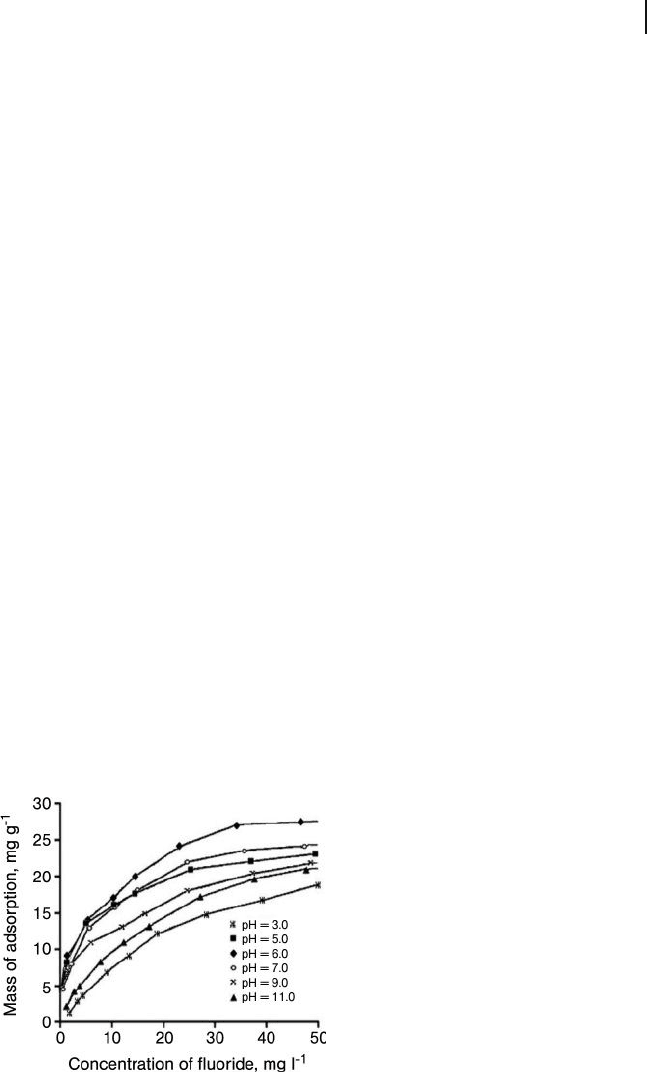
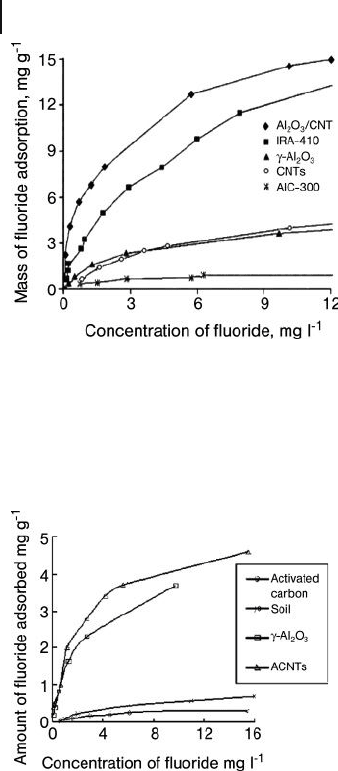
tial for removing fl uoride from drinking water. Based on their adsorption isotherms
(Figures 19.16 and 19.17 ), Li and coworkers found that Al
2
O
3
/CNT composites
showed a high fl uoride adsorption capacity over a pH range from 5.0 to 9.0. They
also found the adsorption capacity for the Al
2
O
3
/CNT composite to be about 13.5 -
fold higher than that of activated carbon, fourfold higher than for γ - alumina, and
also higher than that of the commercial polymeric resin, IRA - 410. This broad range
of pH values and high adsorption capacities observed for the Al
2
O
3
/CNT composite
makes this material very attractive for fl uoride removal from water.
Figure 19.16 Effect of solution pH on fl uoride adsorption
onto an Al
2
O
3
/CNT composite. Reproduced from Ref. [52] .
636 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
A year later, the same group presented improved results using an array of
aligned MWNTs [10] . For this, the authors studied the kinetics of the fl uoride
adsorption process, and the effect of pH on fl uoride adsorption capacity. The
kinetic data indicated that the fl uoride adsorption rate was rapid during the fi rst
60 min, and quickly reached an adsorption capacity of 3.0 mg g
− 1
, with equilibrium
achieved after 180 min. A mild dependence of the adsorption capacity on the pH
of the solution was also observed, with the highest adsorption capacity being
observed at pH 7, and reaching 4.5 mg g
− 1
at fl uoride concentrations of 15 mg l
− 1
.
The adsorption isotherms obtained for this material, compared to those obtained
in γ - Al
2
O
3
, activated carbon, and a soil, are shown in Figure 19.18 . At low fl uoride
Figure 19.17 Adsorption isotherms of fl uoride on activated
carbon (AlC - 300), CNT, γ - Al
2
O
3
, a commercial resin (IRA - 410)
and Al
2
O
3
/CNT composite. The data for activated carbon and
IRA - 410 were fi tted with a Langmuir isotherm, while the data
for CNT and Al
2
O
3
/CNTs were fi tted with a Freundlich
isotherm. Reproduced from Ref. [52] .
Figure 19.18 Adsorption isotherm of fl uoride on CNTs (at pH
7 and 25 ° C) compared with γ - Al
2
O
3
, soil, and activated
carbon. Reproduced from Ref. [10] .
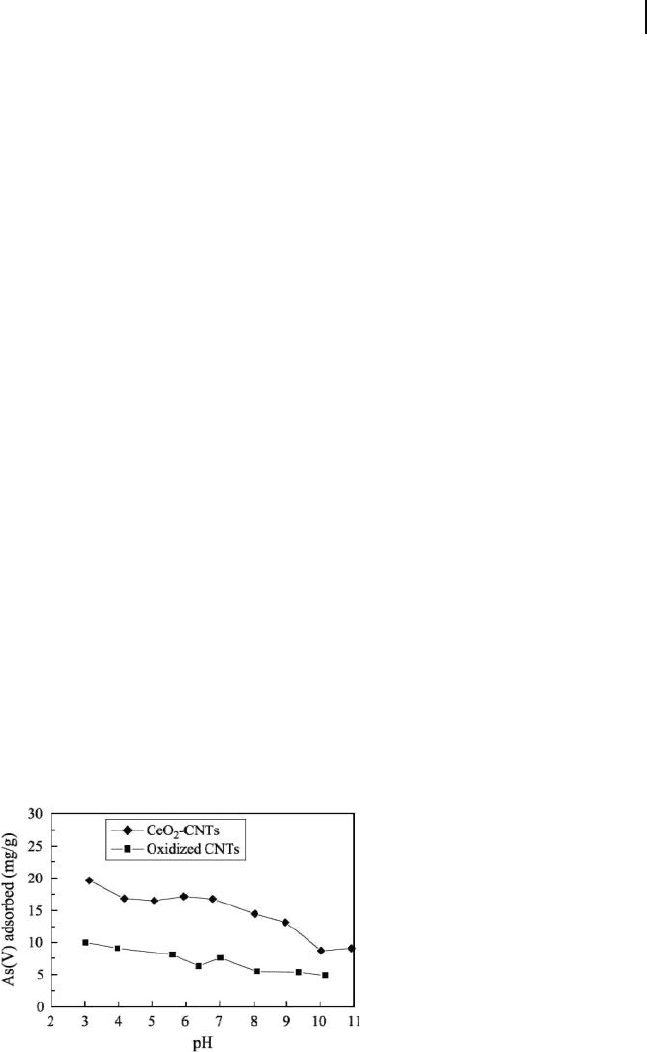
19.4 Carbon Nanotubes as Adsorbents 637
initial concentrations ( < 1 mg l
− 1
), the nanotube material and alumina had the same
adsorption capacities, but at higher fl uoride concentrations the fl uoride adsorption
capacity of the CNTs was higher. These results again indicated the potential of
CNTs in fl uoride removal.
19.4.2.2 Adsorption of Arsenic
Arsenic is required as a micronutrient for the human body, yet it may be carcino-
genic if consumed constantly. Thus, it is of great importance to remove arsenic
from water before it is used for drinking. Two main forms of arsenic are encoun-
tered in natural water, namely trivalent (As(III), arsenite) and the higher - oxidized
form, pentavalent (As(V), arsenate). Whilst either of these species can be found in
natural waters, As(III) is more common in groundwater, and As(V) is more
common in surface water [82] .
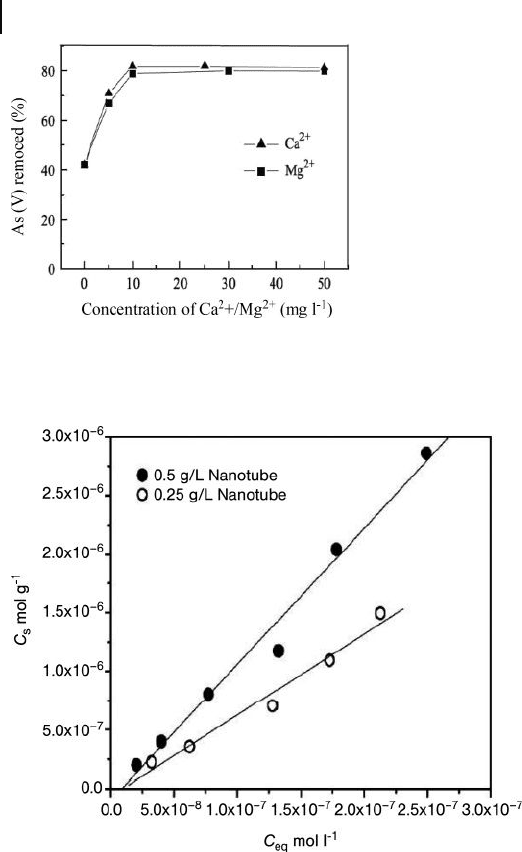
When a novel ceria – CNT composite was proposed as an alternative for the
removal of arsenate from water [83] , the results indicated that arsenate adsorption
on these materials was pH - dependent. The presence of Ca(II) and Mg(II) also
signifi cantly enhanced the adsorption capacity. These very promising results
suggest that these materials might represent a promising adsorbent for drinking
water purifi cation. The effect of pH on the adsorption of As(V) onto CeO
2
/CNT is
shown in Figure 19.19 .
These results indicate that the adsorption of As(V) is pH - dependent, and that
the pristine composite has a higher adsorption capacity than the chemically modi-
fi ed nanotubes. The authors proposed that the dependence of adsorption on pH
was due to variations in the surface charge on the nanotube composites. This was
corroborated by zeta potential measurements. Moreover, the authors also meas-
ured the infl uence of Ca(II) and Mg(II) on the adsorption capability of the CeO
2
/
CNT material. From the results shown in Figure 19.20 it is clear that both Ca(II)
and Mg(II) signifi cantly enhance the adsorption capacity of the nanotube compos-
ite. In fact, an increase from 0 to 10 mg l
− 1
in the concentration of Ca(II) and Mg(II)
resulted in an almost one order of magnitude increase in the amount of As(V)
Figure 19.19 Effect of pH on As(V) adsorption by chemically
treated CNTs (
䊏
) and CeO
2
/CNT composite (
䉬
). Reproduced
from Ref. [83] .
638 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
adsorbed. The authors explained this observed increase in adsorption capacity by
proposing the formation of a ternary surface complex between calcium or magne-
sium, arsenate, and the ceria surface [84] .
19.4.2.3 Adsorption of Americium - 243 ( III )
Wang and collaborators [85] examined the use of MWNTs as adsorbents for the
radionuclide americium - 243 (III). The data obtained (see Figure 19.21 ) indicated
Figure 19.20 Effect of Ca(II) and Mg(II) on As(V) adsorption
(initial concentration of As(V) = 20 mg l
− 1
). Reproduced from
Ref. [83] .
Figure 19.21 Adsorption isotherms for
243
Am(III) onto
MWNTs in polyethylene tubes using Milli - Q water in the
presence of 0.1 M NaClO. The contact time was 4 days.
Reproduced from Ref. [85] .
