Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

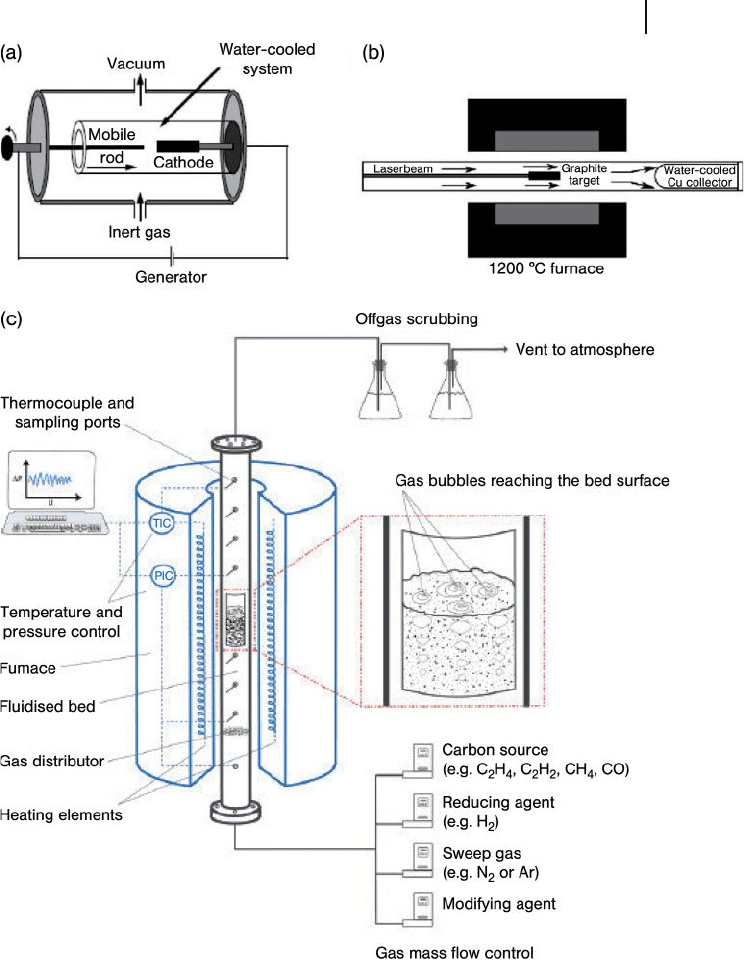
19.2 Structure and Synthesis of Carbon Nanotubes 619
Figure 19.3 Schematic representations of various processes
used to produce CNTs. (a) Electric - arc method apparatus.
Reproduced from Ref. [22] ; (b) Schematic representation of an
oven laser - vaporization apparatus. Reproduced from Ref. [26] ;
(c) A chemical vapor deposition set - up in which the catalytic
bed is fl uidized. Reproduced from C.H. See, A.T. Harris, Ind.
Eng. Chem. Res. (2007), 46 , 997 – 1012.

620 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
19.3
Properties of Carbon Nanotubes
As described above, CNTs have unique structures, with remarkable properties that
can be grouped as electronic, mechanical, thermal, and optical. Most of the phys-
ico - mechanical properties of CNTs are dependent on the sp
2
bond network present
on their structure [36] and their diameter, length, and chirality. As these properties
have been discussed extensively elsewhere [37 – 39] , some of the most important
results in this area will be presented briefl y, with emphasis placed on those proper-
ties that affect the adsorption capacity of these materials.
19.3.1
Mechanical, Thermal, Electrical, and Optical Properties of Carbon Nanotubes
The mechanical properties of a solid must ultimately depend on the strength of
its interatomic bonds. Both, experimental and theoretical, results have predicted
that CNTs have the highest Young ’ s modulus of all different types of nanostruc-
tures, with similar tubular forms such as BN, BC
3
, BC
2
N, C
3
N
4
, CN, and so on.
Furthermore, due to the high in - plane tensile strength of graphite, both single and
multiwalled CNTs, are expected to have large bending constants. The results of
experimental and theoretical studies have indeed indicated that CNTs can be very
fl exible, able to elongate, twist, fl atten, or bend into circles, before fracturing [37] .
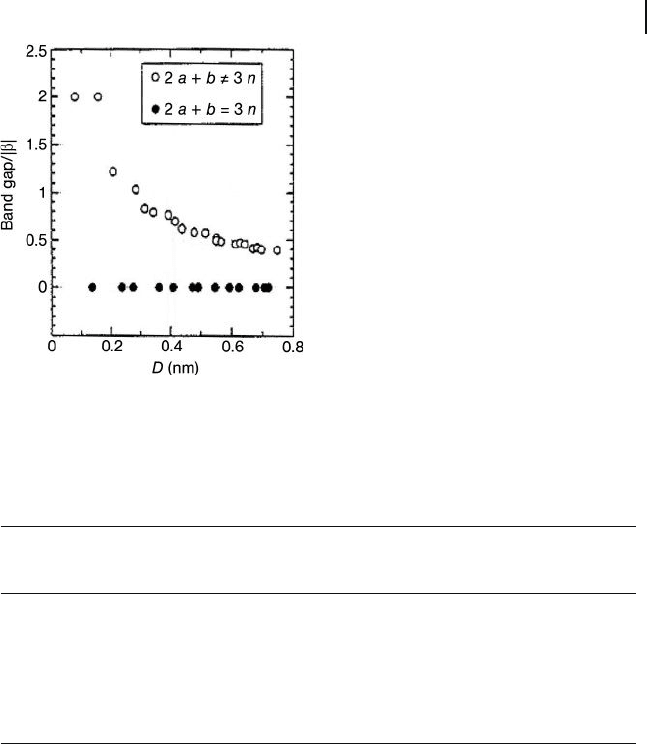
The thermal and electrical properties of CNTs include conductivity. The specifi c
heat and thermal conductivity are determined primarily by the nanotube ’ s elec-
tronic and phononic structures [38] , with theoretical and experimental results
demonstrating superior electrical properties for these materials. Carbon nano-
tubes have electric current - carrying capacities which are 1000 - fold higher than that
of copper wires [40] . In fact, theoretical calculations based on the tight - binding
model approximation within the zone folding scheme show that one - third of the
possible SWNT structures are metallic, while two thirds are semi - conducting
(Figure 19.4 ) [37, 41] .
19.3.2
Adsorption - Related Properties of Carbon Nanotubes
Early studies investigating the adsorption of nitrogen onto both MWNTs [42, 43]
and SWNTs [44] have highlighted the porous nature of these materials. Indeed,
due to their uniformity in size and surface properties, CNTs are considered as
ideal model sorbent systems to study the effect of nanopore size and surface mor-
phology on sorption and transport properties.
The surface area of a CNT has a very broad range, depending on the nanotube
number of walls, the diameter, length, wall defects and, in the case of SWNTs, the
number of nanotubes in a nanotube bundle [45] . An isolated SWNT with an open
end (this may be achieved through oxidation treatment) has a theoretical surface
area equal to that of a single, fl at graphene sheet of 2700 m
2
g
− 1
[46] ; however,
reported experimental values indicate lower adsorption capacities [47] . In the case
19.3 Properties of Carbon Nanotubes 621
Table 19.1 Adsorption properties and type of adsorption sites in SWNT s and MWNT s .
Reproduced from Ref. [49] .
Type of nanotube
Porosity (cm
3
g
− 1
)
Surface area
(m
2
g
− 1
)
Adsorption site Surface area per site
(m
2
g
− 1
)
SWNT bundle Microporous
Vmicro: 0.15 – 0.3
400 – 900 Surface groove 483
Pore 783
Interstitial 45
MWNT Mesoporous 200 – 400 Surface pore;
aggregated pores
–
V
micro
= micropore volume.
of SWNTs, the diameters of the tubes and number of tubes in the bundle have
the strongest effects on the nanotube surface area. In the case of MWNTs, chemi-
cal treatments are reported to be useful for promoting microporosity. Surface areas
as high as 1050 m
2
g
− 1
have been reported for MWNT subjected to alkaline treat-
ment [48] . A two - step activation treatment (acid + CO
2
activation) has been also
reported to increase the specifi c surface area of MWNT materials. It has been
proposed that these treatments open the ends of the nanotube structure, enabling
adsorption onto the nanotube inner openings [49] . Some representative results of
the surface area and pore volume of SWNTs and MWNTs are listed in Table 19.1 .
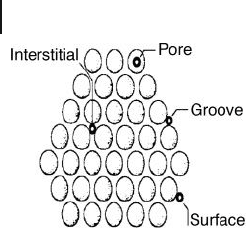
An important issue to address when considering adsorption onto nanotubes is
to identify the adsorption sites. For instance, the adsorption of gases into a SWNT
bundle can occur inside the nanotubes (pore), in the interstitial triangular chan-
nels between the tubes, on the outer surface of the bundle, or in the grooves
formed at the contacts between adjacent tubes outside of the bundle (Figure 19.5 ).
Figure 19.4 Band - gap values versus nanotube diameters
defi ning SWNTs as metallic (
䊉
) or semi - conducting (
䊊
).
Reproduced from Ref. [41] .
622 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
In addition, the surface functionalization of CNTs by chemical methods has been
found to be a powerful tool for improving the adsorption capacity. Selective adsorp-
tion can be achieved through a controlled modifi cation of the nanotube ’ s physical
and chemical properties, such as surface area, hydrophilicity, and permeability.
For instance, Vermisoglou and Georgakilas [50] have studied the sorption proper-
ties of pristine and chemically modifi ed [functionalized with oleylamine and
poly(sodium 4 - styrene sulfonate)] nanotubes using adsorbates with different polar-
ities. Based on their measurements, these authors concluded that the sorption
behavior of the CNTs was greatly modifi ed by chemical treatment. In fact, a chemi-
cal modifi cation that increased the hydrophilicity of the nanotube walls enhanced
the adsorption selectivity for water over n - hexane. Chemical modifi cation of the
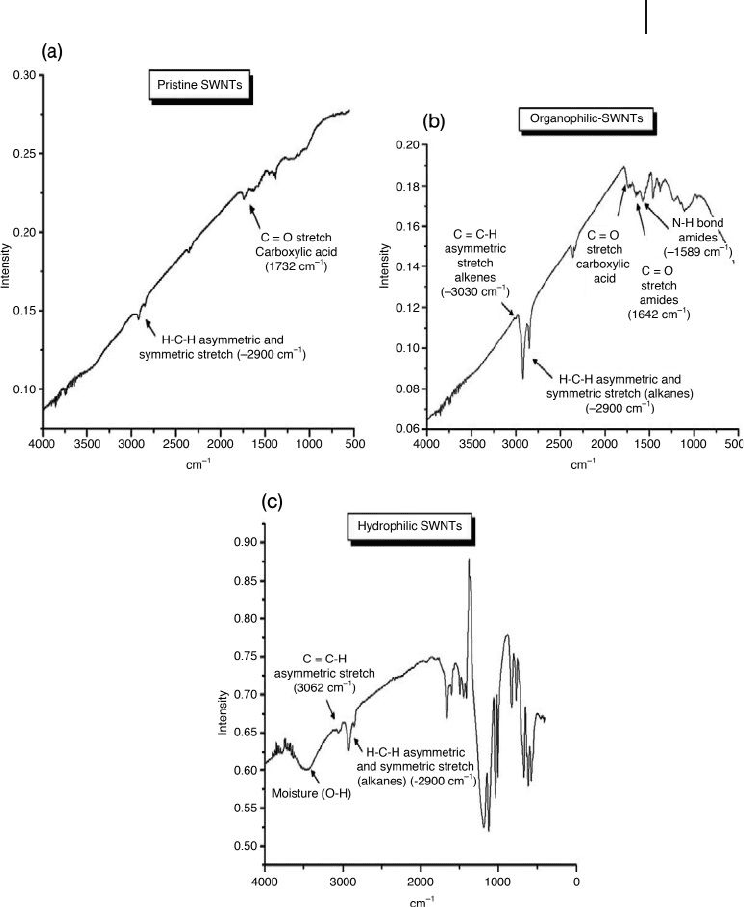
nanotube wall was verifi ed using infrared ( IR ) spectroscopy (Figure 19.6 ). When
comparing the IR spectra of pristine SWNTs with those of chemically treated
CNTs, new peaks corresponding to aliphatic chains were observed in the case of
hydrophobic nanotubes, whereas peaks corresponding to more polar bonds were
observed in the case of hydrophilic materials.
Yu et al. [51] have investigated the adsorptive performance on modifi ed MWNTs
by using mechanical ball milling. For these materials, the adsorptive performance
for aniline in aqueous solution indicated that the adsorptive capacity of milled,
short open - ended MWNTs increased from 15 mg g
− 1
to 36 mg g
− 1
compared to the
unmilled MWNTs. The measurements of pore size distribution proved that the
inner pore diameter of 3 nm remained constant after milling, but the aggregated
pore diameter had decreased.
19.4
Carbon Nanotubes as Adsorbents
Carbon nanotubes have superior capabilities for the adsorption of a wide range of
toxic substances. The earliest reports of CNT use related to the removal of organic
pollutants, notably dioxins, from water [8] , though later they were reported also as
Figure 19.5 Sketch of the cross - sectional view of a SWNT
bundle, illustrating the four different adsorption sites.
Reproduced from B. Bhushan, Springer Handbook of
Nanotechnology , 2nd revision, Springer, Berlin, New York
(2007).
19.4 Carbon Nanotubes as Adsorbents 623
Figure 19.6 Infrared spectra of (a) pristine and (b,c) modifi ed
CNTs, illustrating the characteristic peaks for hydrophilic
(b) and organophilic (c) samples. Reproduced from Ref. [51] .
having an exceptional ability to adsorb inorganic contaminants, such as fl uoride
[52] . In both cases, the CNTs displayed a superior performance compared to “ tra-
ditional ” adsorbents such as activated carbon. These pioneering studies opened a
new fi eld of CNT applications, with many subsequent reports noting CNTs to be
excellent adsorbents for the removal of other contaminants. For example, CNTs

624 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
were shown to adsorb up to 30 mg of a trihalomethane molecule per gram from
a 20 mg l
− 1
solution [11] . Other reports indicated that SWNTs could act as “ molecu-
lar sponges ” for small organic molecules such as CCl
4
[53] . A similar case was
demonstrated for inorganic contaminants, with CNTs again showing superior
performance; measurements of the adsorption capacity of a MWNT material
showed that it could adsorb 13.5 - fold more fl uoride than a typical high - surface - area
alumina adsorbent (see below). These early results led to the suggestion that CNTs
might indeed serve as effective adsorbents for removing polluting agents from
water. Consequently during the past few years some extensive laboratory studies
have established the role of CNTs as effective adsorbents for common contami-
nants from water, including a wide variety of organic compounds and inorganic
ions. Although a large number of studies have been conducted into the use of
CNTs as adsorbents of gas contaminants [54 – 56] , this chapter will focus on the
removal of contaminants from surface water (the process being complementary
to the role of adsorbates in the gas phase). Consequently, below is presented a
discussion of recent results demonstrating the huge potential of CNTs for the
removal of contaminants from surface water.
19.4.1
Adsorption of Heavy Metal Ions
The effects of heavy metals such as lead, copper, zinc, nickel, and chromium on
human health have been widely studied, based on fi ndings that the ingestion of
some of these species can cause accumulative poisoning, cancer, and nervous
system damage [57] . A variety of technologies exist for the removal of heavy metals,
including fi ltration, surface complexation, chemical precipitation, ion exchange,
adsorption, electrode deposition, and membrane processing [58] . Among these
procedures, adsorption is considered one of the most attractive processes for heavy
metal removal from solution, as the adsorbents are generally easier to handle and
provide a greater operating fl exibility [59] . Recent increasingly stringent standards
for the quality of drinking water have also catalyzed a growing effort in the devel-
opment of new, highly effi cient adsorbents. The high surface area and chemical
stability of CNTs offer exciting possibilities for a new generation of adsorbents;
hence, some recently acquired data relating to the adsorption of a wide variety of
water contaminants are reviewed below. Here, emphasis is placed on the CNT
processing method, the adsorption capacities observed, and the prospects for their
successful application.
19.4.1.1 Adsorption of Lead ( II )
Li and collaborators [9] were the fi rst to report experimental data on lead adsorp-
tion from water using CNTs. Lead is ubiquitous in the environment, and its inges-
tion is extremely hazardous; the consumption of drinking water containing high
levels of lead causes serious disorders, including anemia, kidney disease, and
mental retardation [60] . Li and colleagues have shown CNTs to show exceptional
adsorption capacities and a high adsorption effi ciency of Pb(II) removal from
19.4 Carbon Nanotubes as Adsorbents 625
water. The CNTs used in these investigations were pretreated with nitric acid in
order to increase the adsorption capacities and to remove most of the catalyst
particles within the raw material. The amount of Pb(II) adsorbed onto the CNTs
was determined by the difference between the initial Pb(II) concentration and the
equilibrium Pb(II) concentration of the solution. The authors also monitored the
effect of varying the pH of the solution on lead adsorption.
The acid treatment was seen to have a major impact on the nanotubes ’ adsorp-
tion capacities. For example, whereas pristine as - grown CNTs had an adsorption
capacity for Pb(II) of 1 mg g
− 1
at pH 5, the capacity was increased remarkably (to
15.6 mg g
− 1
) when the CNTs were refl uxed with concentrated nitric acid, again at
pH 5. It appears that acid oxidation of the CNTs leads to the introduction of many
functional groups, such as hydroxyl, carboxyl, and carbonyl onto the CNT surface
[61] , which in turn leads to improved adsorption capacity. Li also reported that the
removal of Pb(II) from water by acid - refl uxed CNTs was highly dependent on the
solution pH, as this affects the surface charge of the adsorbents and the degree of
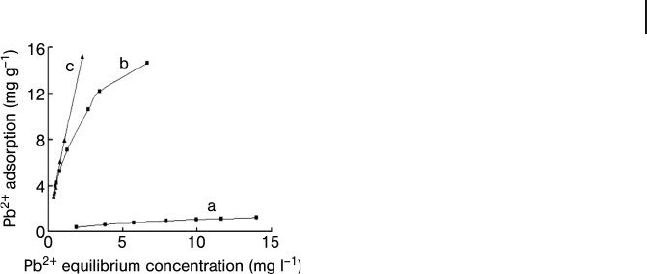
ionization and speciation of the adsorbates. The data in Figure 19.7 show that the
Pb(II) adsorption capacity of the CNTs was increased as the pH value was increased
from 3.0 to 7.0. It was proposed that, at low pH values, the adsorption of Pb(II)
was very weak due to the competition of H
+
with Pb(II) species for the adsorption
sites (Figure 19.7 , curve a). It was also proposed that, at pH 5, the adsorption
capability had increased due to role of functional groups present on the nanotube
surface (Figure 19.7 , curve b), and was further increased at pH 7 (Figure 19.7 ,
curve c). This might be the result of a combined effect of adsorption and a change
in the speciation of the lead ions. The results of other experiments have indicated
that, at pH 5 and room temperature, the amount of Pb(II) adsorbed onto the acid -
refl uxed CNTs increased rapidly during the fi rst 8 min (16.4 mg g
− 1
adsorbent,
81.6% removal), with equilibrium being reached after 40 min (17.5 mg g
− 1
, 87.8%
removal).
More recently, the same group [62] reported the adsorption thermodynamics
and kinetics of Pb(II) adsorption on CNTs, by evaluating various thermodynamic
parameters and employing a pseudo second - order kinetic model to describe the
Figure 19.7 Isotherms for Pb(II) adsorption by acid - refl uxed
CNTs at different pH values. Curve a, pH = 3.0; curve b,
pH = 5.0; curve c, pH = 7.0. Reproduced from Ref. [9] .
626 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
adsorption processes. Based on their results, the authors concluded that the
adsorption of Pb(II) onto CNTs was endothermic; they also suggested that the
CNT material not only possessed a larger adsorption capacity but also showed a
good desorption rate. Such benefi ts could result in a signifi cant reduction in the
overall costs for adsorbent recycling. Desorption studies also revealed that Pb(II)
could be easily removed from the CNTs by altering the pH values of the solution
using both HCl and HNO
3
.
The results of another series of studies has further underlined the infl uence of
the morphologies of CNTs on the removal of lead, in terms of specifi c surface
area, particle size distribution, and type of functional group introduced to the CNT
wall [63] . The specifi c surface area and pore volume of CNTs exposed to four dif-
ferent types of oxidation treatments were compared, and the pore and particle size
distributions of the CNTs evaluated. The results (as summarized in Table 19.2 )
indicated that the CNTs with the highest surface area, smallest particle size and
with a relative larger number of functional groups attached to their walls, showed
a maximum adsorption capability of 82.6 mg g
− 1
from a solution with an initial lead
concentration of 10 mg l
− 1
. Under the same conditions, the adsorption capacities
of samples with a lower number of functional groups attached, and with larger
wall defects, achieved adsorption capacities of only approximately 10 mg g
− 1
.
19.4.1.2 Adsorption of Chromium ( VI )
Chromium (VI) is one of the most toxic metals found in various industrial waste-
waters. Public health considerations of chromium are mostly related to Cr(VI)
compounds that are strong irritants due to their high solubility and diffusivity in
tissue; certain Cr(VI) compounds have also been shown to be carcinogenic and
mutagenic. The toxic effects of Cr(VI) ions in humans include liver damage, inter-
nal hemorrhages, respiratory disorders, dermatitis, skin ulceration, and chromo-
some aberrations [64] .
Di and collaborators [65] have investigated the suitability of CNTs for Cr(VI) (as
dichromate oxoanion) adsorption, and compared their behavior to that of activated
Table 19.2 Summary of results for lead adsorption in terms of
nanotube surface area, pore specifi c volume, pore size
distribution, and particle size distribution. Adapted from
Ref. [63] .
S
BET
(m
2
g
− 1
) V
p
(cm
3
g
− 1
) D
p
(cm
3
g
− 1
)
S
p
( μ m)
Sample 1 47 0.18 3.4 30 and 570
Sample 2 62 0.26 2.4 and 3.2 23 and 450
Sample 3 154 0.58 3.6 8 and 55
Sample 4 145 0.54 3.6 19 and 70
S
BET
(m
2
g
− 1
) = BET surface area; V
p
(cm
3
g
− 1
) = pore specifi c volume; D
p
(cm
3
g
− 1
) = mean pore
diameter: S
p
( μ m) = particle size.
19.4 Carbon Nanotubes as Adsorbents 627
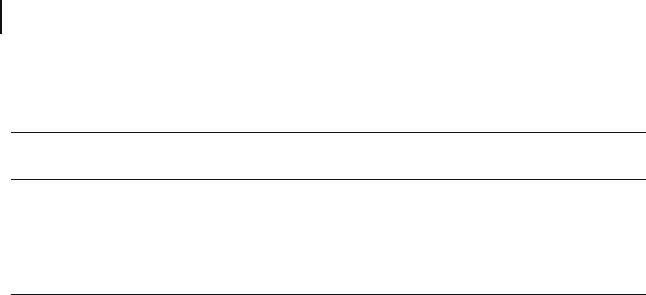
Figure 19.8 Adsorption of Cr(VI) ions onto CNTs (
䉬
) and
activated carbon (
䉱
) as a function of pH. Cr(VI) ion
concentration = 5 mg g
− 1
CNT; carbon nanotube
concentration = 1 g l
− 1
; contact time = 12 h. Reproduced from
Ref. [65] .
carbon. In these studies, following pretreatment of both as - prepared CNTs and
activated carbon with nitric acid and hydrofl uoric acid, the CNTs were shown to
have superior adsorption capabilities and effi ciencies for the removal of Cr(VI)
ions from water over the pH range 4.0 to 7.5 (see Figure 19.8 ). Activated carbon
was seen to adsorb chromate ions very rapidly at low pH values, but the adsorption
capacity declined very sharply at pH 3.5. In contrast, the adsorption effi ciency of
the CNTs was maintained at over 90% over a wide pH range, although the Cr(VI)
ion adsorption capacity of CNTs fell sharply at pH 8. The authors attributed this
behavior to the infl uence of the nanotube ’ s zeta potential. Subsequently, it was
shown experimentally that the isoelectric point of the CNT material was 7.7; at
higher pH values the surfaces of the CNTs became negatively charged and thus
inaccessible to the chromate anions. The authors also proposed competition of the
hydroxyl ions for the few adsorption sites available at these pH values. The data
in Figure 19.8 indicate that the highest Cr(VI) ion adsorption capacity was observed
over the pH range 4.0 to 7.5. At pH ≤ 3, the CNTs and activated carbon showed a
similar adsorption capacity, but at a higher pH the capacity of the CNTs was
greater. The maximum CNT adsorption capacity (20.56 mg g
− 1
) occurred at pH 7.5,
when the Cr(VI) concentration was 33.28 mg l
− 1
. The kinetic curves showed the
adsorption rate of Cr(VI) ions to be relatively high over the fi rst 20 min, reaching
an adsorption capacity of 15 mg g
− 1
.
A few months later, improved values for chromium adsorption were reported
in a new study [66] . Here, a composite of aligned CNTs supported in ceria nano-
particles (CeO
2
/CNTs) was used as the adsorbent, the material being prepared by
the chemical reaction of CeCl
3
with NaOH in the presence of a CNT suspension,
followed by heat treatment. Scanning electron microscopy ( SEM ) images showed
628 19 Carbon Nanotubes as Adsorbents for the Removal of Surface Water Contaminants
that the CNT alignment was uniform, with lengths reaching 200 μ m and diameters
ranging from 20 to 80 nm. Transmission electron microscopy ( TEM ) analysis
indicated a homogeneous distribution on the ceria particles in the CNT network.
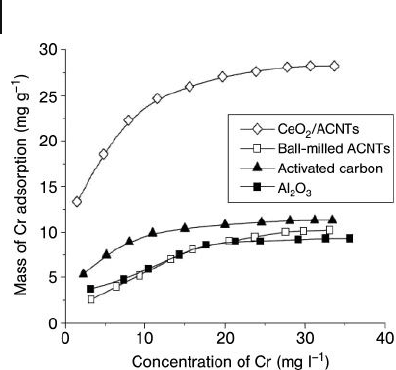
Overall, the study results indicated that highest capacity for Cr(VI) adsorption
occurred at pH values ranging from 3.0 to 7.4, with values of 30.3 mg Cr(VI) g
− 1
nanotube being observed at pH 7.0. The authors also compared the Cr(VI) adsorp-
tion isotherm obtained on the CeO
2
/CNT material with those obtained on activated
carbon and γ - Al
2
O
3
. As shown in Figure 19.9 , the adsorption capacity of the CeO
2
/
CNT material was 1.5 - fold higher than that observed for activated carbon, and
twofold larger than for Al
2
O
3
.
The authors proposed that the small size of the CeO
2
particles, and their uniform
distribution on the surface of the aligned nanotubes, contributed to the observed
high Cr(VI) adsorption. They also suggested that nanotube wall defects, produced
by the CVD synthesis process, could offer active sites for Cr(VI) adsorption on the
outer surfaces of the aligned nanotube array. The inner cavities and the opened
ends present in the inter - aligned nanotube space might also have contributed to
the effective adsorption of Cr(VI) ions.
19.4.1.3 Adsorption of Cadmium ( II )
Cadmium (II) represents a very high risk to human health due to its extremely
high toxicity, even in very small quantities. Drinking water with a cadmium content
in excess of permitted levels (0.005 mg l
− 1
) can cause nausea, salivation, diarrhea,
muscular cramps, renal degradation, lung insuffi ciency, bone lesions, cancer, and
hypertension [67] . Li et al. [68] have analyzed the suitability of CNT materials for
Cd(II) adsorption and its removal from water; the same group evaluated the effi cacy
of several chemical treatments using three different oxidizing agents, namely H
2
O
2
,
KMnO
4
, and HNO
3
. The chemically treated CNTs were shown to have a larger
Figure 19.9 Adsorption isotherms of Cr(VI) on CeO
2
/CNT
materials compared with activated carbon, Al
2
O
3
and
ball - milled CNTs (at pH 5.0 and 25 ° C). Reproduced from
Ref. [66] .
