Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


13.2 Polyolefi n-Based Nanocomposites 425
and are “ confi ned ” (and thus are not extractable, with a higher T
g
than pristine
PO and the starting composite). Here, the Gordon – Taylor equation was employed
to calculate the amounts of both polymer phases by considering the T
g
of the
polymer in the nanocomposite (as determined by the T
g
of the free polymer), and
of the confi ned polymer. This analysis provided results which, in terms of their
behavior, were in agreement with experimental data.
Interestingly, it was demonstrated that the unextractable polymer was respon-
sible for reductions in the oxygen permeability of LDPE and EPM/OLS nano-
composites prepared by using functionalized matrices [85] . In fact, by assuming
a negligible oxygen permeability through the polymer phase adsorbed onto
the surface or trapped inside the galleries of the OLS, these experimental
data were fi nely correlated with the theoretical data predicted from mathemati-
cal models.
Besides the nature of the functional groups, it was also clear that the amount
of functional groups grafted onto the PO could infl uence the fi nal morphology of
the PO/OLS nanocomposite. In fact, the hydrophilicity of the compatibilizer was
seen to increase with the grafting level of MAH, thus improving the level of inter-
action between the polymer and the clay. In the case of the most common PO - g -
MAH compatibilizers, the level of grafting ranged typically between 0.1 and 2 wt%
[50, 85, 92, 93] . In the case of MAH - functionalized PO, there was seen to be a limit
value for the grafting level (ca. 0.1 wt%), for achieving a good improvement in
morphology. In the case of both PE and PP/OLS nanocomposites, if the grafting
level was higher than this limit value, a shift or even a disappearance of the basal
refl ection peak of the OLS on the XRD diffraction pattern could be observed [51,
72, 77, 78, 92] .
In general, because of the low grafting percentage of polar groups onto the
common commercial compatibilizers, a large amount of functionalized polymer
is added to the polyolefi n matrix in order to achieve the necessary polarity and
hence compatibility with the organoclay. Besides the degree of functionalization
( DF ), the molecular weight and structure of the compatibilizer, as well as its melt
viscosity and rheological properties, are responsible for the composite fi nal mor-
phology [93, 94] . The best results are generally obtained by using a compatibilizer
for which the rheological properties are similar to those of the matrix; otherwise,
the miscibility between the two polymers might be compromised and the intercala-
tion/exfoliation process inhibited. A typical example is that of PP, for which a high
amount of compatibilizer is required to obtain an increase in the OLS basal
spacing, but this may cause a deterioration in the properties of the nanocomposite
due to the low molecular weight of the commonly used PP - g - MAH samples.
Unfortunately, functionalized PPs with high molecular weights are yet not avail-
able commercially. The conventional radical functionalization process with perox-
ide and MAH in the case of PP causes a dramatic decrease in the molecular weight
of the polymer, leading to severe damage of its rheological and mechanical proper-
ties. Notably, PP is very sensitive to degradation reactions when treated with
peroxides above its melting temperature, even in the presence of commonly used
maleate functionalizing agents [79 – 81, 94] .

426 13 Nanocomposites Based on Phyllosilicates
Consequently, conventional PP - g - MAH compatibilizers will in general have a
low DF and molecular weight. The problem of obtaining appropriately functional-
ized PP can be overcome by a new radical functionalization approach involving
the use of a furan derivative (BFA). This is added as coagent during the radical
functionalization of PP with MAH, which can yield a wide range of PP - g - MAH
samples with different DFs and molecular weights, by controlling the macroradical
formation and content [86, 87] . These new functionalized PP samples were recently
tested in the preparation of PP/OLS nanocomposites, both as a matrix or as a
compatibilizer of the system, the aim being to study the effects of both polarity
and chain structure/architecture on clay dispersion and the ultimate properties of
the corresponding nanocomposites. The results showed that PP - g - MAH with a
low molecular weight and a high DF ( > 2 mol%) had an excellent ability to disperse
clay at the nanometric level, especially when used as the matrix of the correspond-
ing nanocomposite. Those samples characterized by a high DF value ( > 2 mol%)
and a branched structure/architecture produced nanocomposites with a lower
degree of exfoliation. However, those nanocomposites with a composition of
90/5/5 PP/compatibilizer (the functionalized PP)/organomodifi ed layered silicate,
where the sample contained the compatibilizer characterized by a high DF; pre-
pared using a grafting procedure to avoid PP degradation reactions) provided the
best performance in terms of morphological and thermomechanical properties.
These results not only confi rmed the important role of the DF, but also highlighted
the fact that the control of molecular weight and structure/architecture during
functionalization ensures a good compatibility of the compatibilizer with the PP
matrix, which in turn has a positive effect on the ultimate properties of the PP/
OLS. In particular, elongation at break point (which usually are poor for similar
systems) reached values in excess of 500%, with an excellent reproducibility [88] .
13.2.3
The One - Step Process
In addition to a correctly selected OLS and compatibilizer, it is also necessary to
utilize an effective mixing protocol (e.g., processing parameters such as mean
residence time, screw speed, etc.) capable of promoting both high shear stress and
shear rate, in order to assist the OLS dispersion into a PO matrix [82, 95] . It has
been shown that the residence time and kneading force applied during extrusion
can have a direct effect on the extent of the layered silicate exfoliation [96] . In the
case of LDPE/OLS nanocomposites, a correct balance between dispersive and
distributive actions, by fi ne - tuning the screw profi le, allowed an optimal nanocom-
posite morphology [82] . A good dispersion of clay layers was assessed by preparing
a master - batch PE - g - MAH/OLS, which highlighted the importance of the shear
stress for dispersing, intercalating, and exfoliating lamellae when the correct inter-
face interactions have been provided. A further improvement in dispersion degree
was observed during a second processing step, as the melt viscosity of the two
polymer components was accurately selected with the aim of improving their
compatibility.

13.2 Polyolefi n-Based Nanocomposites 427
Besides the machine parameters, the method selected for feeding the
extruder – and therefore also the order of addition for components – can infl uence
the morphology of the fi nal material. In most reports, the best stack delamination
was observed when a master batch between the functionalized PO (compatibilizer)
and a large proportion of clay (10 – 50 wt%) was fi rst prepared, followed by dilution
in the nonpolar PO matrix (master batch process). The advantage of this method,
compared to a direct melt blending of all ingredients together, is that it facilitates
the intercalation of the polar polymer chains between the clay stacks, thus obtain-
ing a form of pre - intercalated material.
One rarely discussed aspect is the possibility of obtaining, in a single step, both
functionalization of the PO and nanocomposite formation. Such a method would
be especially interesting because its successful application would not only avoid
selecting the compatibilizer but also save a preparative step. In fact, as noted above,
selection of the best compatibilizer may require special care, as this must be suf-
fi ciently polar to allow interaction with the clay, but not so hydrophilic as to favor
phase separations. Not least, it should have similar rheological properties as the
matrix, in order to avoid any deterioration of the composite ’ s ultimate mechanical
properties.
Consequently, two different synthetic approaches were very recently compared
for the preparation of EPM and PP OLS nanocomposites [89] (Figure 13.9 ):
• The fi rst approach is a classical procedure where the dispersion of OLS is
achieved by using, as the compatibilizer of the system or as a matrix, a previously
functionalized PO bearing carboxylate groups on the backbone (two - step
process, Method 1). This method involves a fi rst step when the functionalized
PO is prepared. Frequently, the traditional approach for the preparation of
functionalized PO is by free radical grafting of polar unsaturated molecules.
• The second process is carried out by contemporaneously performing the
functionalization of the PO (by using maleic anhydride and/or diethylmaleate
and a peroxide as radical initiator) and the dispersion of the fi ller (one - step
process, Methods 2 and 3).
The results obtained using the two - step process (Method 1) indicated that, by using
the functionalized PO as matrix, only a fraction of the polar groups on the poly-
olefi n macromolecules would be effective in establishing a bonding with the sili-
cate surface. In order to facilitate the functionalized polymer – clay interactions, it
seems convenient to perform the functionalization during mixing of the PO with
the clay. In this way, the small polar molecules could more easily penetrate the
clay channel, with grafting to the organic macromolecules occurring successively.
Comparative experiments were also carried out according to the one - step proce-
dure, following two different routes – Methods 2 and 3 (see Figure 13.9 ).
In Method 2, the functionalizing reagents and organically modifi ed montmo-
rillonite ( OMMT ) were premixed and added contemporarily to the molten polymer
in a Brabender mixer, such that functionalization and intercalation/exfoliation
could occur simultaneously. In Method 3, the functionalizing reagents (initiator,
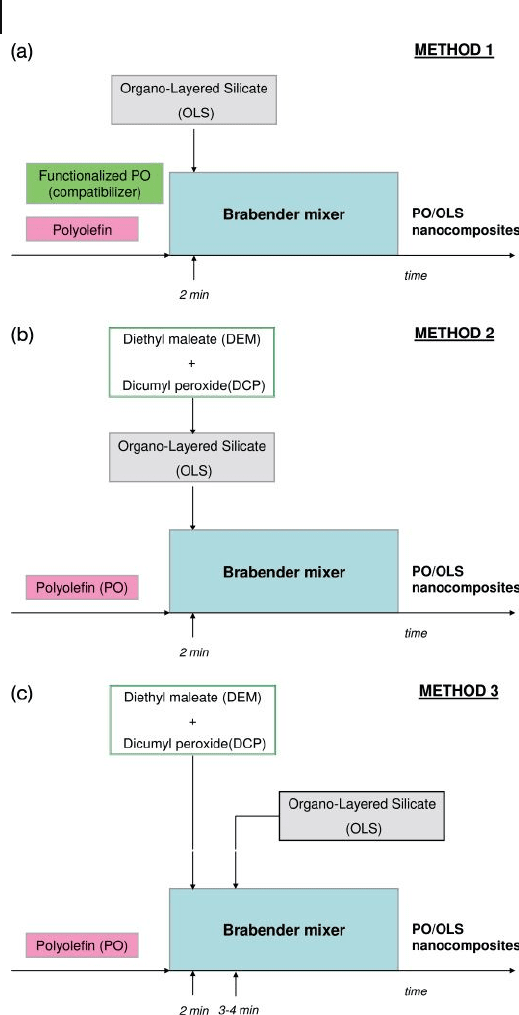
428 13 Nanocomposites Based on Phyllosilicates
Figure 13.9 Different methods of preparation for compatibilized PO/OLS nanocomposites.

13.3 Poly(Ethylene Terephthalate)-Based Nanocomposites 429
DEM and, occasionally, MAH) were added to the molten polymer, with the OMMT
being added after a few minutes. In the former case, the operating conditions the
aim essentially was fi rst to have intercalation of the monomer, followed by grafting
to the macromolecules. In the latter case, the procedure was essentially similar to
the two - step method (Method 1), although for kinetic reasons there was a degree
of overlapping within the time scale between the grafting of DEM to PO, and
intercalation.
The results obtained indicated that, in terms of the role played by the differ-
ent parameters (including the polyolefi n chemical structure), the process was
very complex. In the case of the random ethylene/propylene copolymer (EPM),
the simultaneous addition of a functionalizing reagent and OMMT to the molten
polymer (Method 2) was the most effective approach, even if an optimal ratio
between the DF and clay basal spacing enlargement appeared to exist. In the
case of PP, however, the application of Method 2 did not provide the same
results, with a better dispersion being obtained via Method 3. Indeed, the polymer
functionalization was seen to be favored, and degradation controlled, by the
presence of clay. However, the best polymer chain intercalation was obtained
when degradation was signifi cant during the functionalization process of PP,
indicating that a reduction in molecular weight which favored intercalation
would probably be more effective than the presence of a larger amount of
grafted polar groups.
13.3
Poly(Ethylene Terephthalate) - Based Nanocomposites
Poly(ethylene terephthalate) ( PET ), which is one of the most diffused polymers, is
used in a wide variety of applications, notably the packaging and textile industries.
The need to improve the stiffness, surface properties and/or gas barrier properties
of PET has led to a plethora of investigations, in both academia and industry, into
PET - based nanocomposites. Within this area, those nanocomposites which contain
phyllosilicates are considered superior for providing improved gas barrier proper-
ties, due mainly to the strong effect of confi nement as the result of a high
surface : volume ratio (i.e., reducing chain mobility and permeability [35] ), as well
as to the enhancement of tortuosity [97] of the path required for small molecules
to permeate through a polymer fi lm due to the presence of silicate lamellae. Flame
retardancy and gas barrier functions are much - required properties in the area of
textiles, the main aim being to develop fabrics with fl ame self - extinguishing prop-
erties [98] . Consequently, many research groups have recently investigated PET/
phyllosilicate nanocomposites in an attempt to provide solutions to these impor-
tant technological problems [99 – 102] .
One especially important fi eld of application is that of recycling post - consumer
PET [103] . The recovery of PET from post - consumer packaging products (e.g.,
beverage bottles) allows fl akes to be obtained which have purity levels suitable for
reprocessing. Unfortunately, in many countries (e.g., Italy) the recovered material

430 13 Nanocomposites Based on Phyllosilicates
cannot be recycled via a bottle - to - bottle approach, as legislation decrees that post -
consumer polymers cannot remain in contact with food. Consequently, there is a
need to develop new materials based on post - consumer PET fl akes, in order to
achieve an effective recycling. At present, blending these fl akes with other poly-
mers, especially rubber [104 – 111] , to achieve toughened blends, and/or adding
fi llers [112] , represent the most frequently investigated strategies for producing
materials with added value by utilizing post - consumer fl akes. This fi nal point is
especially important because, if recycling cannot be used to provide materials that
perform similarly to commonly used plastic materials, then landfi lling or energy
recovery using plastic wastes will become inevitable. Nanofi llers – and in particular
phyllosilicate - based nanocomposites – may well provide the means of modulating
the mechanical, thermal, and fl ame - retardancy properties of post - consumer PET,
thus broadening its fi eld of application.
Today, both technological and environmental driving forces continue to attract
the attention of scientifi c research towards PET – phyllosilicate nanocomposites.
13.3.1
In Situ Polymerization
The preparation of PET/MMT nanocomposites by in situ polymerization has
been the object of several investigations. In particular, Hwang et al . [113] reported
the preparation of PET nanocomposites containing pristine and organically modi-
fi ed MMT by in situ polymerization, since this technique has been shown to
produce a homogeneous dispersion of MMT particles in the polymer matrix.
The polymerization of PET was carried out through polycondensation [114, 115] .
In an esterifi cation tube, ethylene glycol and sodium or organically modifi ed
MMT and a catalyst of transesterifi cation (zinc acetate) were subjected to ultra-
sonication before the ester interchange reaction was initiated. Dimethyl tereph-
thalate was then added to the ethylene glycol slurry to obtain a homogeneously
dispersed system. The mixture was then heated to 210 ° C to initiate esterifi cation
between the silicate layers in the clay. The ester exchange reaction was carried
out for 3 h while continuously removing methanol. Finally, polycondensation
was performed at reduced pressure, at temperatures between 180 and 285 ° C,
by adding antimony (III) oxide catalyst as polycondensation catalyst.
The degree of exfoliation was determined by TEM measurements at 20 nm
resolution; these showed a clear separation between the silicate layers of the
clay, enhancing differences between the composites prepared using either
sodium - MMT or OMMT which had been modifi ed with dimethyl, benzyl,
hydrogenated tallow, quaternary ammonium. The TEM images revealed the
presence in 1% MMT composites of uniformly oriented sodium - MMT particles
with complete layer stacks containing an average of 66 silicate sheets per
particle, whereas particles in PET/OMMT were largely exfoliated, with a larger
proportion of intercalated PET, as evidenced by signifi cantly smaller particles
composed of only four to fi ve randomly oriented silicate sheets. Scanning
electron microscopy ( SEM ) images of the nanocomposites confi rmed that the

13.3 Poly(Ethylene Terephthalate)-Based Nanocomposites 431
organophilic modifi er had signifi cantly improved the compatibility between
PET and MMT.
DSC analyses showed that the crystallization temperature of PET/sodium - MMT
during cooling gradually increased, while the degree of supercooling decreased
with clay loading. This suggested that the sodium - MMT had acted as a nucleating
agent and accelerated the crystallization rate of the PET matrix. In subsequent
heating runs, the nanocomposites exhibited two melting peaks compared to pure
PET. The lower melting temperature peak was attributable to the melting of
imperfect crystals, which formed as a result of the nucleation effect of sodium -
MMT during the cooling run. The higher melting temperature peak corresponded
to melting of the melt - reorganized crystal.
DSC curves obtained for the PET/organophilic MMT exhibited an increase in
the crystallization peak intensity during the cooling run at 0.5 wt% loading. This
peak decreased with increasing clay content, although it maintained a higher
intensity than that of neat PET. This indicated that the nucleating effect of organo-
philic MMT was lower than that of sodium - MMT. This may be attributable to
interference by the alkyl groups on the organophilic MMT surfaces with secondary
nucleation and diffusion of PET molecules, resulting in a decrease in the DSC
crystallization peak.
The dispersion of rigid, exfoliated MMT layers served to enhance the macroscale
stiffness of the composite material. Elongation at break values was drastically
decreased due to increased stiffness and the formation of microvoids around the
clay particles during tensile testing. The main factor contributing to the enhance-
ment of mechanical properties in PET/clay nanocomposites was not the quantity
of clay, but rather the degree of dispersion and exfoliation of the clay in the PET
matrix.
A very similar in situ polymerization procedure was adopted by Chang and Mun
[100] , who prepared PET nanocomposites by using dodecyltriphenylphosphonium -
modifi ed MMT as a reinforcing fi ller in the fabrication of PET hybrid fi bers. The
group studied the properties of the composites obtained, for organoclay contents
ranging from 0 to 5 wt%, by applying different draw ratios. The modifi ed MMT
displayed well - dispersed individual clay layers in the PET matrix, although some
particles appeared to be agglomerated at size levels greater than approximately
10 nm.
Ke et al . [116] also reported the properties of PET/MMT nanocomposites pre-
pared by in situ polymerization of PET onto organophilic - modifi ed MMT. The
refi ned clay, reduced to particles of 40 μ m average diameter, was made into a
slurry, and formed a solution with an intercalated reagent, which reacted directly
with PET monomers in an autoclave [117, 118] . Although, this preparation method
led mainly to intercalated nanocomposites, a similar nucleating effect with respect
to the report of Chang and Munn was observed.
An alternative in situ polymerization method was provided by Lee et al . [118] ,
who successfully polymerized ethylene terephthalate cyclic oligomer s ( ETCO s) to
form a high - molecular - weight PET in the presence of OMMT by employing the
advantages of the low viscosity of cyclic oligomers and lack of chemical emissions
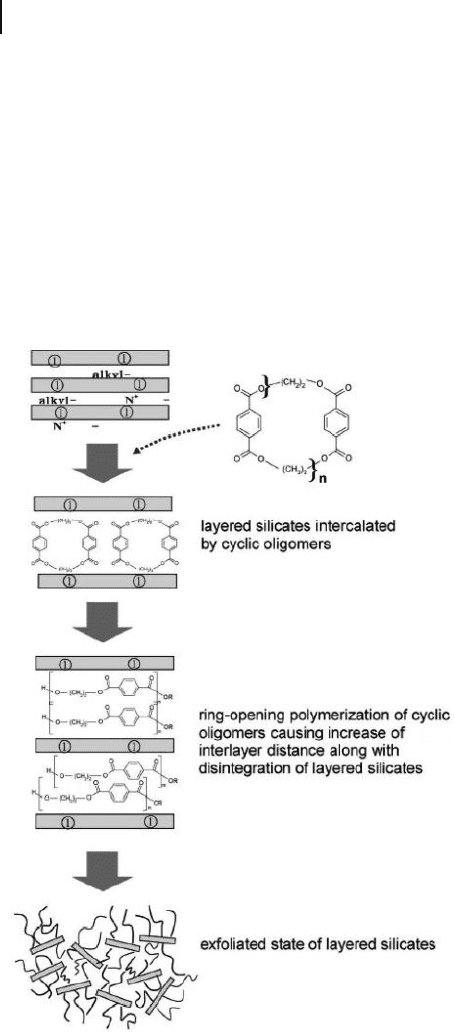
432 13 Nanocomposites Based on Phyllosilicates
during polymerization (Figure 13.10 ). Here, the OMMT was prepared via the
cation exchange of sodium - MMT with N , N , N - trimethyl octadecylammonium
bromide.
The application of ring - opening polymerization ( ROP ) is usually hindered by
the limited effi ciencies for synthesizing and isolating cyclic oligomers. Therefore,
various types of preparative methods have recently been developed. The present
authors followed the direct reaction of acid chlorides with glycols in dilute solution,
which provides high yields of ETCs [119] . Subsequently, ETC, OMMT and Ti(O -
1 - C
3
H
7
)
4
were dissolved in dichloromethane and the solution maintained at room
temperature for 12 h. The oligomers, OMMT and Ti(O - 1 - C3H7)4, dissolved in
dichloromethane, were maintained at room temperature for 12 h; the solvent was
Figure 13.10 Schematic representation of nanocomposites
formation by ring - opening reaction of cyclic oligomers
in - between silicate layers. Reproduced with permission from
Ref. [118] ; © 2005, Elsevier Ltd.
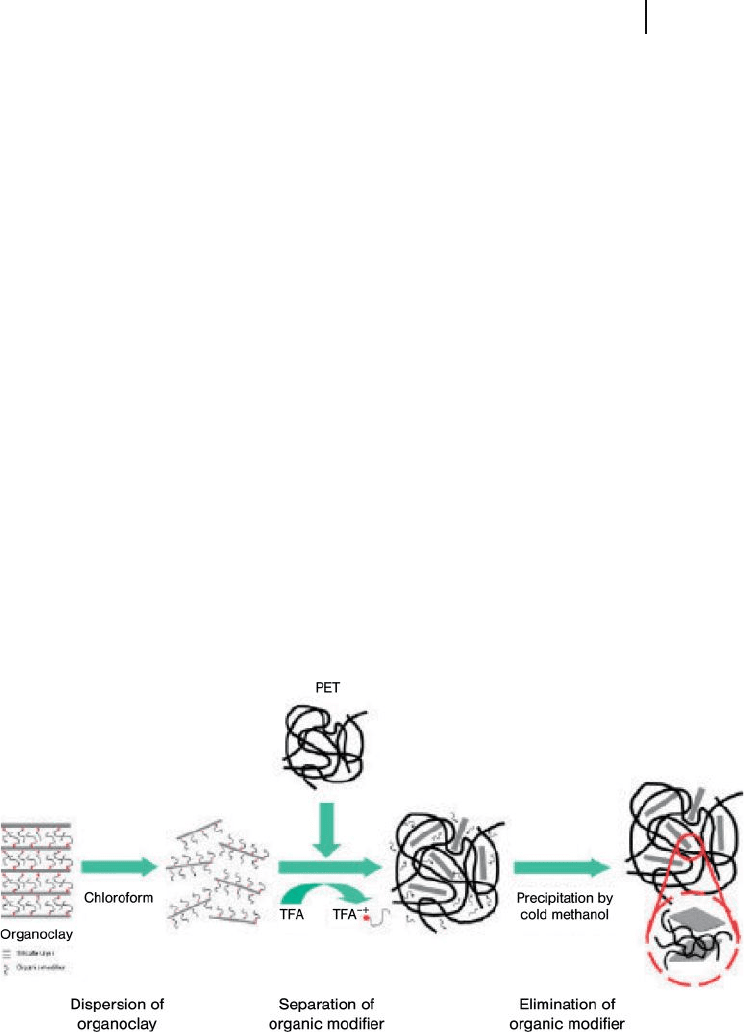
13.3 Poly(Ethylene Terephthalate)-Based Nanocomposites 433
Figure 13.11 Schematic illustration of the preparation of
exfoliated PET nanocomposites excluding organic modifi er by
means of the solvent – nonsolvent system. Reproduced with
permission from Ref. [120] ; © 2008, John Wiley & Sons, Ltd.
then removed, dried under reduced pressure and polymerized in a high - vacuum
system at temperatures ranging from 240 to 310 ° C.
Due to their low molecular weight and low viscosity, the ETCs are successfully
intercalated into the clay galleries, as evidenced by XRD analyses which showed a
down - shift of the basal plane peak of layered silicate, in good agreement with the
results of TEM investigations. The successive ROP of ETCs in - between silicate
layers yielded a PET matrix of high molecular weight, along with high disruption
of the layered silicate structure and a homogeneous dispersion of the latter in the
matrix. However, the coexistence of exfoliation and intercalation states of silicate
layers was revealed by morphological investigations. Interestingly, the highest
investigated polymerization temperature provided the nanocomposite with the
PET having the greatest molecular weight (11 000 mol g
− 1
).
13.3.2
Intercalation in Solution
To date, only one report has been made [120] detailing the preparation of PET/
phyllosilicate nanocomposites in solution. Exfoliated PET - layered silicate nano-
composites excluding and including organic modifi ers were obtained by solution
methods in chloroform/ trifl uoroacetic acid ( TFA ) mixtures, with and without
solvent – nonsolvent systems, respectively. For this, the PET/OLS/chloroform/
TFA solution was added dropwise to the cold methanol to obtain PET nanocom-
posites excluding an organic modifi er; the precipitated materials were then col-
lected by fi ltration and dried in a vacuum oven (Figure 13.11 ). In contrast, the PET

434 13 Nanocomposites Based on Phyllosilicates
nanocomposite including an organic modifi er was prepared by removing both
solvents from the prepared PET/OLS/chloroform/TFA solution.
Based on wide - angle X - ray diffraction (WAXRD) and high - resolution TEM anal-
yses, both types of composite were found to have an exfoliated structure that was
attributed to a suffi cient dispersion of silicate in prepared solvents, regardless of
the sample preparation method.
The DSC results indicated that both types of nanocomposite had higher degrees
of crystallinity and shorter crystallization half - times than neat PET, since the dis-
persed silicate layers had acted as nucleating agents in both situations. However,
those nanocomposites prepared with the organic modifi er exhibited a lower degree
of crystallinity and a longer half - time of crystallization than those without an
organic modifi er. This difference in crystallization behavior between the two nano-
composites was ascribed to the organic modifi er, which may have acted as an
inhibitor of crystallization.
13.3.3
Intercalation in the Melt
The most frequently reported method of intercalation has been within the melt,
mainly because it is signifi cantly more economical and simpler than other
methods, and is also compatible with existing processes. In fact, melt processing
allows nanocomposites to be formulated directly using ordinary compounding
devices such as extruders or mixers, without the need to involve resin production.
Consequently, nanocomposite production can be shifted downstream, providing
end - use manufacturers with many degrees of freedom with regards to their fi nal
product specifi cations. Notably, melt processing is also environmentally friendly,
as no solvents are required. It also enhances the specifi city of intercalation of the
polymer, by eliminating any competing host – solvent and polymer – solvent
interactions.
One topic which has been widely investigated is the infl uence of an organo-
philic modifi cation of nanoclay on the morphology of composites. Pegoretti et
al . [121] compared the effect of a nonmodifi ed natural MMT clay (Cloisite - Na+)
and an ion - exchanged clay modifi ed with quaternary ammonium salt (Cloisite
25A) (Table 13.5 , row h) in a recycled PET matrix. The Cloisite 25A is a MMT
modifi ed with dimethyl, hydrogenated tallow, 2 - ethylhexyl quaternary ammonium
cations. Following preparation of the composites in an injection - molding
machine, subsequent TEM images of the composite fracture surfaces indicated
that the particles of Cloisite 25A were much better dispersed in the recycled PET
matrix than those of Cloisite - Na+. Moreover, wide - angle X - ray scattering ( WAXS )
measurements indicated the intercalation of recycled PET between the silicate
layers (lamellae) of the clay. In addition, improvements in mechanical properties
were more evident in Cloisite 25A nanocomposites than in those containing
Cloisite - Na+. More recently, the effects of different modifi ed commercial MMTs
on the morphology of PET composites prepared in a twin - screw extruder were
investigated [122] . In particular, within a scale of increasing hydrophobicity of
