Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


3.3 Endogenous Triggers 95
Protonation of the block copolymer backbone can also trigger destabilization of
the micelles. Block copolymers with such characteristics normally contain l - histi-
dine [20, 21] , pyridine [22] , and tertiary amine groups in their hydrophobic seg-
ments [23] . In these systems, polymeric micelles are formed at a pH above the p K
a
of the protonatable group, and therefore the hydrophobic segment essentially is
uncharged. As the pH falls below the p K
a
, however, ionization of the polymer
causes increased hydrophilicity and electrostatic repulsions of the polymers,
leading to destabilization of the micelles. In this way, PEG - b - poly( l - histidine)
(PEG - b - P(His)) was used to prepare pH - sensitive polymeric micelles incorporating
DOX [20] . The prepared micelles showed an accelerated release of drug as the pH
was decreased, with ionization of the P(His) block forming the micelle core deter-
mining the pH - dependent critical micelle concentration ( CMC ) and stability of the
system. Moreover, control of the transition pH is possible by combining different
block copolymers. In light of these fi ndings, Lee et al. prepared mixed micelles
from PEG - b - P(His) and PEG - b - poly(lactic acid) ( PEG - b - PLA ) [21] . The PEG - b -
P(His) micelles destabilized at physiological pH, whereas the mixed polymeric
micelles of PEG - b - P(His) and PEG - b - PLA showed improved micellar stability
at pH 7.4 and dissociated over a pH range of 6.0 to 7.2, depending on the propor-
tion of PEG - b - PLA present. Similar pH - sensitive mixed micelles were prepared
from biotin - P(His) - b - PEG - b - PLLA (poly(
L - lactic acid)) and PEG - b - P(His). At pH > 7,
the P(His) attached to the biotin was mostly deionized and became hydrophobic,
thus interacting with the micellar PLA core. However, as the pH was slowly
decreased the P(His) segments became progressively ionized and extended out-
wards through the polyethylene ( PEG ) brush surrounding the core, thus exposing
the biotin moieties for ligand – receptor interactions. At pH values < 6.5, protona-
tion of P(His) in the PEG - b - P(His) block copolymer contained in the core caused
the induction of micellar dissociation.
The above - described pH - sensitive nanoassemblies release their contents after
dissociation of the micelles. However, a different type of pH - sensitive nanoassem-
bly was designed to release the encapsulated contents after aggregation or collapse
of the nanoassemblies. As an example, Leroux et al. prepared random copolymers
of N - isopropylacrylamide ( NIPAAm ) and methacrylic acid ( MAA ) substituted
with alkyl chains at either the terminal chain ends, or distributed randomly over
the copolymer chain to induce micelle formation [24] . When chloroaluminum
phthalocyanine ( AlClPc ), a widely used photosensitizer for the photodynamic
treatment of cancer, was incorporated into these micelles [25] , the addition of
5 mol% MAA to the copolymers caused the hydrophobic core to distort following
neutralization of the MAA as the pH fell below 5.7 – 5.8 at 37 ° C. This phenomenon
was thought to cause the release of the entrapped photosensitizer and to alter the
intracellular localization of the drug in a favorable way, making it more
photoactive.
Smart polymeric micelles may also represent a promising approach for the oral
delivery of hydrophobic drug molecules. Sant et al. developed pH - sensitive micelles
composed of block copolymers of PEG as the hydrophilic block and poly(alkyl
acrylate - co - methacrylic acid) [PEG - b - (PAA - co - MAA)] [26] . Due to the presence of

96 3 Smart Nanoassemblies of Block Copolymers for Drug and Gene Delivery
pendant carboxylic groups on the MAA segments in the core, the copolymers self -
assembled at pH < 4.7, whereas, above this value the micelles dissociated owing
to ionization of the COOH moieties. The pH at which micellization occurred was
decreased with a reduction in the length of the hydrophobic block. Three poorly
water - soluble drugs, namely indomethacin, fenofi brate, and progesterone, were
successfully loaded into these micelles. It was also possible to trigger drug release
in a pH - dependent manner by changing the pH of the release medium from 1.2
to 7.2; this clearly demonstrated the potential of pH - responsive polymeric micelles
for targeting drugs to the intestine following oral administration.
3.3.1.2 Gene Delivery
Gene therapy refers to the potential use of nucleic acids, irrespective of whether
this involves plasmid DNA, antisense oligonucleotides or siRNA, to modulate the
expression of genes in cells for therapeutic purposes. Among the highlights of
gene therapy are:
• The replacement of a defi cient gene in a genetically inherited disease, with a
normal copy restoring the production of a functional protein.
• The correction of genetic defects beyond inherited disorders, as modulation of
the regulation of gene expression is involved in numerous acquired diseases.
• The integration of functions in cells that are not originally present, and which
could serve a therapeutic purpose.
Gene delivery may be divided into two main categories, depending on the vectors
used for nucleic acid transfer, namely viral and nonviral . The fi rst vectors to be
developed were based on the use of viruses or pseudoviral particles. Viral vectors
may pose serious problems in terms of immunogenicity, toxicity and potential
oncogenicity, thus risking their use as therapeutic drugs [27] . Nonviral gene deliv-
ery involves the use of cationic lipids and cationic polymers to deliver the genes.
Synthetic self - assembled gene vectors based on cationic polymers, termed poly-
plexes , are considerably safer and easier to produce. Polyplexes have also been
progressively ameliorated as gene vectors, and specifi c DNA delivery to several
tissues has been achieved in vivo by using either systemic or localized delivery [28] .
Cationic polymers mask the negative charge of the plasmid DNA ( pDNA ) and
package it into small particles, thus protecting the pDNA from both enzymatic
and hydrolytic degradation [29 – 31] . Polycations with a relatively low p K
a
value,
such as poly(ethylenimine) ( PEI ), present a high transfection activity, most likely
because they buffer the endosomal acidifi cation and produce an increase in the
ion osmotic pressure in endosomes. This is followed by protonation of the amines,
which leads in turn to a disruption of the endosomal membranes and release of
the endosomal contents into the cytoplasm. This whole process is referred to as
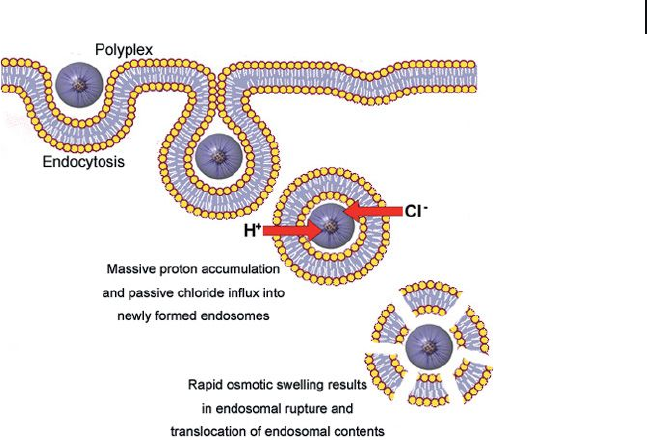
the “ proton - sponge effect ” (Figure 3.3 ) [32] . Such polycations require a high ratio
of cationic amino groups in the polycations to phosphate anions in the DNA (N/P
ratio), in order to form a polyplex which has a high stability and effi cient transfec-
tion activity. Moreover, although free PEI contributes to the increased gene expres-
sion, it also produces a considerable increase in the toxicity of the carrier [33] .
3.3 Endogenous Triggers 97
Conversely, polycations with a p K
a
> 9.0, such as poly( l - lysine) (P(Lys)), form stable
polyplexes even at a relatively lower N/P ratios [34] . The introduction of buffering
units into a polycation with high p K
a
value improves the transfection effi ciency
based on the proton - sponge effect, but decreases the stability of the complex due
to a lower affi nity towards DNA [35] . The buffering capacity of these units is also
lessened due to protonation of the polycations following their complexation with
DNA. Thus, as the effi ciencies of the polyplexes are still too low for clinical use,
the next crucial point of gene delivery will be to construct virus - like polyplexes
using smart polymer conjugates. By using that approach, the creation of effective
gene vectors for clinical applications should resolve the problems of poor stability
and high toxicity of the current polyplexes, and also provide the buffering capacity
to enhance transfection, without an excess of free polymers.
It is essential that the smart gene nanostructures recognize the biological
signals and undergo designed structural changes which match the different steps
of gene delivery. In this respect, Oishi et al. reported the creation of hepatocyte -
targeted polyion complex ( PIC ) micelles with a pH - sensitive PEG shell as a smart
delivery system for antisense oligodeoxynucleotide s ( ODN s) [36] . These PIC
micelles were prepared from P(Lys) and a lactosylated PEG - ODN conjugate ( Lac -
PEG - ODN ), which had an acid - labile linkage ( β - thiopropionate) between the PEG
and ODN segments. The lactose - PIC micelles achieved an elevated antisense effect
against luciferase gene expression in human hepatoma (HuH - 7) cells, this being
signifi cantly higher than that produced by either ODN or Lac - PEG – ODN alone,
Figure 3.3 The “ proton sponge effect. ”
Polyplexes containing weak base components
buffer the endosomal acidifi cation and
produce an increase in the ion osmotic
pressure in endosomes; this leads to
disruption of the endosomal membranes.
Finally, the endosomal contents are delivered
into the cytoplasm.

98 3 Smart Nanoassemblies of Block Copolymers for Drug and Gene Delivery
and also the lactose - free PIC micelle, most likely due to an asialoglycoprotein
receptor - mediated endocytosis process. In addition, a signifi cant decrease in the
antisense effect was observed for a lactosylated PIC micelle without the acid - labile
linkage. This suggested that the pH - sensitive release of the active antisense ODN
molecules into the cytoplasm is a key event in the antisense effect of this micelle.
Conversely, the use of a polycation with low p K
a
, such as branched PEI instead of
the P(Lys), to prepare the PIC micelle led to a decrease in the antisense effect,
most likely due to a buffer effect of the branched PEI in the endosomal compart-
ment that prevented cleavage of the acid - labile linkage in the conjugate.
Another approach for developing a smart gene delivery system consists of an
A – B – C - type triblock copolymer using a biocompatible fragment in the A - frag-
ment, a polycation with low p K
a
value and buffering effect as the B - fragment, and
a polycation with high p K
a
to condense the DNA as the C - fragment. Fukushima
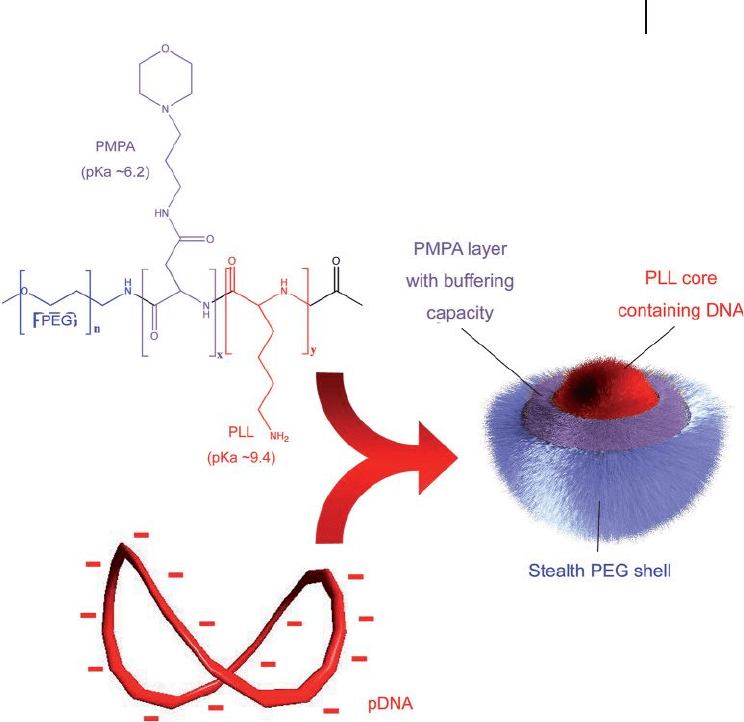
et al. showed that PEG - b - poly[(3 - morpholinopropyl) aspartamide] (PMPA;
p K
a
= 6.2) - b - P(Lys) (PEG - b - PMPA - b - P(Lys)) formed smart PIC micelles where the
P(Lys) backbone condensed DNA and the uncomplexed PMPA backbone covered
the P(Lys)/DNA polyplex core (Figure 3.4 ) [37] . These PIC micelles had a diameter
of 88.7 nm, a zeta potential of 7.3 mV, and exhibited much higher transfection
effi ciency against HuH - 7 cells than did micelles prepared from PEG - b - PLL
(poly(lactic acid)) or the combination of PEG - b - PLL and PEG - b - PMPA. The
improvement in transfection effi ciency of these three - layered polyplex micelles can
be related to the buffering capacity of the PMPA segment in the polyplex micelle.
Nevertheless, positively charged polyplexes might potentially induce cytotoxicity
and form aggregates with the plasma proteins present in the biological media,
thus restricting their in vivo applicability. In order to overcome this problem, two
strategies have been followed:
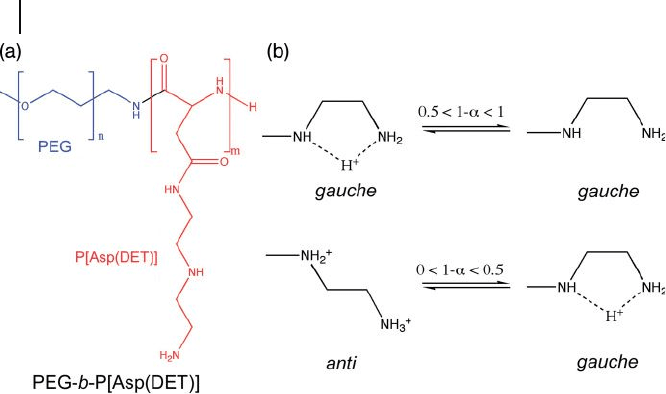
• The fi rst approach consists of using a block copolymer of a PEG segment and a
cationic polyaspartamide segment carrying an ethylenediamine unit at the side
chain (PEG - b - P[Asp(DET)]) (Figure 3.5 ) [38] . This block copolymer led to the
formation of stable polyplexes with a core of tightly packed pDNA.
Ethylenediamine undergoes a clear, two - step protonation with a characteristic
gauche – anti conformational transition providing an effective buffering function
in the acidic endosomal compartment. Thus, after endocytosis of these
polyplexes, the ethylenediamine unit in the block copolymer is expected to
facilitate the effi cient translocation of the micelle towards the cytoplasm due to
the proton - sponge effect. The PIC micelles prepared from PEG - b - P[Asp(DET)]
accomplished an appreciably high gene transfection effi cacy and a remarkably
low cytotoxicity against several cell lines, including primary osteoblasts.
Moreover, these polyplexes showed a high effi ciency in vivo for the treatment of
vascular lesions, with markedly reduced cytotoxicity and thrombogenicity [39] .
• A second approach was proposed by Lee et al. using polymeric micelles of PEG -
b - poly[( N ′ - citraconyl - 2 - aminoethyl)aspartamide] (PEG - b - P(Asp(EDA - Cit))) [40] .
This block copolymer has the ability to switch the charge from anionic to
cationic at the endosomal pH due to degradation of the citraconic amide side
chain at pH 5.5. This rapid charge - conversion can cause the PIC micelles to
3.3 Endogenous Triggers 99
promptly release the loaded protein in response to the endosomal pH.
Consequently, this pH - sensitive charge - conversion polymer has shown great
promise for the design of gene carriers that become cationic at the early
endosomal stage, yet still have the ability to achieve endosomal escape due to the
proton - sponge effect.
3.3.2
Oxidation - and Reduction - Sensitive Polymeric Nanoassemblies
Redox - sensitive nanostructures from block copolymers can result in a change of
the assembly morphology and the selective release of encapsulated drugs when
an electric current is applied externally. Moreover, the redox - triggering can occur
Figure 3.4 Chemical structure of the PEG - PMPA - PLL triblock
copolymer, and schematic illustration of the three - layered
polyplex micelles with spatially regulated structure [37] .
100 3 Smart Nanoassemblies of Block Copolymers for Drug and Gene Delivery
at infl ammation sites and solid tumors, since those pathologies present activated
macrophages that release oxygen - reactive species. Hubbell et al. synthesized
amphiphilic A – B – A block copolymers which consisted of the hydrophobic
poly(propylene sulfi de) and PEG (PEG - b - PPS - b - PEG), which formed polymeric
vesicles in water [41] . Following exposure of these vesicles to oxidative agents, the
thioethers in the PPS block were oxidized to poly(propylene sulfoxide) and eventu-
ally to poly(propylene sulfone), leading in turn to hydrophilization of the originally
hydrophobic block. As a result, the vesicles became destabilized. This oxidative
conversion was also accomplished by incorporating glucose oxidase ( GOx ) into the
vesicles [42] . After incubation in 0.1 M glucose solution, the GOx - containing poly-
mersomes were disassembled due to the oxidation of glucose by GOx to produce
H
2
O
2
. Another possibility would be to take advantage of the redox tunability of
metal - containing compounds. In this way, redox - active micelles were prepared
from amphiphilic block copolymers bearing a hydrophobic ferrocenylalkyl moiety
(FPEG) [43] . Oxidation of the ferrocenyl moiety caused the micelles to be disrupted
into unimers. However, when loaded with perylene, these redox - active FPEG
micelles released the drug in a controlled manner by applying a selective and
electrochemical oxidation of the FPEG.
Another type of reduction - sensitive nanostructures is represented by polymeric
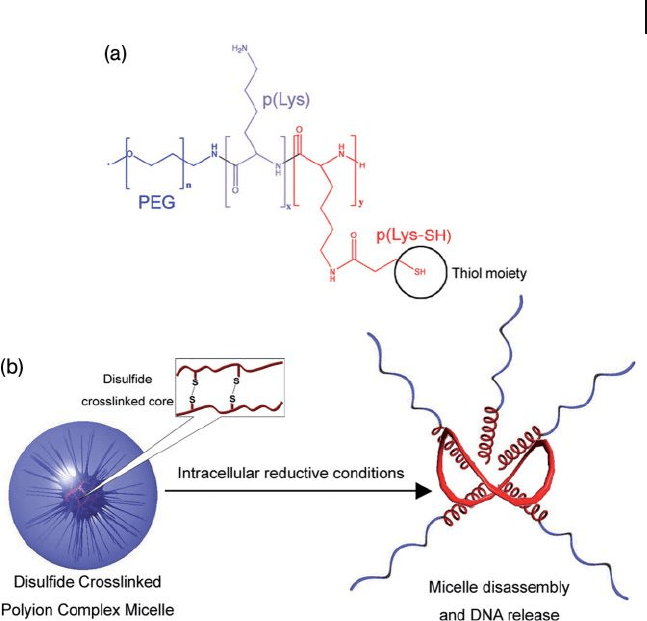
assemblies containing disulfi de bonds. Polyplex micelles with a disulfi de -
crosslinked core effi ciently release the loaded pDNA in response to reductive
intracellular conditions; that is, at 50 - to 1000 - fold higher glutathione concentra-
tions than are encountered in the extracellular environment (Figure 3.6 ) [44 – 47] .
Figure 3.5 (a) PEG - b - P[Asp(DET)] copolymer
bearing an ethylenediamine unit at the side
chain leads to the formation of stable
polyplexes with smart buffering properties;
(b) Ethylenediamine presents a two - step
protonation with a unique gauche – anti
conformational transition providing an
effective buffering function at the endosome.
The proton - free form may take both gauche
and anti conformation [38] .
3.3 Endogenous Triggers 101
The intracellular glutathione reductively cleaves the disulfi de links, which leads to
a destabilization of the system. This type of micelle also achieved suffi cient toler-
ability against destabilization by anionically charged biocomponents, and induced
an effi cient transfection in the cell. As a result, the transfection effi ciencies
achieved in vivo were relatively high [47] .
3.3.3
Other Endogenous Triggers
Several other internal triggers, including enzymes or peptides, can be used to
control the structure and properties of smart block copolymer nanoassemblies.
The construction of smart polyplexes with two types of gene - transfection activation
has been reported [48] . These systems utilize cationic polymers which are respon-
sive to cyclic AMP - dependent protein kinase A ( PKA ) or to caspase - 3, PAK, and
Figure 3.6 Disulfi de crosslinking is used to stabilize PIC
micelles. (a) Molecular structure of the PEG - P( L - Lysine - SH)
block copolymer; (b) Under intracellular reductive conditions,
the disulfi de bonds are cleaved and the PIC micelles release
their contents.

102 3 Smart Nanoassemblies of Block Copolymers for Drug and Gene Delivery
PAC, respectively. The PAK polymer incorporates a substrate for PKA, ARRASLG,
while the PAC polymer has a substrate sequence for caspase - 3, DEVD, and a cati-
onic oligolysine, KKKKKK. These polymers formed stable complexes with
DNA. However, the PKA or caspase - 3 signal breaks up the PAK – DNA or PAC –
DNA complexes, respectively, releasing the DNA and activating the gene transfec-
tion activity.
3.4
External Stimuli
3.4.1
Temperature
As local heating can also be exploited to destabilize smart nanoassemblies, several
thermosensitive copolymers that use this approach are currently undergoing
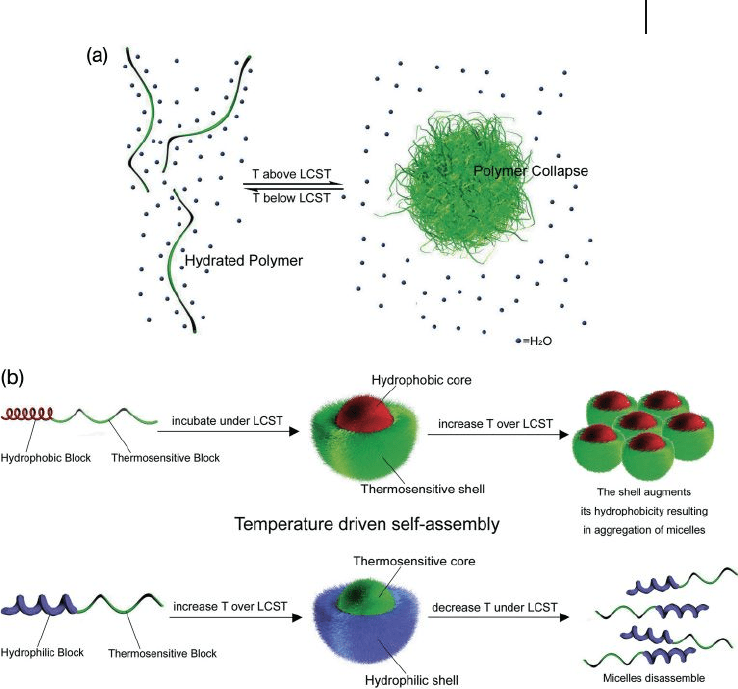
investigation for biomedical applications. The thermosensitive nanoassemblies
are characterized by a lower critical solution temperature ( LCST ), below which
water is bound to the thermosensitive polymer block so as to prevent both intrap-
olymer and interpolymer interactions, thus rendering the nanoassembly water -
soluble. Whilst the formation of hydrogen bonds between the thermosensitive
polymer and water lowers the free energy of mixing, the ordered molecular orien-
tations of water on the polymer lead to negative entropy changes and positive
contributions to free energy. The nonpolar hydrophobic groups in the thermosen-
sitive polymer are incompatible with water, and facilitate an ordered clustering of
the surrounding water molecules so as to decrease the entropy. Increasing the
temperature of the system causes the water cluster to become destabilized to
compensate the thermal energy. Above the LCST, water is released from the
polymer chain (Figure 3.7 a). The positive entropic contribution then grows and
dominates the heat term, which causes the monophase system to be come unbal-
anced and leads to phase separation due to polymer association. To date, the most
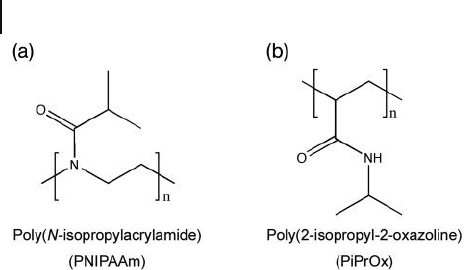
extensively investigated thermosensitive polymer is poly( N - isopropylacrylamide)
(PNIPAAm; Figure 3.8 a); this molecule has a sharp LCST in water at approxi-
mately 32 ° C (slightly lower than body temperature), which makes it extremely
attractive for the design of thermosensitive drug delivery systems [49] . The LCST
of a thermosensitive polymer can also be modulated by copolymerizing it with
hydrophobic comonomers to reduce the LCST, or with hydrophilic comonomers
to increase the LCST. PNIPAAm copolymers may be used either as a hydrophilic
segment or as a hydrophobic segment of polymeric micelles, switching the dis-
persivity depending on the LCST (Figure 3.7 b). By using this approach, Okano et
al. were able to prepare DOX - loaded polymeric micelles of PNIPAAm - b - poly(butyl
methacrylate) (PNIPAAm - b - PBMA) and PNIPAAm - b - poly(styrene) (PNIPAAm - b -
PS) [50] . Below the LCST of PNIPAAm, this system demonstrated a core – shell
micellar structure, but on heating above the LCST the DOX was rapidly released
from the PNIPAAm - b - PBMA micelles as a result of structural distortion of the
relatively fl exible PBMA core, caused by collapse of the PNIPAAm shell. In con-
3.4 External Stimuli 103
Figure 3.7 (a) Thermosensitive polymers can undergo
reversible transformation from hydrated to hydrophobic/
collapse state upon heating; (b) Several strategies can be
used with thermosensitive polymers, such as using them as
the micelle shell below the LCST or the micelle core over the
LCST.
trast, the PNIPAAm - b - PS micelles did not show any enhanced DOX release when
the temperature was increased above the LCST, mainly because the rigid PS core
was insensitive to collapse of the PNIPAAm. As PNIPAAm exists in its precipi-
tated form at body temperature, this system proved not to be suitable for in vivo
application without modifi cation. Nevertheless, the copolymerization of NIPAAm
with the hydrophilic dimethylacrylamide ( DMAAm ) resulted in a random copoly-
mer (P(NIPAAm - co - DMAAm)) with a LCST slightly above body temperature
(40 ° C) [51] . The release of DOX from P(NIPAAm - co - DMAAm) - b - PLA micelles was
very slow at 37 ° C, but showed a sudden increase at 42.5 ° C, which suggested that
this system might have potential benefi t for hyperthermic treatments.
A system in which PNIPAAm was utilized as the core - forming block was
reported by Feijen et al. [52] . Here, the PEG - b - PNIPAAm block copolymer was
104 3 Smart Nanoassemblies of Block Copolymers for Drug and Gene Delivery
water - soluble below the LCST of PNIPAAm, but above this temperature it formed
polymeric micelles with a collapsed PNIPAAm core and a PEG outer shell. The
temperature at which micelles are formed is known as the critical micelle tem-
perature ( CMT ). The heating rate is a critical parameter for the size of PEG - b -
PNIPAAm micelle, as a higher heating rate causes rapid dehydration of the
thermosensitive segments. Any subsequent collapse of these segments precedes
the aggregation between polymers and, as a result, micelles with a well - defi ned
core – shell structure are formed.
Poly(2 - isopropyl - 2 - oxazoline) ( PiPrOx ) is another promising thermosensitive
polymer (Figure 3.8 b). This material possess an isopropyl group in the side 2 -
position and, as PNIPAAm, the aqueous solutions of PiPrOx have a LCST at
near - physiological conditions [53, 54] . Park et al. prepared novel thermosensitive
PIC micelles in an aqueous medium via the complexation of a pair of oppositely
charged block copolymers containing the thermosensitive PiPrOx segments,
PiPrOx - b - P(Lys) and PiPrOx - b - P(Asp) [55] . These PIC micelles had a constant
cloud - point temperature of approximately 32 ° C under physiological conditions,
regardless of their concentration. Since the LCST of PiProx can be modulated
by copolymerization [56] , these PiPrOx - PIC micelles have high potential as a
size - regulated, temperature - responsive nanocontainers for loading charged
compounds.
The main drawback of thermosensitive drug delivery systems is that the thermal
treatment required for the controlled destabilization of the micelles and subse-
quent drug release is not always feasible in clinical practice. However, this issue
can be overcome using secondary external triggers. Sershen et al. developed a
photothermally modulated hydrogel using NIPAAm - b - acrylamide in combination
with photoactive gold nanoshells [57] . The nanoshells strongly absorbed near -
infrared ( NIR ) irradiation (1064 nm) and converted it to heat, resulting in a collapse
of the hydrogel. As an example, laser irradiation of this system led to the controlled
release of methylene blue and proteins.
Figure 3.8 Two examples of thermosensitive polymers having
a LCST. (a) Poly( N - isopropylacrylamide) ( PNIPAAm );
(b) Poly(2 - isopropyl - 2 - oxazoline) ( PiPrOx ). Both polymers
have been used in the construction of nanoassemblies of
block copolymers.
