Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c018” — 2007/4/9 — 15:51 — page6—#6
18-6 Tissue Engineering
(b)(a)
(d)(c)
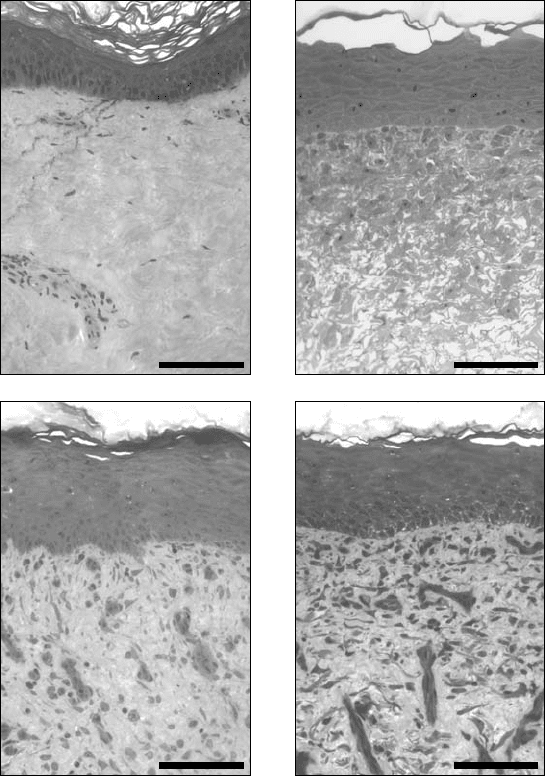
FIGURE 18.1 Histological comparison of native skin and cultured skin substitute prepared for treatment of pediatric
burn patient. (a) Native human skin. (b) Cultured skin substitute in vitro, shown after 6 days of incubation. Graft
was transplanted to patient after 10 days of in vitro incubation. (c),(d) Healed autograft (c), and healed cultured skin
substitute (d), biopsied 3 weeks after grafting to excised full-thickness burn wounds. Scale bars = 0.1 mm.
has been in the treatment of patients with burns affecting greater than 50% TBSA. In these patients, donor
sites for autografting are extremely limited and adjunctive treatments for wound coverage are required.
For preparation of CSS, primary cultures of keratinocytes and fibroblasts are isolated using standard
techniques from a small split-thickness skin biopsy that is usually taken during a patient’s first autograft-
ing procedure [26,27,71,72,78,79]. Selective in vitro culture of keratinocytes and fibroblasts stimulates
exponential increases in cell numbers, resulting in very large populations of cells in only 2 to 3 weeks
of culture [78]. Grafting to patients can generally be performed within 2 weeks of inoculation of CSS,
which corresponds to 4 to 5 weeks after the initial patient biopsy. Because the CSS contains both dermal
and epidermal layers and develops a functional basement membrane in vitro, grafting of CSS can replace
both skin layers in a single surgical procedure (Figure 18.1) [80]. Culture of CSS at the air–liquid interface
promotes development of a stratified epidermal layer with functional barrier properties in vitro, providing
protection of the wound immediately after grafting [68,80,81]. CSS have been used with favorable results

mikos: “9026_c018” — 2007/4/9 — 15:51 — page7—#7
Skin Substitutes 18-7
as an adjunctive treatment for the healing of large burn wounds, and have been shown to significantly
reduce the requirement for autograft and shorten the number of surgical procedures needed for definitive
wound closure [27,67,68]. CSS have also been used to a limited extent for treatment of chronic wounds
and congenital giant nevi [28,29].
18.4 Clinical Considerations
Multiple clinical factors can determine whether treatment of wounds with engineered skin substitutes
will result in skin repair. Modifications of care protocols for wounds must be used to compensate for the
anatomic and physiologic deficiencies in engineered skin. Currently available skin substitutes are avascular,
tend to heal more slowly than skin autograft, and may be mechanically fragile. Factors that affect the clinical
outcome with bioengineered skin replacements include, but are not limited to: composition at the time
of grafting; wound bed preparation; control of microbial contamination; dressings and nursing care; and
survival of transplanted cells during vascularization of grafts.
Attachment of cultured epithelium to a dermal substitute in vitro is advantageous because both epi-
dermal and dermal components can be applied in a single procedure, similar to skin autograft. Culture
conditions can be optimized to promote deposition of basement membrane proteins at the dermal–
epidermal junction prior to grafting, thereby eliminating the problem of blistering that is frequently
observed after grafting of epithelial sheets [33,80]. Alternatively, dermal and epidermal components of
skin substitutes may be applied in two stages: first, application of a dermal substitute followed by vascu-
larization; and second, grafting of an autologous epidermal substitute [45,82,83]. This two-step approach
increases the density of blood vessels and extracellular matrix in the wound bed, and has been reported
to improve engraftment of cultured keratinocyte sheets. However, it requires two surgical procedures to
achieve permanent wound closure.
The lack of a vascular plexus is a major limitation of all skin replacements currently available. Split-
thickness skin contains a vascular plexus and adheres to debrided wounds by coagulum. Inosculation of
vessels in the graft to vessels in the wound occurs within 2 to 3 days [84]. In the absence of microbial
contamination or mechanical damage, autograft skin is generally engrafted and reperfused within 1 week
after transplantation. In contrast, current clinical models of engineered skin substitutes are avascular,
requiring reperfusion from de novo angiogenesis. The time required for perfusion is proportional to the
thickness of the dermal component of the skin substitute, and is longer than perfusion of split-thickness
skin. Vascularization can be accelerated by secretion of angiogenic factors from engineered skin containing
keratinocytes and fibroblasts, but growth factors alone cannot compensate for the lack of a vascular plexus
prior to grafting [85,86]. The additional time required for vascularization may contribute to epithelial
loss from microbial destruction and nutrient deprivation.
Due to the delayed vascularization of skin replacements, control of contamination is critical for engraft-
ment. Topical antimicrobials are more effective for control of wound contamination than parenteral agents
[87]. Topical treatments must provide effective coverage of a broad spectrum of microorganisms, but must
have low cytotoxicity to allow healing to proceed. It is also important to avoid overlap of topical agents
with parenteral drugs used for treatment of sepsis, which could facilitate development of resistant organ-
isms. Several studies have identified individual agents, and mixtures of multiple agents, which are effective
against common wound organisms but are not inhibitory to proliferation of keratinocytes and fibroblasts
[88–90].
An additional limitation of engineered skin substitutes is mechanical fragility, which contributes to
graft failure due to shear and maceration. For delicate grafts, a backing material can facilitate handling
and attachment to the wound. For example, CEA are routinely attached to petrolatum-impregnated gauze
for surgical application [91]. However, this material may not be compatible with wet dressings used to
manage infection. CSS may be handled and stapled to wounds with a backing of N-Terface™, a relatively
strong, nonadherent, highly porous material [25,26]. Porous dressings do not interfere with the delivery
of topical solutions and permit drainage of wound exudate.

mikos: “9026_c018” — 2007/4/9 — 15:51 — page8—#8
18-8 Tissue Engineering
An important practical obstacle to the routine clinical use of skin substitutes, as with any engineered
tissue, remains the high cost of their preparation and care. Estimates for the cost of keratinocyte sheets
range from $1,000 to $13,000 for each percent of absolute TBSA [63,92]. If a dermal substitute is also
included, the cost can be expected to approximately double [8,45]. Therefore, expense can become
a limiting factor for treatment of very large wounds, such as those seen in severely burned patients.
Unfortunately, these are the patients most in need of skin substitutes. Although the use of skin substitutes
can theoretically reduce the number of surgeries required to heal large burns, which should decrease the
total time of hospitalization, there are currently no studies that clearly demonstrate a decrease in costs by
use of skin substitutes of any kind. Engineered skin grafts remain an important adjunct to conventional
skin grafting, particularly in the treatment of burns, but cannot be used as a primary modality of wound
closure except in the most extreme cases [61].
18.5 Assessment
The outcome after treatment of wounds with bioengineered skin substitutes must be measured to determ-
ine whether the benefits justify any risks associated with the therapy. Qualitative outcome, which relies
heavily on the trained eye of the clinician, can be assessed through clinical evaluation integrating multiple
properties of the wound. For example, the Vancouver Scale is used for assessment of burn scar by trained
clinicians, and provides an ordinal score for properties of skin including pigmentation, vascularity, pli-
ability, and scar height [93]. Such scales assign quantitative values to qualitative measurements and can
provide a relative comparison for evaluation, but they are inherently subjective and dependent on the
examiner.
Objectivity may be increased by use of noninvasive instruments to measure biophysical properties
of skin, including vascular perfusion, epidermal barrier, pliability, color, and surface pH. Quantitative
assessment of skin substitutes can highlight deficiencies compared to normal skin or split-thickness
autograft, or can be used to assess the advantage of skin substitutes to the patient without interfering
with recovery. Although no single biophysical property is definitive, multiple measurements can provide a
general assessment for evaluation of outcome. For example, measurements of surface electrical capacitance
(SEC) can be used to define the degree of skin barrier development [94]. SEC is measured using a dermal
phase meter, an instrument that is easily used in a clinical setting, with minimal pain or discomfort for the
patient [95]. Pigmentation of grafted wounds treated with engineered skin substitutes can be measured
using a chromameter. Multiple parameters of skin function must be measured to quantify overall benefit
from treatment with skin substitutes.
18.6 Regulatory Issues
In the United States, it is the responsibility of the Food and Drug Administration (FDA) to protect the
public from health risks associated with new medical therapies. FDA approval requires that new therapies
be safe and effective, and that the probable benefits to health outweigh the probable risks of the therapy
or of the untreated disease or condition [96]. Safety considerations for engineered skin substitutes must
take several factors into account, including media composition, tissue acquisition, graft fabrication and
storage, and sterility testing of the final product [96]. For example, cell culture media must be of the
highest purity and free from toxic contaminants. Cells derived from allogeneic donors must test negative
for transmissible pathogens. Autologous cells must be handled carefully as well. Because autologous tissues
are not routinely screened for pathogens, universal precautions to protect laboratory personnel must be
practiced. Xenogeneic components, such as bovine collagen, must not only be free from pathogens that
can cross species boundaries, but must also be nonimmunogenic. If xenogeneic cells, such as irradiated
3T3 mouse fibroblasts, are used to facilitate initiation of keratinocytes cultures, compliance with safety
standards for xenogeneic transplantation must be assured [97]. Although these common cells are generally

mikos: “9026_c018” — 2007/4/9 — 15:51 — page9—#9
Skin Substitutes 18-9
thought to be free from risk of disease transmission, unknown risks may exist, and hence patients are
excluded from future donation of blood or body parts [36,98].
Bioengineered skin substitutes may be regulated as either devices or biologics, depending on their
composition and “primary mode of action.” Skin substitutes consisting of autologous cells only, or an
acellular human tissue matrix, may not require collection of effectiveness data for regulatory approval.
Living autologous cell populations intended for structural repair are consideredto be inherently efficacious
[99]. However, if no effectiveness data are collected, no claims of effectivenesscan be made. Skin substitutes
that combine cells with biopolymers are currently considered class III (significant risk) devices that require
demonstration of effectiveness in addition to safety [96].
18.7 Future Directions
Despite encouraging clinical results with bioengineered skin for the adjunctive treatment of burns, chronic
wounds, and other skin deficiencies, skin substitutes containing just two cell types are limited by anatomic
and physiologic deficiencies compared to split-thickness skin autograft. Several areas of preclinical invest-
igation suggest that skin substitutes can be further engineered to increase homology to native human skin.
These include the incorporation of additional cell types to improve functional and cosmetic outcome, and
the use of genetically modified skin cells to enhance performance after grafting.
18.7.1 Pigmentation
Normal skin pigmentation results from the appropriate epidermal distribution and function of melano-
cytes. The most critical function of melanocytes is protection from ultraviolet irradiation, but they have
psychological importance as well, as a patient’s body image and personal identity can impact recovery from
massive skin injury [100–102]. Pigmentation of cultured skin may result from transplantation of “passen-
ger” melanocytes, which may persist in selective cultures of epidermal keratinocytes [67,79]. Melanocytes
can survive under conditions used for keratinocyte culture, though they proliferate at slower rates and are
depleted upon serial passage or cryopreservation [103–105]. In CSS grafted to excised burns, pigmented
areas resulting from passenger melanocytes have been observed as individual foci within two months after
transplantation [79]. By 1 to 2 years after healing, the foci increase in area, occasionally fusing together
to form larger pigmented regions. Uniform pigmentation was demonstrated in preclinical studies with
CSS deliberately populated with selectively cultured human melanocytes [106–108]. Future studies will
be needed to address regulation of the level of pigmentation in uniformly pigmented cultured skin.
18.7.2 In Vitro Angiogenesis
The absence of a vascular plexus in bioengineered skin necessitates vascularization to occur de novo, rather
than through inosculation of the graft with the wound, increasing the time of nutrient deprivation and
susceptibility to microbial contamination after grafting. This limitation can be indirectly addressed in a
clinical setting by irrigating the graft with solutions of nutrients and antimicrobial agents for several days
after transplantation [27,67,109]. A direct approach would be to initiate angiogenesis in the skin substitutes
in vitro, prior to grafting. This would permit vascularization to occur through both inosculation of existing
vessels and also neovascularization, as occurs for grafted split-thickness skin [84].
Initiation of angiogenesis in vitro requires the addition of endothelial cells to the engineered skin.
Endothelial cells may organize into vascular structures in culture with the aid of biomaterial supports and
coculture with accessory cells. For example, engineered blood vessels have been constructed in vitro using
mixed cultures of fibroblasts, human umbilical vein endothelial cells (HUVEC), and vascular smooth
muscle cells in a collagen matrix [110]. In preclinical studies, transplantation of engineered blood vessels
constructed by culture of HUVEC in three-dimensional collagen/fibronectin gels has been reported [111].
More recently, a composite cultured skin containing HUVEC and keratinocytes in a human dermal matrix
was reported, which displayed evidence of perfusion after grafting to mice [112]. These studies illustrate

mikos: “9026_c018” — 2007/4/9 — 15:51 — page 10 — #10
18-10 Tissue Engineering
the feasibility of grafting synthetic vessels in cultured skin, but overexpression of Bcl-2 through retroviral
modification was required to promote survival of the transplanted endothelial cells [111]. In addition,
another potential limitation of these studies that will impede their clinical application is the reliance on
nondermal or nonautologous endothelial cells. Ideally, multiple cell types (keratinocytes, fibroblasts, and
human dermal microvascular endothelial cells, or HDMEC) could be derived from a single autologous skin
sample. Transplantation of HDMEC in a composite skin substitute containing isogenic keratinocytes and
fibroblasts was demonstrated in an athymic mouse model, though perfusion was not observed [113]. The
transplantation of HDMEC in a clinically relevant cultured skin model showed the feasibility of preparing
autologous cultured skin containing HDMEC, but future studies must demonstrate inosculation of vessels
in the graft with vessels in the wound bed to yield improved performance after grafting.
18.7.3 Cutaneous Gene Therapy
Keratinocytes and fibroblasts are amenable to genetic modification in vitro by a variety of methods.
Genetically modified cells can be used to populate engineered skin substitutes. This is termed “ex vivo”
gene therapy because cells are removed from the body and genetically modified in culture before being
transplanted back to the recipient. There has been a great deal of interest in the use of genetically modi-
fied skin substitutes for the treatment of cutaneous diseases. For example, preclinical studies suggest that
ex vivo gene therapy can be useful for treatment of lamellar ichthyosis, a condition characterized by defect-
ive epidermal barrier, and the blistering skin disease junctional epidermolysis bullosa (JEB) [114–116].
Theoretically, genetically modified keratinocytes can be transplanted for secretion of circulating factors
to treat systemic diseases. Keratinocytes have been genetically modified to secrete human growth hor-
mone and clotting factor IX, but therapeutic protein levels have been difficult to obtain after grafting
[117–121].
Another application of cutaneous gene therapy is the regulation of wound healing. Hypothetically,
genetic modification may be used to overcome anatomic limitations or to enhance their biological activ-
ity. For example, keratinocytes modified to overexpress the mesenchymal cell mitogen Platelet Derived
Growth Factor-A (PGDF-A), seeded on an acellular dermal matrix, showed increased cellularity, vascu-
larization, and collagen deposition after grafting to mice, suggesting improved function due to PDGF-A
overexpression [122]. In other studies, keratinocytes were genetically modified by retroviral transduc-
tion to overexpress the angiogenic cytokine Vascular Endothelial Growth Factor (VEGF) [85,86]. After
transplantation to athymic mice, skin substitutes containing fibroblasts and VEGF-modified keratinocytes
showed enhanced and accelerated vascularization, decreased contraction, and increased engraftment com-
pared to control grafts containing unmodified cells [85,86]. Thus, genetic modification of keratinocytes
can hypothetically be used to overcome the lack of a vascular plexus in engineered skin grafts.
A particularly promising avenue of research involves the genetic modification of cells in cultured skin
grafts to reduce or eliminate immune rejection. Preclinical studies have shown that reduced expression
of major histocompatibility complex (MHC) class I and II antigens can prolong engraftment of skin
grafts in mouse allograft models [123]. In one study, fetal skin was used because it exhibited substantially
reduced MHC class I and II expression compared with neonatal skin. In another study, keratinocytes
were genetically modified to overexpress indoleamine 2,3-deoxygenase (IDO), a tryptophan-catalyzing
enzyme that functions to prevent fetal rejection during pregnancy [124]. Increased IDO expression in
keratinocytes led to a down-regulation of MHC class I expression. These studies suggest a possible
mechanism for preparation of allogeneic skin substitutes that would escape immune rejection, and may
someday lead to universal donor cultured skin grafts.
18.8 Conclusions
Technological advances in the fabrication of biomaterials and the culture of skin cells have permitted
the production of bioengineered skin substitutes. These have provided improved therapeutic options for
mikos: “9026_c018” — 2007/4/9 — 15:51 — page 11 — #11
Skin Substitutes 18-11
patients suffering from acute or chronic wounds, and offer the promise of new treatments for inherited
cutaneous diseases. Continued research will be needed to identify more efficient methods to utilize pre-
cious autologous tissue, which will provide greater amounts of skin substitutes for grafting as well as
shorten the time required for their preparation. Increasing the complexity of skin substitutes, from acellu-
lar biopolymers to composite materials with multiple cell types, will result in continued improvements in
anatomy and physiology, working toward greater homology to native human skin. These improvements
will lead to enhanced performance of engineered skin grafts, greater clinical efficacy, and reduction of
morbidity and mortality for patients with wounds or cutaneous disease.
References
[1] Brigham, P. and McLoughlin, E., Burn incidence and medical care use in the United States:
estimates, trends, and data sources, J. Burn Care Rehabil., 17, 95, 1996.
[2] Berthod, F. and Damour, O., In vitro reconstructed skin models for wound coverage in deep burns,
Br. J. Dermatol., 136, 809, 1997.
[3] Phillips, T.J., Chronic cutaneous ulcers: etiology and epidemiology, J. Invest. Dermatol., 102, 38S,
1994.
[4] Sorrell, J.M., Caterson, B., Caplan, A.I., Davis, B., and Schafer, I.A., Human keratinocytes contain
carbohydrates that are recognized by keratan sulfate-specific monoclonal antibodies, J. Invest.
Dermatol., 95, 347, 1990.
[5] Schurer, N.Y. and Elias, P.M., The biochemistry and role of epidermal lipid synthesis, Adv. Lipid
Res., 24, 27, 1991.
[6] Elias, P.M., Stratum corneum architecture, metabolic activity and interactivity with subjacent cell
layers, Exp. Dermatol., 5, 191, 1996.
[7] Gallico, G.G.I., Biologic skin substitutes, Clin. Plast. Surg., 17, 519, 1990.
[8] Wainwright, D., Madden, M., Luterman, A., Hunt, J., Monafo, W., Heimbach, D., Kagan, R.,
Sittig, K., Dimick, A., and Herndon, D., Clinical evaluation of an acellular allograft dermal matrix
in full-thickness burns, J. Burn Care Rehabil., 17, 124, 1996.
[9] Sheridan, R., Choucair, R., Donelan, M., Lydon, M., Petras, L., and Tompkins, R., Acellular
allodermis in burns surgery: 1-year results of a pilot trial, J. Burn Care Rehabil., 19, 528,
1998.
[10] Menon, N.G., Rodrigues, E.D., Byrnes, C.K., Girotto, J.A., Goldberg, N.H., and Silverman, R.P.,
Revascularization of human acellular dermis in full-thickness abdominal wall reconstruction in
the rabbit model, Ann. Plast. Surg., 50, 523, 2003.
[11] Lorenz, R.R., Dean, R.L., Hurley, D.B., Chuang, J., and Citardi, M.J., Endoscopic reconstruction
of anterior and middle cranial fossa defects using acellular dermal allograft, Laryngoscope, 113,
496, 2003.
[12] Falanga, V., Margolis, D.J., Alvarez, O., Auletta, M., Maggiacomo, F., Altman, M., Jensen, J.,
Sabolinski, M., and Hardin-Young, J., Rapid healing of venous ulcers and lack of clinical rejection
with an allogeneic cultured human skin equivalent, Arch. Dermatol., 134, 293, 1998.
[13] Eaglstein, W.H., Iriondo, M., and Laszlo, K., A composite skin substitute (Graftskin) for surgical
wounds: a clinical experience, Dermatol. Surg., 21, 839, 1995.
[14] Sams, H.H., Chen, J., and King, L.E., Graftskin treatment of difficult to heal diabetic foot ulcers:
one center’s experience, Dermatol. Surg., 28, 698, 2002.
[15] Curran, M.P. and Plosker, G.L., Bilayered bioengineered skin substitute (Apligraf): a review of its
use in the treatment of venous leg ulcers and diabetic foot ulcers, BioDrugs, 16, 439, 2002.
[16] Phillips, T.J., Manzoor, J., Rojas, A., Isaacs, C., Carson, P., Sabolinski, M., Young, J., and Falanga, V.,
The longevity of a bilayered skin substitute after application to venous ulcers, Arch. Dermatol., 138,
1079, 2002.
[17] Falabella, A.F., Schachner, L.A., Valencia, I.C., and Eaglstein, W.H., The use of tissue-engineered
skin (Apligraf) to treat a newborn with epidermolysis bullosa, Arch. Dermatol., 135, 1219, 1999.
mikos: “9026_c018” — 2007/4/9 — 15:51 — page 12 — #12
18-12 Tissue Engineering
[18] Falabella, A.F., Valencia, I.C., Eaglstein, W.H., and Schachner, L.A., Tissue-engineered skin
(Apligraf) in the healing of patients with epidermolysis bullosa wounds, Arch. Dermatol., 136,
1225, 2000.
[19] Ozerdem, O.R., Wolfe, S.A., and Marshall, D., Use of skin substitutes in pediatric patients,
J. Craniofac. Surg., 14, 517, 2003.
[20] Fivenson, D.P., Scherschun, L., and Cohen, L.V., Apligraf in the treatment of severe mitten
deformity associated with recessive dystrophic epidermolysis bullosa, Plast. Reconstr. Surg., 112,
584, 2003.
[21] Tavis, M.N., Thornton, N.W., Bartlett, R.H., Roth, J.C., and Woodroof, E.A., A new composite
skin prosthesis, Burns, 7, 123, 1980.
[22] Purdue, G.F., Hunt, J.L., Gillespie, R.W., Hansbrough, J.F., Dominic, W.J., Robson, M.C.,
Smith, D.J., MacMillan, B.G., Waymack, J.P., Heradon, D.N. et al., Biosynthetic skin substi-
tute versus frozen human cadaver allograft for temporary coverage of excised burn wounds, J.
Trauma, 27, 155, 1987.
[23] Lal, S., Barrow, R.E., Wolf, S.E., Chinkes, D.L., Hart, D.W., Heggers, J.P., and Herndon, D.N.,
Biobrane improves wound healing in burned children without increased risk of infection, Shock,
14, 314, 2000.
[24] Arevalo, J.M. and Lorente, J.A., Skin coverage with Biobrane biomaterial for the treatment of
patients with toxic epidermal necrolysis, J. Burn Care Rehabil., 20, 406, 1999.
[25] Hansbrough, J.F., Boyce, S.T., Cooper, M.L., and Foreman, T.J., Burn wound closure with cul-
tured autologous keratinocytes and fibroblasts attached to a collagen–glycosaminoglycan substrate,
JAMA, 262, 2125, 1989.
[26] Boyce, S.T., Greenhalgh, D.G., Kagan, R.J., Housinger, T., Sorrell, J.M., Childress, C.P., Rieman,
M., and Warden, G.D., Skin anatomy and antigen expression after burn wound closure with
composite grafts of cultured skin cells and biopolymers, Plast. Reconstr. Surg., 91, 632, 1993.
[27] Boyce, S.T., Goretsky, M.J., Greenhalgh, D.G., Kagan, R.J., Rieman, M.T., and Warden, G.D.,
Comparative assessment of cultured skin substitutes and native skin autograft for treatment of
full-thickness burns, Ann. Surg., 222, 743, 1995.
[28] Boyce, S.T., Glatter, R., and Kitzmiller, W.J., Treatment of chronic wounds with cultured cells and
biopolymers: a pilot study, Wounds, 7, 24, 1995.
[29] Passaretti, D., Billmire, D., Kagan, R., Corcoran, J., and Boyce, S., Autologous cultured skin
substitutes conserve donor site autograft in elective treatment of congenital giant melanocyte
nevus, Plast. Reconstr. Surg. 114, 1523, 2004.
[30] Cooper, M.L., Hansbrough, J.F., Spielvogel, R.L., Cohen, R., Bartel, R.L., and Naughton, G.,
In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a
biodegradable polyglycolic or polyglactin mesh, Biomaterials, 12, 243, 1991.
[31] Hansbrough, J.F., Dore, C., and Hansbrough, W.B., Clinical trials of a living dermal tissue replace-
ment placed beneath meshed, split-thickness skin grafts on excised wounds, J. Burn Care Rehabil.,
13, 519, 1992.
[32] Hanft, J.R. and Surprenant, M.S., Healing of chronic foot ulcers in diabetic patients treated with a
human fibroblast-derived dermis, J. Foot Ankle Surg., 41, 291, 2002.
[33] Carsin, H., Ainaud, P., Le Bever, H., Rives, J., Lakhel, A., Stephanazzi, J., Lambert, F., and Perrot, J.,
Cultured epithelial autografts in extensive burn coverage of severelytraumatized patients: a five year
single-center experience with 30 patients, Burns, 26, 379, 2000.
[34] Gobet, R., Raghunath, M., Altermatt, S., Meuli-Simmen, C., Benathan, M., Dietl, A., and
Meuli, M., Efficacy of cultured epithelial autografts in pediatric burns and reconstructive surgery,
Surgery, 121, 654, 1997.
[35] Compton, C.C., Current concepts in pediatric burn care: the biology of cultured epithelial
autografts: an eight-year study in pediatric burn patients, Eur. J. Pediatr. Surg., 2, 216, 1992.
[36] Gallico III, G.G., O’Connor, N.E., Compton, C.C., Remensynder, J.P., Kehinde, O., and Green, H.,
Cultured epithelial autografts for giant congenital nevi, Plast. Reconstr. Surg., 84, 1, 1989.
mikos: “9026_c018” — 2007/4/9 — 15:51 — page 13 — #13
Skin Substitutes 18-13
[37] Koria, P., Brazeau, D., Kirkwood, K., Hayden, P., Klausner, M., and Andreadis, S.T., Gene expres-
sion profile of tissue engineered skin subjected to acute barrier disruption, J. Invest. Dermatol.,
121, 368, 2003.
[38] Oren, A., Ganz, T., Liu, L., and Meerloo, T., In human epidermis, beta-defensin 2 is packaged in
lamellar bodies, Exp. Mol. Pathol., 74, 180, 2003.
[39] Liu, L., Roberts, A.A., and Ganz, T., By IL-1 signaling, monocyte-derived cells dramatically
enhance the epidermal antimicrobial response to lipopolysaccharide, J. Immunol., 170, 575, 2003.
[40] Liebsch, M., Traue, D., Barrabas, C., Spielmann, H., Uphill, P., Wilkins, S., McPherson, J.P.,
Wiemann, C., Kaufmann, T., Remmele, M., and Holzhutter, H.-G., The ECVAM prevalidation
study on the use of EpiDerm for skin corrosivity testing, AT L A , 28, 371, 2000.
[41] Limat, A., Mauri, D., and Hunziker, T., Successful treatment of chronic leg ulcers with epidermal
equivalents generated from cultured autologous outer root sheath cells, J. Invest. Dermatol., 107,
128, 1996.
[42] Yang, J.S., Lavker, R.M., and Sun, T.T., Upper human hair follicle contains a subpopulation of
keratinocytes with superior in vitro proliferative potential, J. Invest. Dermatol., 101, 652, 2003.
[43] Yannas, I.V. and Burke, J.F., Design of an artificial skin. I. Basic design principles, J. Biomed. Mater.
Res., 14, 65, 1980.
[44] Yannas, I.V., Burke, J.F., Gordon, P.L., Huang, C., and Rubenstein, R.H., Design of an artificial
skin. II. Control of chemical composition, J. Biomed. Mater. Res., 14, 107, 1980.
[45] Heimbach, D., Luterman, A., Burke, J.F., Cram, A., Herndon, D., Hunt, J., Jordon, M., McManus,
W., Solem, L., Warden, G., and Zawacki, B., Artificial dermis for major burns; a multi-center
randomized clinical trial, Ann. Surg., 208, 313, 1988.
[46] Sheridan, R.L., Hegarty, M., Tompkins, R.G., and Burke, J.F., Artificial skin in massive burns —
results to ten years, Eur. J. Plast. Surg., 17, 91, 1994.
[47] Heimbach, D.M., Warden, G.D., Luterman, A., Jordan, M.H., Ozobia, N., Ryan, C.M., Voigt,
D.W., Hickerson, W.L., Saffle, J.R., DeClement, F.A., Sheridan, R.L., and Dimick, A.R., Multicenter
postapproval clinical trial of Integra dermal regeneration template for burn treatment, J. Burn Care
Rehabil., 24, 42, 2003.
[48] Wisser, D. and Steffes, J., Skin replacement with a collagen based dermal substitute, autologous
keratinocytes and fibroblasts in burn trauma, Burns, 29, 375, 2003.
[49] Kopp, J., Magnus, N.E., Rubben, A., Merk, H.F., and Pallua, N., Radical resection of giant
congenital melanocyte nevus and reconstruction with meek-graft covered integra dermal template,
Dermatol. Surg., 29, 653, 2003.
[50] Stephens, R., Wilson, K., and Silverstein, P., A premature infant with skin injury successfully
treated with bilayered cell matrix, Ostomy Wound Manage., 48, 34, 2002.
[51] Still, J., Glat, P., Silverstein, P., Griswold, J., and Mozingo, D., The use of a collagen spong/living
cell composite material to treat donor sites in burn patients, Burns, 29, 837, 2003.
[52] Doucet, O., Robert, C., and Zastrow, L., Use of a serum-free reconstituted epidermis as a skin
pharmacological model, Toxicol. In Vitro, 10, 305, 1996.
[53] De Fraissinette, A.D.B., Picarles, V., Chibout, S.D., Kolopp, M., Medina, J., Burtin, P.,
Ebelin, M.-E., Osborne, S., Mayer, F.K., Spake, A., Rosdy, M., De Wever, B., Ettline, R.A., and
Cordier, A., Predictivity of an in vitro model for acute and chronic skin irritation (SkinEthic)
applied to the testing of topical vehicles, Cell Biol. Toxicol., 15, 121, 1999.
[54] Medina, J., Elsaesser, C., Picarles, V., Grenet, O., Kolopp, M., Chibout, S.D., and De Fraissinette,
A.D.B., Assessment of the phototoxic potential of compounds and finished topical products using
a human reconstructed epidermis, InVitroMol.Toxicol., 14, 157, 2001.
[55] Higham, M.C., Dawson, R., Szabo, M., Short, R., Haddow, D.B., and MacNeil, S., Development
of a stable chemically defined surface for the culture of human keratinocytes under serum-free
conditions for clinical use, Tissue Eng., 9, 919, 2003.
[56] Hansbrough, J.F., Mozingo, D.W., Kealey, G.P., Davis, M., Gidner, A., and Gentzkow, G.D., Clinical
trials of a biosynthetic temporary skin replacement, Dermagraft-transitional covering, compared
mikos: “9026_c018” — 2007/4/9 — 15:51 — page 14 — #14
18-14 Tissue Engineering
with cryopreserved human cadaver skin for temporary coverage of excised burn wounds, J. Burn
Care Rehabil., 18, 43, 1997.
[57] Purdue, G.F., Hunt, J.L., Still Jr, J.M., Law, E.J., Herndon, D.N., Goldfarb, I.W., Schiller, W.R.,
Hansbrough, J.F., Hickerson, W.L., Himel, H.N., Kealey, G.P., Twomey, J., Missavage, A.E., Solem,
L.D., Davis, M., Totoritis, M., and Gentzkow, G.D., A multicenter clinical trial of a biosynthetic skin
replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary
coverage of excised burn wounds, J. Burn Care Rehabil., 18, 52, 1997.
[58] Noordenbos, J., Dore, C., and Hansbrough, J.F., Safety and efficacy of TransCyte for the treatment
of partial-thickness burns, J. Burn Care Rehabil., 20, 275, 1999.
[59] Odessey, R., Addendum: multicenter experience with cultured epithelial autografts for treatment
of burns, J. Burn Care Rehabil., 13, 174, 1992.
[60] Pittelkow, M.R. and Scott, R.E., New techniques for the in vitro culture of human skin keratinocytes
and perspectives on their use for grafting of patients with extensive burns, Mayo Clin. Proc., 61,
771, 1986.
[61] Desai, M.H., Mlakar, J.M., McCauley, R.L., Abdullah, K.M., Rutan, R.L., Waymack, J.P.,
Robson, M.C., and Herndon, D.N., Lack of long term durability of cultured keratinocyte burn
wound coverage: a case report, J. Burn Care Rehabil., 12, 540, 1991.
[62] Williamson, J., Snelling, C., Clugston, P., Mac Donald, I., and Germann, E., Cultured epithelial
autograft: five years of clinical experience with twenty-eight patients, J. Trauma, 39, 309, 1995.
[63] Rue, L.W., Cioffi, W.G., McManus, W.F., and Pruitt, B.A., Wound closure and outcome in
extensively burned patients treated with cultured autologous keratinocytes, J. Trauma, 34, 662,
1993.
[64] Arons, M.S., Management of giant congenital nevi, Plast. Reconstr. Surg., 110, 352, 2002.
[65] Boyce, S.T., Cultured skin substitutes: a review, Tissue Eng., 2, 255, 1996.
[66] Boyce, S.T. and Warden, G.D., Principles and practices for treatment of cutaneous wounds with
cultured skin substitutes, Am.J.Surg., 183, 445, 2002.
[67] Boyce, S.T., Kagan, R.J., Meyer, N.A., Yakuboff, K.P., and Warden, G.D., The 1999 Clinical Research
Award, Cultured skin substitutes combined with Integra to replace native skin autograft and
allograft for closure of full-thickness burns, J. Burn Care Rehabil., 20, 453, 1999.
[68] Boyce, S.T., Kagan, R.J., Yakuboff, K.P., Meyer, N.A., Rieman, M.T., Greenhalgh, D.G., and
Warden, G.D., Cultured skin substitutes reduce donor skin harvesting for closure of excised,
full-thickness burns, Ann. Surg., 235, 269, 2002.
[69] Lukish, J.R., Eichelberger, M.R., Newman, K.D., Pao, M., Nobuhara, K., Keating, M., Golonka,
N., Pratsch, G., Misra, V., Valladares, E., Johnson, P., Gilbert, J.C., Powell, D.M., and Hartman,
G.E., The use of a bioactive skin substitute decreases length of stay for pediatric burn patients, J.
Pediatr. Surg., 36, 1118, 2001.
[70] Klein, R.L., Rothmann, B.F., and Marshall, R., Biobrane — a useful adjunct in the therapy of
outpatient burns, J. Pediatr. Surg., 19, 846, 1984.
[71] Boyce, S.T. and Ham, R.G., Cultivation, frozen storage, and clonal growth of normal human
epidermal keratinocytes in serum-free media, J. Tissue Cult. Meth., 9, 83, 1985.
[72] Boyce, S.T., Methods for serum-free culture of keratinocytes and transplantation of collagen-GAG
based composite grafts, in Methods in Tissue Engineering, Morgan, J.R. and Yarmush, M., Eds.,
Humana Press, Inc., Totowa, NJ, 1998, p. 365.
[73] Boyce, S.T., Methods for the serum-free culture of keratinocytes and transplantation of collagen-
GAG-based skin substitutes, in Methods in Molecular Medicine, Vol. 18: Tissue Engineering Methods
and Protocols, Morgan, J.R. and Yarmush, M.L., Eds., Humana Press Inc., Totowa, NJ, 2001, p. 365.
[74] Munster, A.M., Cultured skin for massive burns: a prospective, controlled trial, Ann. Surg., 224,
372, 1996.
[75] Boyce, S.T., Foreman, T.J., English, K.B., Stayner, N., Cooper, M.L., Sakabu, S., and Hansbrough,
J.F., Skin wound closure in athymic mice with cultured human cells, biopolymers, and growth
factors, Surgery, 110, 866, 1991.
mikos: “9026_c018” — 2007/4/9 — 15:51 — page 15 — #15
Skin Substitutes 18-15
[76] Boyce, S.T., Cultured skin for wound closure, in Skin Substitute Production by Tissue Engineering:
Clinical and Fundamental Applications, Rouahbia, M., Ed., R.G. Landes, Austin, TX, 1997, p. 75.
[77] Boyce, S.T., Skin substitutes from cultured cells and collagen-GAG polymers, Med. Biol. Eng.
Comp., 36, 791, 1998.
[78] Boyce, S.T. and Ham, R.G., Calcium-regulated differentiation of normal human epidermal kerat-
inocytes in chemically defined clonal culture and serum-free serial culture, J. Invest. Dermatol.,81
(Suppl 1), 33s, 1983.
[79] Harriger, M.D., Warden, G.D., Greenhalgh, D.G., Kagan, R.J., and Boyce, S.T., Pigmentation and
microanatomy of skin regenerated from composite grafts of cultured cells and biopolymers applied
to full-thickness burn wounds, Transplantation, 59, 702, 1995.
[80] Boyce, S.T., Supp, A.P., Swope, V.B., and Warden, G.D., Vitamin C regulates keratinocyte viability,
epidermal barrier, and basement membrane formation in vitro, and reduces wound contraction
after grafting of cultured skin substitutes, J. Invest. Dermatol., 118, 565, 2002.
[81] Boyce, S.T., Supp, A.P., Harriger, M.D., Pickens, W.L., Wickett, R.R., and Hoath, S.B., Surface
electrical capacitance as a noninvasive index of epidermal barrier in cultured skin substitutes in
athymic mice, J. Invest. Dermatol., 107, 82, 1996.
[82] Cuono, C., Langdon, R., Birchall, N., Barttelbort, S., and McGuire, J., Composite autologous-
allogeneic skin replacement: development and clinical application, Plast. Reconstr. Surg., 80, 626,
1987.
[83] Burke, J.F., Yannas, I.V., Quinby, W.C., Bondoc, C.C., and Jung, W.K., Successful use of a
physiologically acceptable skin in the treatment of extensive burn injury, Ann. Surg., 194, 413,
1981.
[84] Young, D.M., Greulich, K.M., and Weier, H.G., Species-specific in situ hybridization with
fluorochrome-labeled DNA probes to study vascularization of human skin grafts on athymic
mice, J. Burn Care Rehabil., 17, 305, 1996.
[85] Supp, D.M., Supp, A.P., Bell, S.M., and Boyce, S.T., Enhanced vascularization of cultured skin
substitutes genetically modified to overexpress Vascular Endothelial Growth Factor, J. Invest.
Dermatol., 114, 5, 2000.
[86] Supp, D.M. and Boyce, S.T., Overexpression of vascular endothelial growth factor accelerates early
vascularization and improves healing of genetically modified cultured skin substitutes, J. Burn
Care Rehabil., 23, 10, 2002.
[87] Monafo, W.W. and West, M.A., Current treatment recommendations for topical burn therapy,
Drugs, 40, 364, 1990.
[88] Boyce, S.T. and Holder, I.A., Selection of topical antimicrobial agents for cultured skin for burns
by combined assessment of cellular cytotoxicity and antimicrobial activity, Plast. Reconstr. Surg.,
92, 493, 1993.
[89] Boyce, S.T., Warden, G.D., and Holder, I.A., Cytotoxicity testing of topical antimicrobial agents on
human keratinocytes and fibroblasts for cultured skin grafts, J. Burn Care Rehabil., 16, 97, 1995.
[90] Boyce, S.T., Warden, G.D., and Holder, I.A., Non-cytotoxic combinations of topical antimicrobial
agents for use with cultured skin, Antimicrob. Agents Chemother., 39, 1324, 1995.
[91] Compton, C.C., Wound healing potential of cultured epithelium, Wounds, 5, 97, 1993.
[92] Munster, A.M., Weiner, S.H., and Spence, R.J., Cultured epidermis for coverage of burn wounds:
a single center experience, Ann. Surg., 211, 676, 1990.
[93] Sullivan, T., Smith, H., Kermode, J., Mclver, E., and Courtemanche, D.J., Rating the burn scar,
J. Burn Care Rehabil., 11, 256, 1990.
[94] Supp, A.P., Wickett, R.R., Swope, V.B., Harriger, M.D., Hoath, S.B., and Boyce, S.T., Incuba-
tion of cultured skin substitutes in reduced humidity promotes cornification in vitro and stable
engraftment in athymic mice, Wound Repair Regen., 7, 226, 1999.
[95] Goretsky, M.J., Supp, A.P., Greenhalgh, D.G., Warden, G.D., and Boyce, S.T., The 1995 Young
Investigator Award: Surface electrical capacitance as an index of epidermal barrier properties of
composite skin substitutes and skin autografts, Wound Repair Regen., 3, 419, 1995.
