Ferry M. Direct Stripcasting of Metals and Alloys: Processing, Microstructure and Properties
Подождите немного. Документ загружается.

Chapter
2
Overview
of
Solidification
Processing
2.1
Introduction
Solidification is the transformation of liquid into the solid crystalline state via
the nucleation of the solid phase and its subsequent growth to consume the
liquid. The solidification reaction is fundamental to many technological
processes such as ingot
and
foundry casting, continuous slab casting, net-shape
die casting, welding and joining
and
single crystal growth for the production of
semiconductor devices and turbine blades. The production of strip products by
direct strip casting
(OSC)
is particularly reliant
on
a sound fundamental
understanding of the atomic processes that govern both the nucleation
and
growth stages of solidification. The control of these stages dictates the
microstructure
and
many important properties of the as-cast strip. This chapter
provides an overview of some of the more important theoretical
and
practical
aspects of solidification phenomena applicable to casting of metals and alloys.
2.2 Development
of
solidification microstructure
2.2.1
Nucleation from the melt
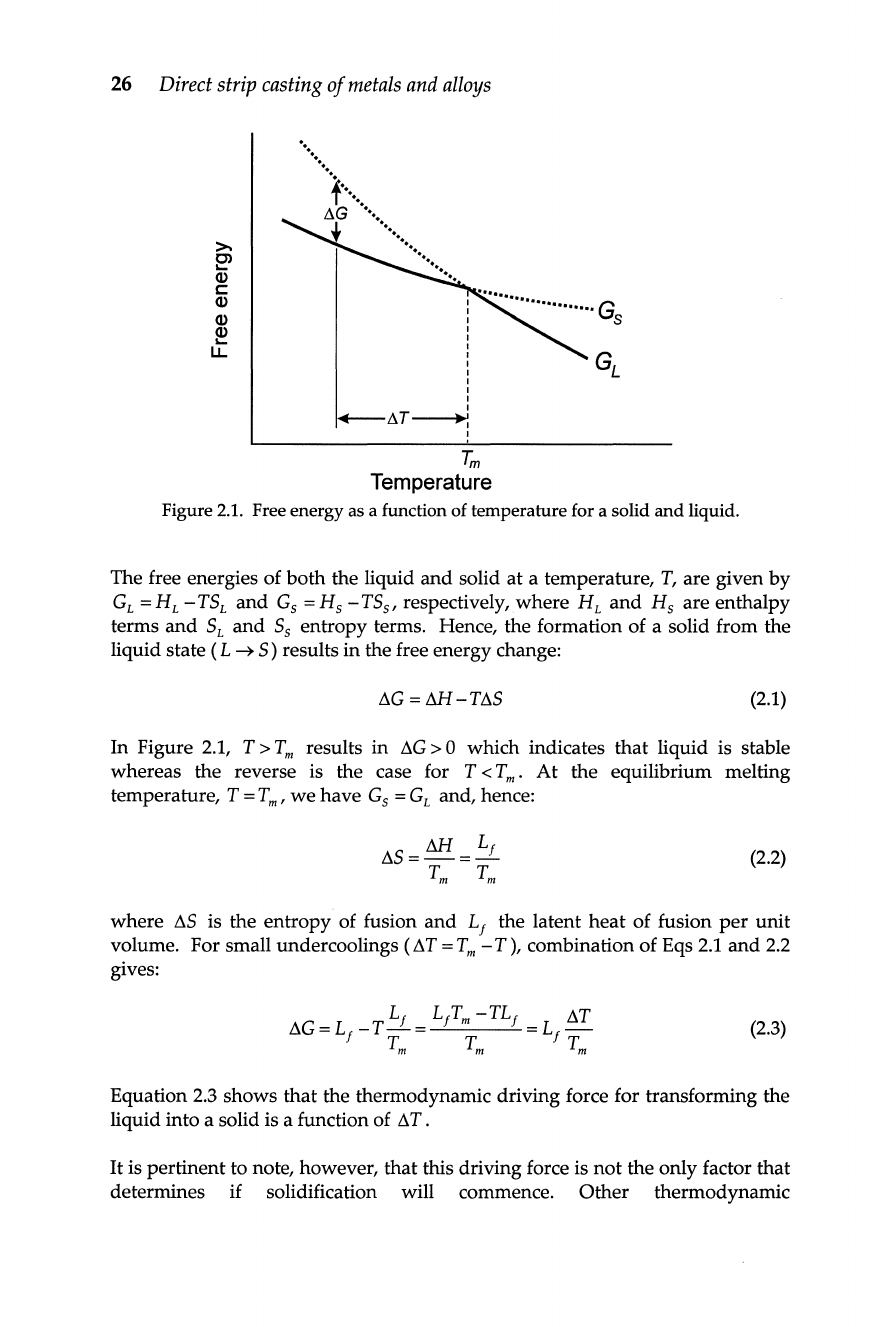
When a liquid is cooled below its equilibrium melting temperature
(Tm),
there is
a thermodynamic driving force for solidification
(l1G). Figure
2.1
shows the
free energy as a function of temperature for a solid
and
liquid phase which
indicates how the change
in
free energy
(l1G)
varies with respect to T m.
25
26
Direct
strip
casting
of
metals
and
alloys
>.
e>
Q)
c
Q)
Q)
~
LL
'.
".
"''''''
T '.
L\G
••••
:t
....
'.
'.
'.
'.
'.
'.
".
:
·················
..
···G
I S
I
I
I
: G
L
I
I
I
I
-+--~T--+:
Tm
Temperature
Figure
2.1.
Free energy as a function of temperature for a solid
and
liquid.
The free energies of both the liquid and solid
at
a temperature,
T,
are given
by
G
L
=H
L
-T5
L
and
G
s
=Hs
-T5
s
'
respectively, where
HL
and
Hs
are enthalpy
terms
and
5
L
and
5
s
entropy terms. Hence, the formation of a solid from the
liquid state (
L
~
5)
results in the free energy change:
L'lG
=
L'lH
-Ti'l5
(2.1)
In Figure 2.1, T >
Tm
results in
L'lG
> 0 which indicates that liquid is stable
whereas the reverse is the case for
T <
Tm'
At
the equilibrium melting
temperature,
T = T
m
,
we
have G
s
= G
L
and, hence:
(2.2)
where
L'l5
is the entropy of fusion
and
L f the latent heat of fusion
per
unit
volume. For small undercoolings
(L'lT
=
Tm
-
T),
combination of Eqs
2.1
and
2.2
gives:
(2.3)
Equation
2.3
shows that the thermodynamic driving force for transforming the
liquid into a solid is a function of
L'lT.
It
is pertinent to note, however, that this driving force is
not
the only factor that
determines if solidification will commence.
Other thermodynamic
Overview
of
solidification
processing
27
considerations such as energy created
by
the generation of solid-liquid
interfaces
and
the thermophysical properties of the fluid
must
also be
considered (Flemings 1974).
2.2.1.1
Homogeneous
nucleation
There are many detailed treatments of the nucleation stage of solidification, for
example: Chalmers
(1967),
Flemings
(1974),
Kurz
and
Fisher
(1989),
Porter and
Easterling
(1992)
and
Christian
(2002).
Homogeneous nucleation may be
defined as the formation of a
new
phase from the parent phase
without
aid of
foreign materials.
While the formation of a solid particle
in
a liquid
at
T <
Tm
results in a natural
decrease in free energy associated
with
the phase change (Eq. 2.3), there is a
corresponding increase in energy
due
to the creation of a solid-liquid interface.
The overall energy change associated with the formation of the solid is:
~Ghom
= -
Vs~G
+
ASLrSL
~
'--v----'
energy energy
decrease
inrease
(2.4a)
where
r
SL
is the surface energy of the particle/liquid interface
and
Vs
and
ASL
are the volume
and
surface area of the solid, respectively. For a spherical
particle,
we
have:
432
~Ghom
=
--m
~G
+
4m
r
SL
3
(2.4b)
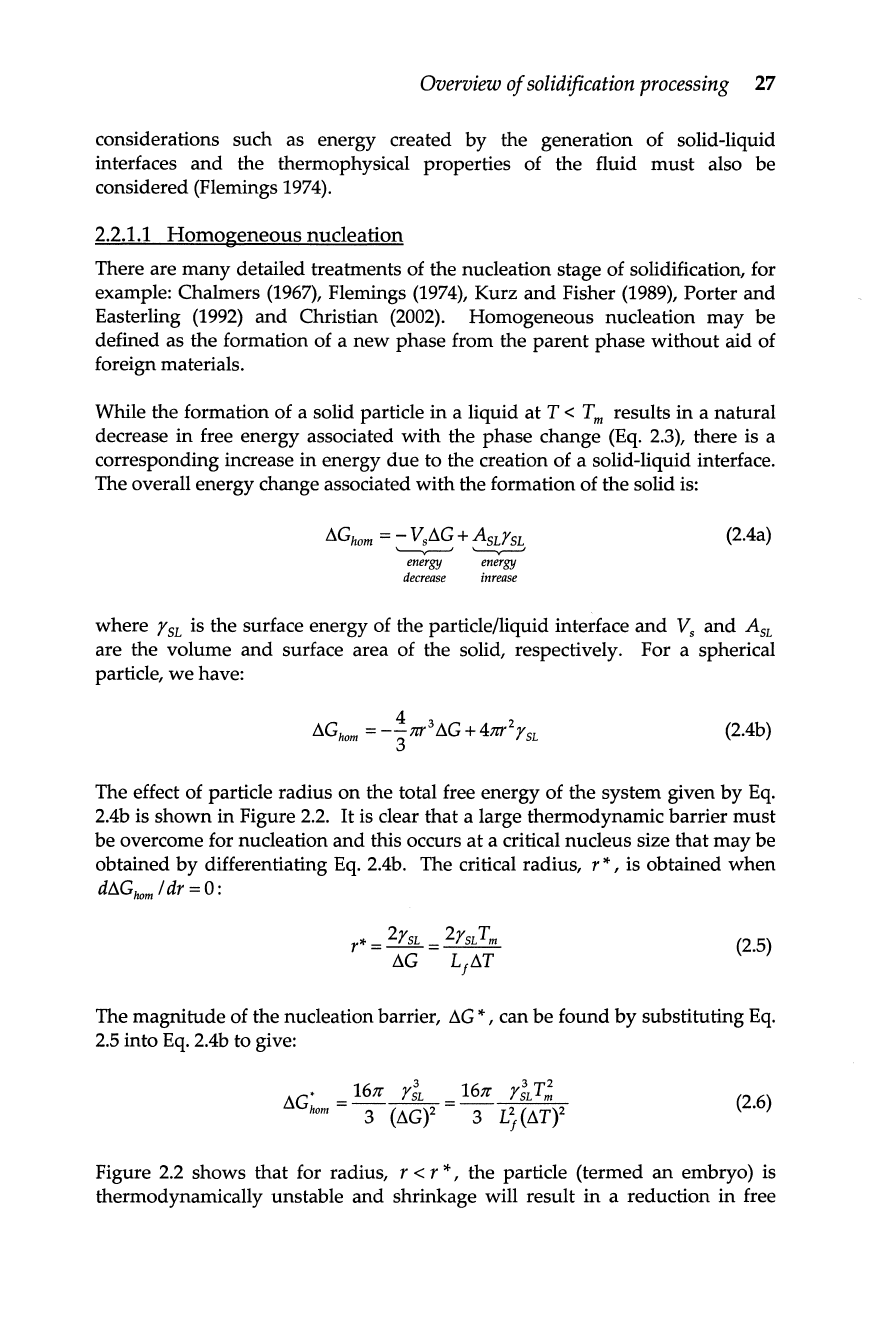
The effect of particle radius
on
the total free energy of the system given
by
Eq.
2.4b is
shown
in Figure
2.2.
It
is clear that a large thermodynamic barrier
must
be overcome for nucleation
and
this occurs
at
a critical nucleus size that
may
be
obtained
by
differentiating Eq. 2.4b. The critical radius, r * , is obtained
when
d~Ghom
/dr
=0:
(2.5)
The magnitude of the nucleation barrier,
~G
* , can be found
by
substituting Eq.
2.5
into
Eq.
2.4b to give:
167l'
r:LT;,
3
L~(~T)2
(2.6)
Figure 2.2 shows that for radius, r < r
*,
the particle (termed
an
embryo) is
thermodynamically unstable
and
shrinkage will result
in
a reduction in free
28
Direct
strip
casting
of
metals
and
alloys
energy. For
r>
r*,
further growth corresponds to a reduction
in
free energy of
the particle, that is, the embryo has exceeded a critical size
and
is
now
stable.
Equation
2.6
shows that the thermodynamic barrier to the formation of a
nucleus,
~G
*,
and critical nucleus size decrease rapidly
with
increasing
undercooling,
~T.
Thus, it may be expected that homogeneous nucleation is
only possible for
T
«Tm
corresponding to a very large undercooling.
+
~~~~------~~----~--~---r
..................
..
..
..
....
.••..
..
-v.D.;····
...
:\
.•.•
\
~Ghom
Figure
2.2.
Free energy as a function of embryo radius illustrating the free energy
barrier to nucleation and critical diameter for nucleation.
The foregoing analysis considers only the nucleation of a single spherical
particle
and
does
not
provide any physical reasons for its formation. In
classical nucleation theory, the critical nucleus is attributed to the stochastic
addition of molecules or atoms to initially sub critical embryos. In the melt,
atoms or molecules undergo random movement but,
due
to the statistical
nature of the process
and
the bonding characteristics of the constituents, at any
given time there is the tendency for short range ordering or clustering of atoms
into a crystalline structure. Hence, small volumes of crystalline material
continually form
and
break
up
in the melt. The number of clusters of a given
radius,
nT' as a function of temperature
is:
nr
=
no
exp(-
~Gr
/ kT)
(2.7)
where
no
is the total number of atoms
in
the system
and
~Gr
is the excess free
energy associated with the cluster. Equation 2.7 indicates that the probability of

Overview
of
solidification
processing
29
producing a cluster of atoms of critical size, r *, near the melting point is
exceedingly low.
The number of clusters per unit volume of liquid of critical radius,
nr
=
n;,
as a
function of undercooling is:
n;
=
no
exp
(-
AG~om
/ kT)
(2.8)
We are now
in
the position to derive a rate equation for homogeneous
nucleation
by
considering the number of clusters that reach the critical size
at
a
given undercooling
and
by
assuming that the addition of a single atom makes
the potential nucleus stable. The rate of formation of these nuclei
per
unit
volume,
IV
hom' is:
(2.9)
where
()}*
is the number of atoms surrounding a critical nucleus
and
v
LS
is the
frequency
at
which atoms jump across the liquid-solid interface. The latter term
takes into account both the vibrational frequency of the atoms
and
the
activation energy for diffusion
in
the liquid.
The classical expression for the rate of homogeneous nucleation
in
bulk liquid
may be written
in
the following form:
(2.10)
where
k\
depends
on
the critical nucleus size and surface energy,
DL
and
DIM
are the diffusivity of the liquid
at
T and T
m
,
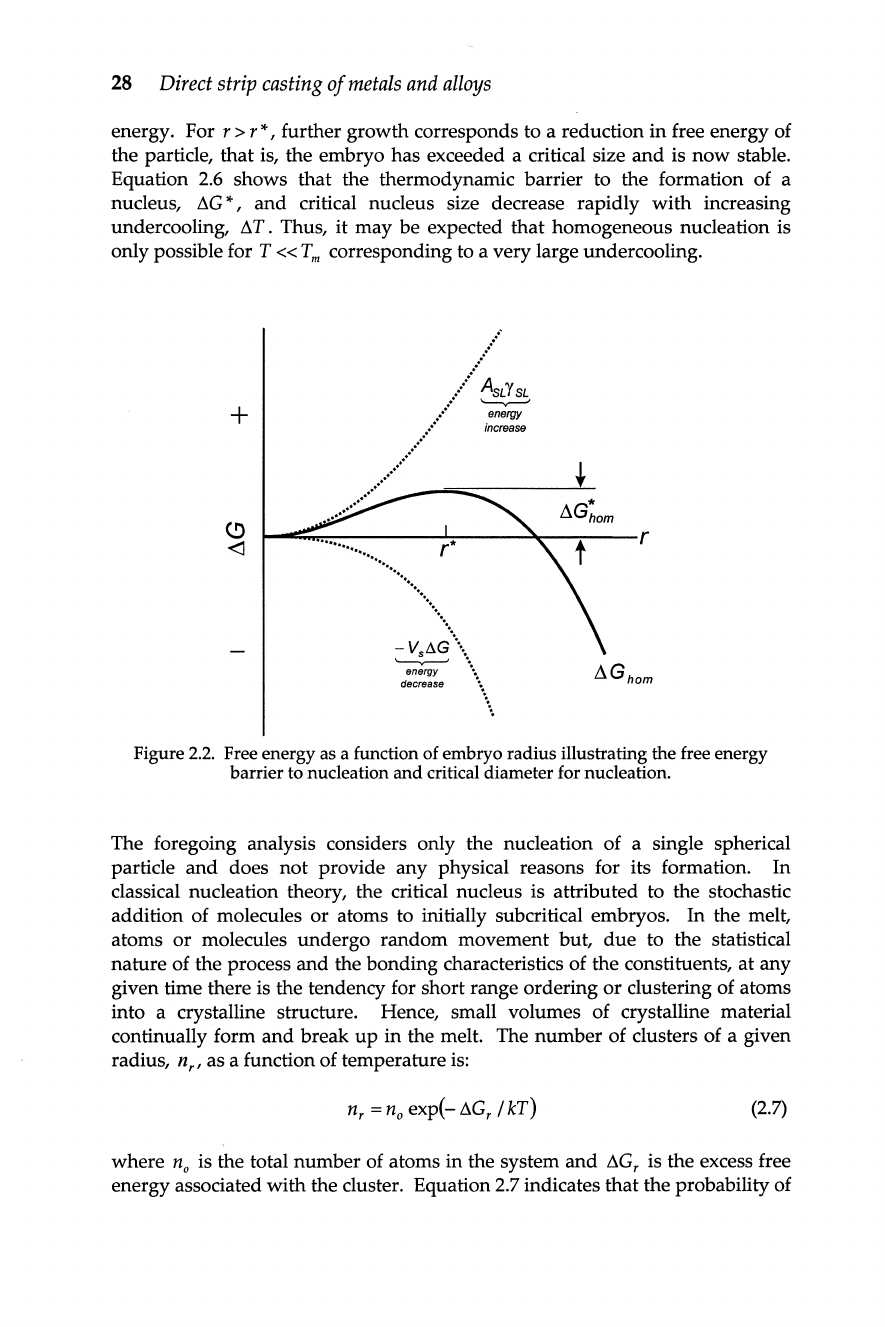
respectively. Table
2.1
provides
experimental data for a range of high purity metals showing that the maximum
undercooling for nucleation
(ATN) is - 0.2T
m;
a value close to that predicted
by
theory.
The ratio,
DL
/
DIM
in
Eq.
2.10
is important for determining whether nucleation
can occur
in
inorganic glasses and polymers as their liquid diffusivity drops
markedly with temperature to become an overriding term. For most liquid
metals,
DL
-
DIM
indicating that atoms remain sufficiently mobile
at
well below
Tm
to generate critical size clusters for nucleation.
On
the other hand, multi-
component metallic alloys often show behaviour similar to inorganic
compounds
(DL < DIM)
and
sufficiently high cooling rates
may
suppress
nucleation altogether thereby generating
an
amorphous structure
at
room
temperature (Porter
and
Easterling
1992;
Jiles
2003).
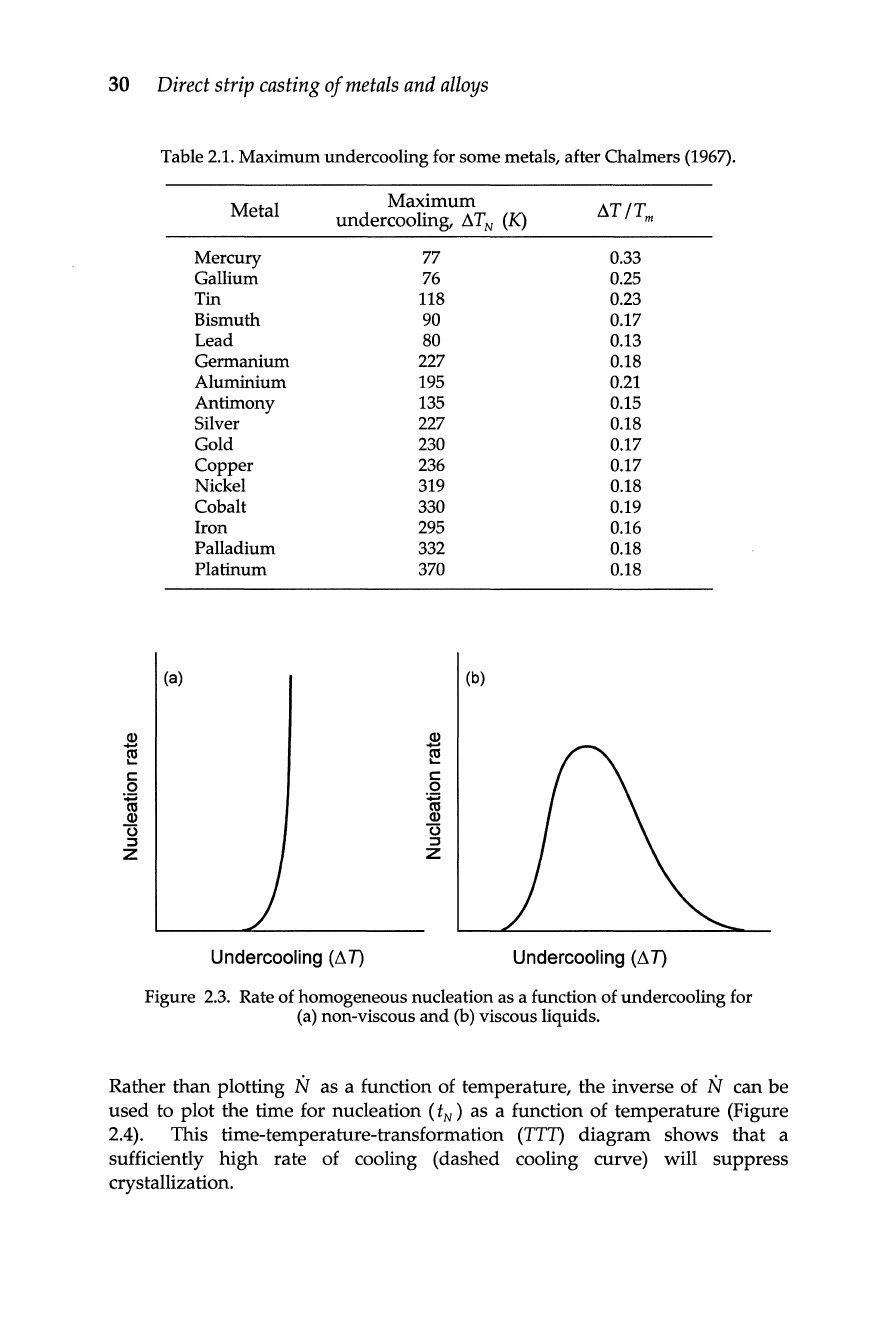
Figure
2.3
shows
schematically the nucleation behaviour for a pure metal
and
a material that
exhibits
an
increase
in
melt viscosity with decreasing temperature.
30
Direct
strip
casting
of
metals
and
alloys
Q)
-
III
...
c:
.Q
Cii
Q)
u
::J
Z
Table
2.1.
Maximum undercooling for some metals, after Chalmers
(1967).
Metal
Maximum
,1T
/Tm
undercooling,
,1T
N
(K)
Mercury
77
0.33
Gallium
76
0.25
Tin
118
0.23
Bismuth
90
0.17
Lead
80
0.13
Germanium
227
0.18
Aluminium
195
0.21
Antimony
135
0.15
Silver
227
0.18
Gold
230
0.17
Copper
236
0.17
Nickel
319
0.18
Cobalt
330
0.19
Iron
295
0.16
Palladium
332
0.18
Platinum
370
0.18
(a)
(b)
Q)
-
e:!
c:
0
:;:::;
III
Q)
U
::J
Z
Undercooling
(,1
T)
Undercooling
(,1
T)
Figure
2.3.
Rate of homogeneous nucleation as a function of undercooling for
(a) non-viscous and
(b)
viscous liquids.
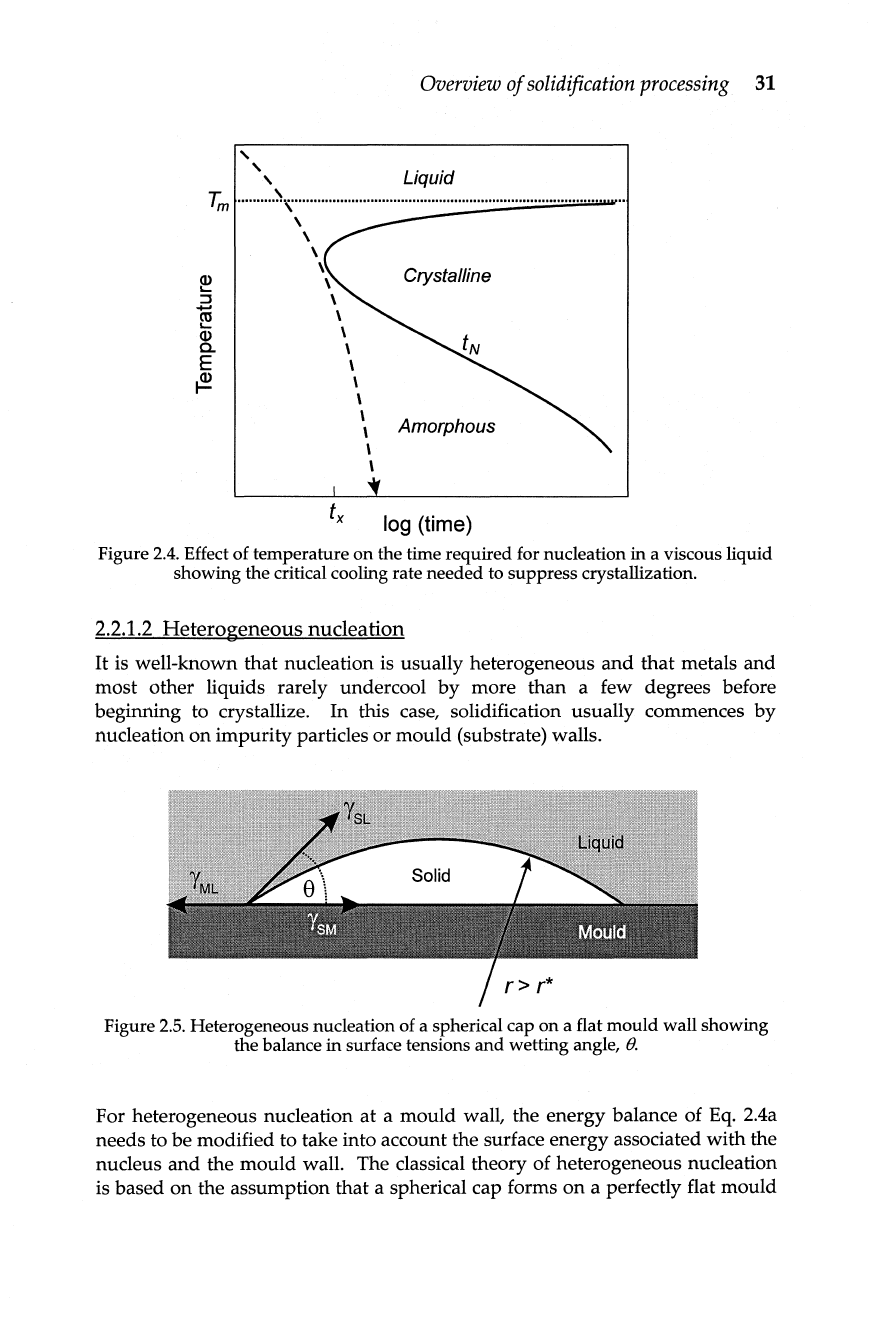
Rather
than
plotting
N
as
a
function
of
temperature,
the
inverse
of
N
can
be
used
to
plot
the
time
for
nucleation
(tN) as a
function
of
temperature
(Figure
2.4).
This
time-temperature-transformation
(TTT)
diagram
shows
that
a
sufficiently
high
rate
of
cooling
(dashed
cooling
curve)
will
suppress
crystallization.
Overview
of
solidification
processing
31
,
" Liquid
Tm
..........
~,
.................................................................................
..
\
\
\
\
\
\
\
\
\
\
\
\
\
\ Amorphous
\
\
log (time)
Figure
2.4.
Effect of temperature on the time required
for
nucleation in a viscous liquid
showing the critical cooling rate needed
to
suppress crystallization.
2.2.1.2
Heterogeneous
nucleation
It
is well-known
that
nucleation is usually heterogeneous
and
that
metals
and
most
other
liquids rarely undercool
by
more
than
a few degrees before
beginning to crystallize.
In
this case, solidification usually commences
by
nucleation
on
impurity
particles
or
mould
(substrate) walls.
Figure
2.5.
Heterogeneous nucleation
of
a spherical cap on a flat mould wall showing
the balance in surface tensions and wetting angle,
B.
For heterogeneous nucleation
at
a
mould
wall, the
energy
balance of Eq. 2.4a
needs
to
be
modified
to
take into account the surface
energy
associated
with
the
nucleus
and
the
mould
wall. The classical theory of
heterogeneous
nucleation
is
based
on
the
assumption
that
a spherical cap forms
on
a perfectly flat
mould

32
Direct
strip
casting
of
metals
and
alloys
wall, as indicated in Figure
2.5.
The total interfacial energy of the embryo in
contact
with
both
the melt
and
mould wall is given as a balance of interfacial
tensions:
(2.11)
where YML
and
YSM
are mould-liquid
and
solid-mould interfacial energies,
respectively,
and
B is the so-called wetting angle.
The total free energy of the system is similar to that given
in
Eq. 2.4a
but
includes some additional terms:
LlG
het
=:-
VsLlG
-
ASMYML,
+ (1SLYSL
+,
ASMYSM,
(2.12)
energy energy
decrease
inrease
where
Vs
is the volume of the spherical cap,
and
ASL
and
ASM
the surface area
of the solid-liquid
and
solid-mould interface, respectively.
This equation differs from Eq. 2.4a since the mould-liquid interface (positive
energy) is removed
by
the formation of the spherical cap. The volume
and
surface areas of the cap are:
Vs
=m
2
(2+cosB)(1-cosBf
/3
ASM
= m
2
sin
2
B
ASL
=
2m
2
(1-cosB}
and
substituting these relations into Eq.
2.12,
it
can be
shown
that:
llG
hel
= llG
hom
. f(B}
where f(B) is a shape factor defined by:
I(O}
=
(2
-3cosO+cos
3
0)/4
(2.13a)
(2.13b)
(2.13c)
(2.14)
(2.15)
Following the same principles that generated Eqs
2.5
and
2.6,
it can
be
shown
that
r;om
=
r;et,
but:
llG~et
=
llG~om
. f(B)
(2.16)
Hence, the critical radius is the same for
both
types of nucleation event,
but
since f(B} < 1 the activation barrier for heterogeneous nucleation is always less
than for homogeneous nucleation (Figure 2.6). For
0 = 180°,
we
have the
condition
where
no wetting occurs, which means that nucleation
at
the mould
